Abstract
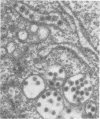
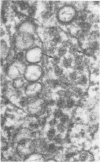
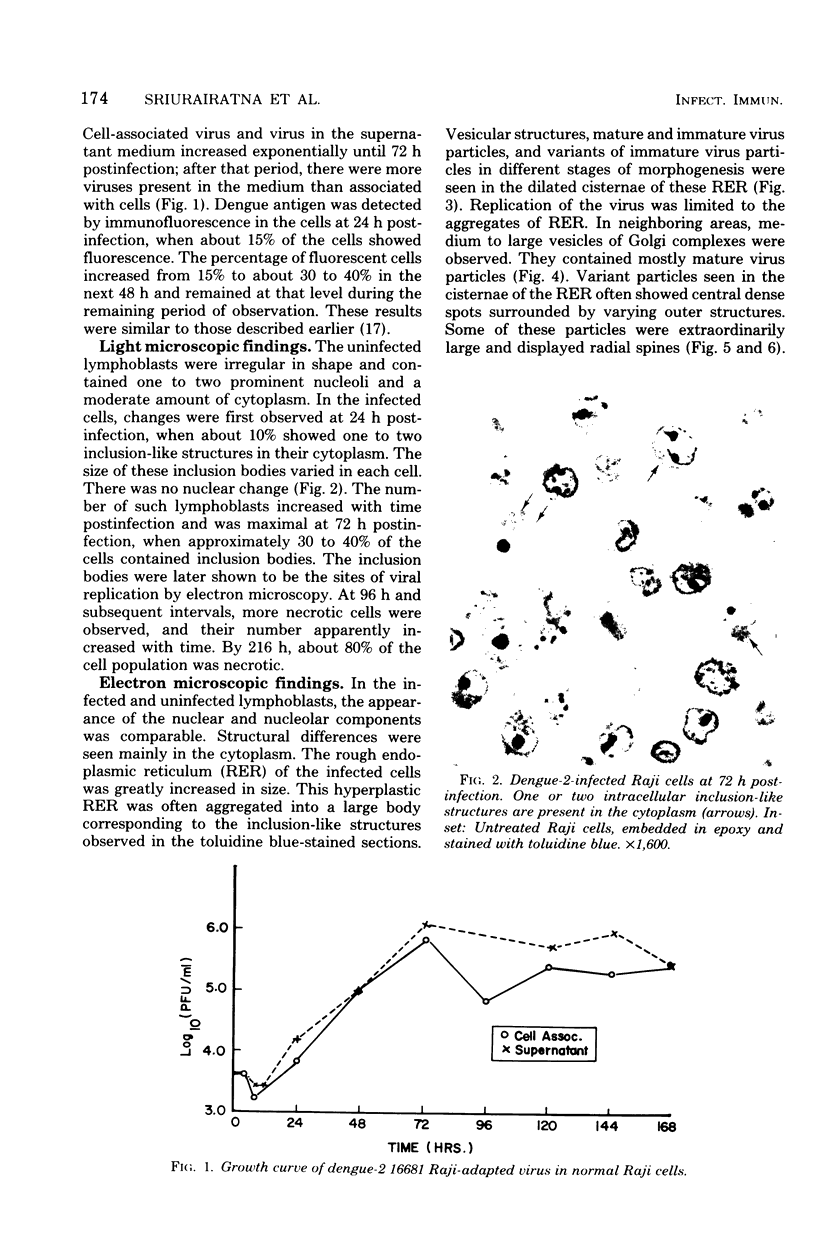
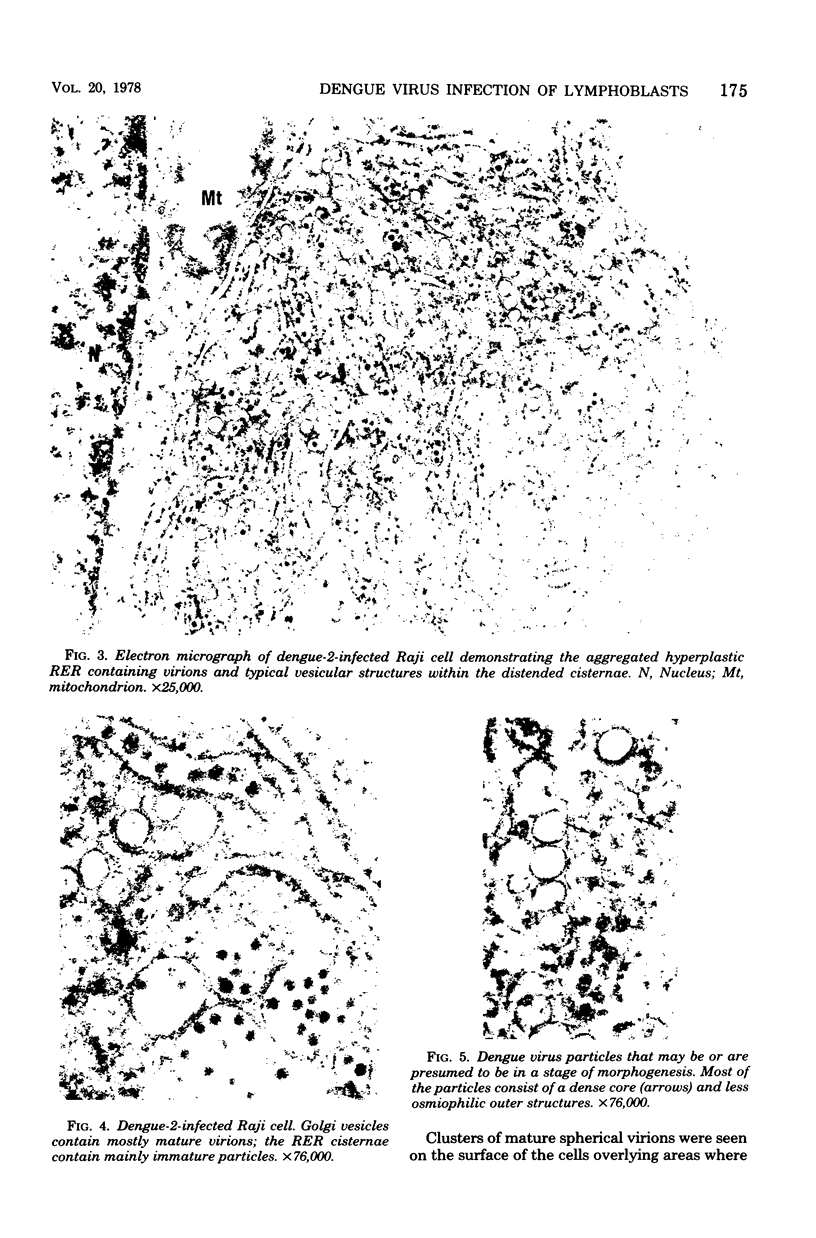
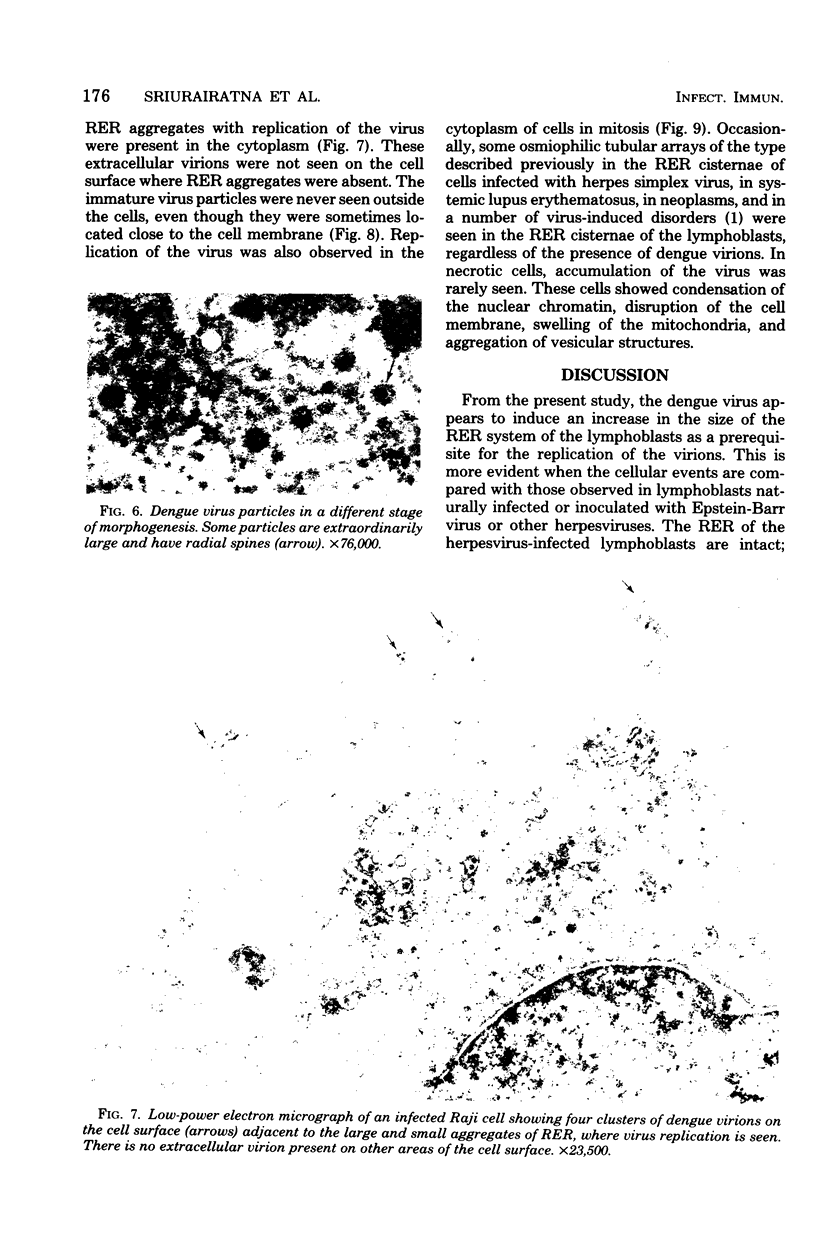
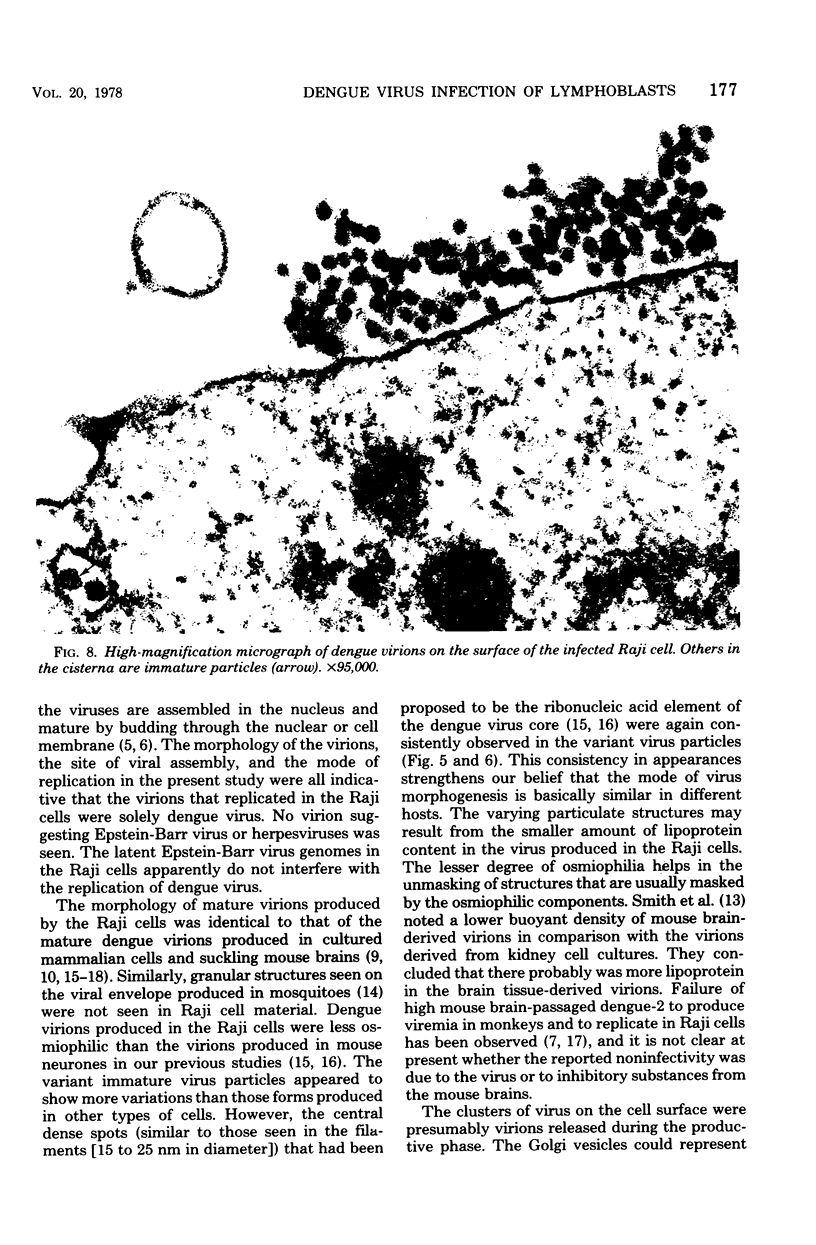
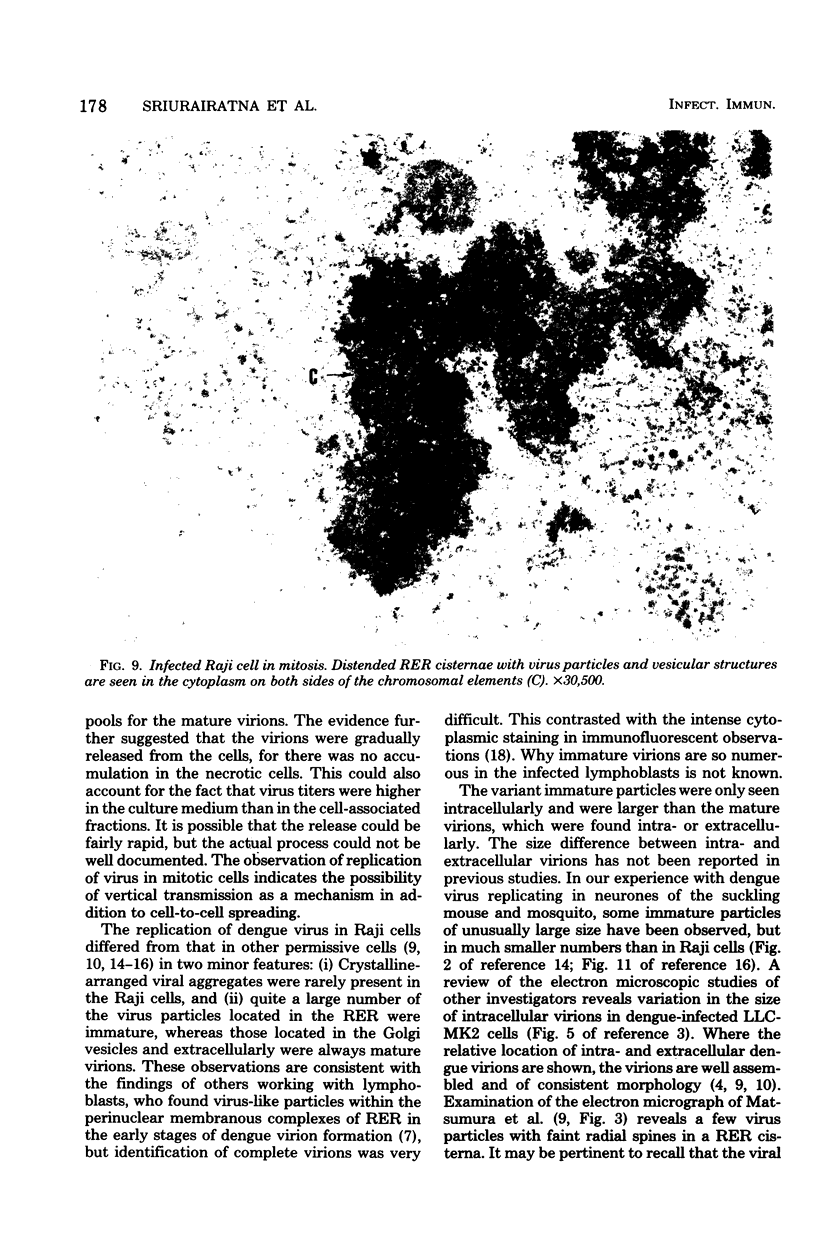
Ultrastructural studies of dengue-2 virus-infected lymphoblastoid Raji cells showed that the virus induced an increase in the size of the rough endoplasmic reticula (RER) and that the replication of the virus was confined to the cisternae of these RER. The proliferating RER formed cytoplasmic inclusions that could be seen by light microscopy. This observation could be used as evidence of a cytopathogenic effect of dengue virus on infected Rajii cells in routine cultures. Accumulation of virions in the infected cells was minimal in comparison with other cell systems, however. Sporadic clusters of mature virions were often seen on the plasma membrane. These extracellular virions were distributed adjacent to the virus-bearing RER and were presumably released virions. Vertical transmission of the virus was evident in mitotic lymphoblasts. The replication pattern of dengue virus in lymphoblastoid cells suggests that efforts should be made to determine whether blast-transformed lymphocytes, numerous in secondary dengue infections, support dengue virus replication in vivo.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R., Swoveland P. Tubular aggregates in endoplasmic reticulum: evidence against their viral nature. J Ultrastruct Res. 1972 Nov;41(3):270–276. doi: 10.1016/s0022-5320(72)90069-x. [DOI] [PubMed] [Google Scholar]
- Boonpucknavig S., Bhamarapravati N., Nimmannitya S., Phalavadhtana A., Siripont J. Immunofluorescent staining of the surfaces of lymphocytes in suspension from patients with dengue hemorrhagic fever. Am J Pathol. 1976 Oct;85(1):37–48. [PMC free article] [PubMed] [Google Scholar]
- Cardiff R. D., Russ S. B., Brandt W. E., Russell P. K. Cytological localization of Dengue-2 antigens: an immunological study with ultrastructural correlation. Infect Immun. 1973 May;7(5):809–816. doi: 10.1128/iai.7.5.809-816.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demsey A., Steere R. L., Brandt W. E., Veltri B. J. Morphology and development of dengue-2 virus employing the freeze-fracture and thin-section techniques. J Ultrastruct Res. 1974 Jan;46(1):103–116. doi: 10.1016/s0022-5320(74)80025-0. [DOI] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G., Pope J. H. Virus in cultured lymphoblasts from a New Guinea Burkitt lymphoma. Br Med J. 1967 Apr 29;2(5547):290–291. doi: 10.1136/bmj.2.5547.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M. A., Achong B. G. The EB virus. Annu Rev Microbiol. 1973;27:413–436. doi: 10.1146/annurev.mi.27.100173.002213. [DOI] [PubMed] [Google Scholar]
- Halstead S. B., Chow J. S., Marchette N. J. Immunological enhancement of dengue virus replication. Nat New Biol. 1973 May 2;243(122):24–26. [PubMed] [Google Scholar]
- Halstead S. B., Udomsakdi S., Simasthien P., Singharaj P., Sukhavachana P., Nisalak A. Observations related to pathogenesis of dengue hemorrhagic fever. I. Experience with classification of dengue viruses. Yale J Biol Med. 1970 Apr;42(5):261–275. [PMC free article] [PubMed] [Google Scholar]
- Matsumura T., Shiraki K., Sashikata T., Hotta S. Morphogenesis of dengue-1 virus in cultures of a human leukemic leukocyte line (J-111). Microbiol Immunol. 1977;21(6):329–334. doi: 10.1111/j.1348-0421.1977.tb00295.x. [DOI] [PubMed] [Google Scholar]
- Matsumura T., Stollar V., Schlesinger R. W. Studies on the nature of dengue viruses. V. Structure and development of dengue virus in Vero cells. Virology. 1971 Nov;46(2):344–355. doi: 10.1016/0042-6822(71)90036-5. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. CYTOLOGY OF BURKITT'S TUMOUR (AFRICAN LYMPHOMA). Lancet. 1964 Feb 1;1(7327):238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- Shapiro D., Brandt W. E., Russell P. K. Change involving a viral membrane glycoprotein during morphogenesis of group B arboviruses. Virology. 1972 Dec;50(3):906–911. doi: 10.1016/0042-6822(72)90445-x. [DOI] [PubMed] [Google Scholar]
- Smith T. J., Brandt W. E., Swanson J. L., McCown J. M., Buescher E. L. Physical and biological properties of dengue-2 virus and associated antigens. J Virol. 1970 Apr;5(4):524–532. doi: 10.1128/jvi.5.4.524-532.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriurairatna S., Bhamarapravati N., Onishi S. Filamentous structures in dengue type 3 virus infected mouse neurones. Biken J. 1974 Dec;17(4):183–191. [PubMed] [Google Scholar]
- Sriurairatna S., Bhamarapravati N., Phalavadhtana O. Dengue virus infection of mice: morphology and morphogenesis of dengue type-2 virus in suckling mouse neurones. Infect Immun. 1973 Dec;8(6):1017–1028. doi: 10.1128/iai.8.6.1017-1028.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriurairatna S., Bhamarapravati N. Replication of dengue-2 virus in Aedes albopictus mosquitoes. An electron microscopic study. Am J Trop Med Hyg. 1977 Nov;26(6 Pt 1):1199–1205. doi: 10.4269/ajtmh.1977.26.1199. [DOI] [PubMed] [Google Scholar]
- Sung J. S., Diwan A. R., Falkler W. A., Jr, Yang H. Y., Halstead S. B. Dengue carrier culture and antigen production in human lymphoblastoid lines. Intervirology. 1975;5(3-4):137–149. doi: 10.1159/000149891. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Brandt W. E., Russell P. K., Dixon F. T. Replication of dengue-2 virus in cultured human lymphoblastoid cells and subpopulations of human peripheral leukocytes. J Immunol. 1976 Sep;117(3):953–961. [PubMed] [Google Scholar]