ABSTRACT
Coordinating exit from the cell cycle with differentiation is crucial for proper development and tissue homeostasis. Failure to do so can lead to aberrant organogenesis and tumorigenesis. However, little is known about the developmental signals that regulate the switch from cell cycle exit to differentiation. Signals downstream of two key developmental pathways, Notch and Salvador–Warts–Hippo (SWH), and signals downstream of myosin activity regulate this switch during the development of the follicle cell epithelium of the Drosophila ovary. Here, we have identified a fourth player, the integrin signaling pathway. Elimination of integrin function blocks the mitosis-to-endocycle switch and differentiation in posterior follicle cells (PFCs), by regulation of the cyclin-dependent kinase inhibitor (CKI) dacapo. In addition, integrin-mutant PFCs show defective Notch signaling and endocytosis. Furthermore, integrins act in PFCs by modulating the activity of the Notch pathway, as reducing the amount of Hairless, the major antagonist of Notch, or misexpressing Notch intracellular domain rescues the cell cycle and differentiation defects. Taken together, our findings reveal a direct involvement of integrin signaling on the spatial and temporal regulation of epithelial cell differentiation during development.
KEY WORDS: Integrins, Proliferation, Differentiation
INTRODUCTION
The processes of cell proliferation and differentiation have been studied independently, but the cell cycle is intimately entwined with differentiation, as the latter is usually accompanied by irreversible cell cycle exit. However, in some cell types, a modified cell cycle occurs during differentiation, in which the DNA is replicated without concomitant cell division, resulting in an increase in nuclear DNA content. This process, called endoreplication, occurs in many cell types, including plant, invertebrate and vertebrate epidermis and in platelet-producing megakaryocytes and placental trophoblast cells in mammals. Furthermore, recent work indicates that progression through the endoreplication cycle is an important aspect of cell fate acquisition and cell differentiation in Arabidopsis (Bramsiepe et al., 2010). However, although considerable progress has been made in understanding how the core cell cycle machinery is modified to convert a mitotic cycle into an endocycle, little is known about the developmental signals that couple the mitosis-to-endocycle switch with differentiation.
The follicular epithelium of the Drosophila ovary constitutes an excellent model system for the in vivo study of the molecular and cellular mechanisms regulating the mitotic to endocycle switch and its association with cell differentiation. The Drosophila ovary is composed of ∼15 ovarioles, each containing a germarium at their proximal end and progressively older egg chambers at their distal end. Each egg chamber is composed of 15 nurse cells and one oocyte surrounded by a single layer of follicle cells, which constitute the follicular epithelium (King, 1970). Follicle cells originate from the asymmetric division of the follicle stem cells (FCSs), which are located in the germarium (Margolis and Spradling, 1995). At the time that the egg chamber buds from the germarium, ∼80 follicle cells surround the germline cyst (King, 1970). Follicle cells continue to divide mitotically until stage 6 (S6). At this point, they exit the mitotic cycle and switch to an endocycle (Calvi et al., 1998). Between S7 and S10, follicle cells undergo three rounds of endoreplication, become polyploid and increase their size. At S10B, these cells undergo their third cell cycle regimen, gene amplification, during which four regions containing genes encoding chorion proteins are locally amplified (Calvi et al., 1998). These switches in cell cycle are coupled with changes in cell growth and differentiation.
A major pathway controlling cell proliferation and differentiation in follicle cells is the Notch pathway. The Notch pathway is evolutionary conserved and operates iteratively in an enormous diversity of developmental and physiological processes (Andersson et al., 2011; Bray, 2006). This indicates that, despite its simple molecular design, the Notch pathway must be able to elicit a large variety of appropriate responses in many spatially and temporally distinct cellular contexts. This versatility in signaling output is, in part, achieved by modulation of the pathway at different levels in the signal transduction cascade, such as post-translational modifications and trafficking of both the receptor itself and its ligands (reviewed in Bray, 2006). Notch activity is also highly sensitive to chromatin modification and histone rearrangements (reviewed in Andersson et al., 2011; Bray, 2006). Finally, a number of reports have indicated that crosstalk with other signaling pathways also influences the Notch signaling output, adding additional levels of regulation (reviewed in Guruharsha et al., 2012). In the context of this work, the Notch pathway has been shown to regulate the mitotic-to-endocycle switch, as well as the transition from undifferentiated to mature follicle cells (reviewed in Klusza and Deng, 2011). At around S6, increased Delta (Dl) expression in the germ line activates Notch in follicle cells, promoting the mitotic-to-endocycle switch (Deng et al., 2001; López-Schier and St Johnston, 2001). Loss of function of Notch in follicle cells, or Dl in the germ line, leads to prolonged mitosis at the expense of endocycles and to a block in cell differentiation. Notch signaling negatively regulates the Drosophila Cdc25 phosphatase String (Stg). Because Stg activates the CycA–Cdk1 and CycB–Cdk1 complexes and an inhibitor of CycE–Cdk2 complexes – the p27Cip/Kip cyclin-dependent kinase inhibitor (CKI) protein Dacapo (Dap) – Notch signaling allows CycE to oscillate, driving cells into the endocycle. In addition, Notch positively regulates Fizzy-related (Fzr), an adaptor for the APC/C E3 ligase, which allows the degradation of the mitotic cyclins, thereby ensuring complete transition to endocycle (Schaeffer et al., 2004; Shcherbata et al., 2004). One pathway that has been shown to modulate Notch activity in this context is the Salvador–Warts–Hippo (SWH) pathway (Yu et al., 2008). SWH-mutant follicle cells continue proliferating, fail to enter into the endocycle and remain undifferentiated (Meignin et al., 2007; Polesello and Tapon, 2007; Yu et al., 2008). Furthermore, the SWH pathway has been proposed to promote Notch signaling in follicle cells by regulating Notch endocytic trafficking (Meignin et al., 2007; Polesello and Tapon, 2007; Yu et al., 2008). The Notch pathway can also be modulated by myosin activity in follicle cells. As it is the case for SWH, elimination of PP1β, a phosphatase that dephosphorylates and inactivates the non-muscle myosin II light chain, also results in defective Notch signaling, owing to defects in the endosomal trafficking of the receptor (Sun et al., 2011). Interestingly, myosin activity does not affect the SWH pathway. These results highlight the complexity of the genetic circuitry that affects Notch signaling during follicle cell differentiation.
In this work, we have identified a fourth pathway regulating the cell-proliferation-to-differentiation switch in follicle cells – signaling downstream of integrins. Integrins are αβ heterodimeric cell-surface receptors that connect the extracellular matrix (ECM) to the cytoskeleton (Hynes, 2002). Although in vertebrates there are at least eight β subunits and 18 α subunits, in Drosophila, there are only two β subunits, βPS and βn, and five α subunits, αPS1 to αPS5. We have shown previously that elimination of myospheroid (mys), the gene encoding the βPS subunit (the only β subunit present in the ovary), from follicle cells results in multilayering and polarity defects at both poles of the egg chamber (Fernández-Miñán et al., 2008). Here, we report a new role for integrins in follicle cells – the control of the coupling of mitosis-to-endocycle switch with differentiation. We show that elimination of integrin function results in aberrant PFC endoreplication and differentiation. Furthermore, we show that integrins regulate proper exit from the cell cycle and cell differentiation by regulating the expression of the CDK inhibitor dacapo. Our results also demonstrate that integrins act in follicle cells by modulating the activity of the Notch pathway through the regulation of its intracellular trafficking and/or processing. In summary, we believe that our work reveals that cell–ECM interactions mediated by integrins contribute to the temporal coupling of cell-cycle arrest with differentiation during development.
RESULTS
Integrins regulate follicle cell differentiation
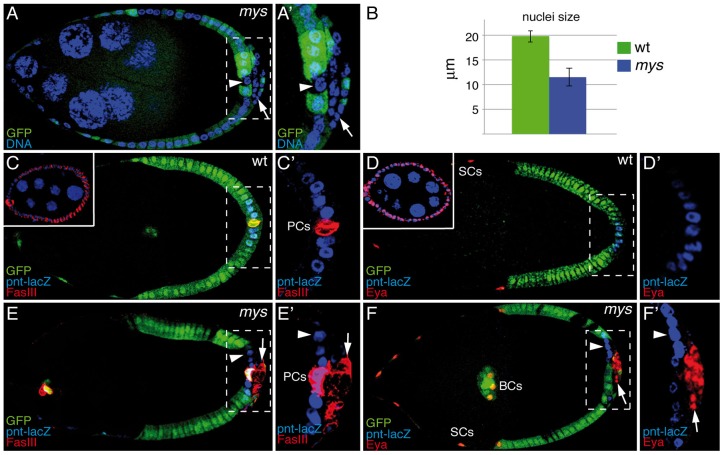
We have previously reported that mosaic egg chambers containing mys mutant follicle cell clones very often grow extra layers at both poles of the egg chamber (Fernández-Miñán et al., 2007). In addition, we have shown that mys-mutant follicle cells located at the posterior pole, hereafter referred to as mys PFCs, show an aberrant distribution of polarity markers (Fernández-Miñán et al., 2008). Interestingly, this polarity defect is only observed in mys PFCs located in the ectopic layers, whereas mys PFCs in contact with the germline are correctly polarized (Fernández-Miñán et al., 2008). Recently, we noticed that the nuclear diameter of mys PFCs (as identified by the absence of GFP) was significantly smaller than that of their wild-type counterparts (Fig. 1A,B). As was the case for the polarity defects, the nuclear size phenotype was only observed in mutant PFCs that have lost contact with the germline (Fig. 1A, white arrow), whereas mys PFCs in contact with the germline show no phenotype (Fig. 1, white arrowhead). Small nuclear size is a potential indication of a failure to differentiate and to transit into the endocycle. Thus, we checked whether integrins were required for proper follicle cell differentiation and mitotis-to-endocycle switch. In order to do this, we generated follicle cell clones for two unrelated null alleles mys11 and mys10, and we analyzed the expression of markers normally found in undifferentiated cells [FasIII and Eyes absent (Eya)] or in differentiated PFCs [pointed-lacZ (pnt-lacZ) (Bai and Montell, 2002; González-Reyes and St Johnston, 1998; Morimoto et al., 1996; Snow et al., 1989)]. Both alleles gave rise to indistinguishable phenotypes; however, in the text and in the figures we refer only to results obtained with mys11. In wild-type egg chambers, FasIII and Eya are detected in all follicle cells up to S6 (insets in Fig. 1C,D, respectively). From S6 onwards, they are expressed only in polar cells (FasIII) or border cells and stretched cells (Eya) (Fig. 1C,D). By contrast, pnt-lacZ is specifically expressed in wild-type PFCs from S6 onwards (Fig. 1C,D). However, in contrast to the wild type, we found that in S6 and older egg chambers, most mys PFCs express FasIII and Eya (Fig. 1E, 83% n = 37; Fig. 1F, 80%, n = 42, respectively) and fail to activate pnt-lacZ (Fig. 1E,F, 100%, n = 31). Again, the effects on FasIII, Eya and pnt-lacZ expression were only observed in mys PFCs located in the ectopic layers (Fig. 1E,F, arrows). Finally, we found that the RNA-binding protein Staufen (Stau), which localises to the posterior of the oocyte during S9 (St Johnston et al., 1991; supplementary material Fig. S1A), was properly localised in mosaic egg chambers containing mys PFCs (supplementary material Fig. S1B, arrowhead), most likely owing to the normal differentiation of mys PFCs in contact with the germ line. Taken together, these results strongly suggest that integrins are required for proper PFC maturation when contact with the germline is lost.
Fig. 1.
Integrins regulate PFC differentiation. (A) An S9 mosaic egg chamber carrying mys mutant clones (GFP−) was stained for anti-GFP and the DNA marker TO-PRO-3 (blue). Although mys PFCs in contact with the germline (GFP−, arrowhead) have normal nuclear size, the nuclear size of those located in ectopic layers (GFP−, arrow) is smaller than that of surrounding wild-type (wt) cells (GFP+). (B) Quantification of the nuclear size. Data show the mean±s.d. (C–F) S9–S10 wild-type and mosaic egg chambers carrying mys mutant clones expressing the posterior cell fate marker pnt-lacZ, and stained with anti-GFP (green), anti-β-galactosidase (C–F; blue), anti-FasIII (C,E; red) and anti-Eya (D,F; red). In S9–S10 wild-type egg chambers, FasIII (C) and Eya (D) are restricted to polar cells (PCs; FasIII) or to border cells (BCs) and stretched cells (SCs; Eya), whereas pnt-lacZ is specifically expressed in PFCs from S6 onwards (C,D). However, in mys PFCs located in ectopic layers (GFP−, arrow), FasIII (E) and Eya (F) are maintained, whereas pnt-lacZ is inhibited (E,F). mys PFCs in contact with the germline (GFP−, arrowhead in E,F) behave as wild-type follicle cells. Upper-left panels in C and D show the expression of FasIII and Eya in S4 egg chambers, respectively. Anterior is to the left in all figures. (A′–F′) Magnifications of the white boxes in A–F, respectively.
Integrins are required in follicle cells for the mitosis-to-endocycle switch and for gene amplification
Differentiation and withdrawal from the mitotic cycle are coupled in follicle cells. However, whether initiation of the differentiation program in follicle cells depends on exit from cell cycle has not yet been formally proven. Here, we decided to directly address this by blocking exit from the cell cycle through the simultaneous overexpression of E2F, Dp (DP transcription factor), CycE and Stg. This combination is sufficient to abrogate cell cycle exit in Drosophila eye and wing disc cells, where cell cycle arrest is not required for terminal differentiation (Buttitta et al., 2007). Here, we found that overexpression of those four genes in follicle cells was also sufficient to bypass cell cycle exit, as visualized by ectopic phosphorylated histone 3 (PH3) staining after S6 (supplementary material Fig. S2B). However, in contrast to the wing and eye, differentiation in follicle cells was blocked, as revealed by the maintenance of high levels of FasIII expression after S6 (supplementary material Fig. S2B). These results strongly suggest that follicle cells need to exit the mitotic cycle to differentiate.
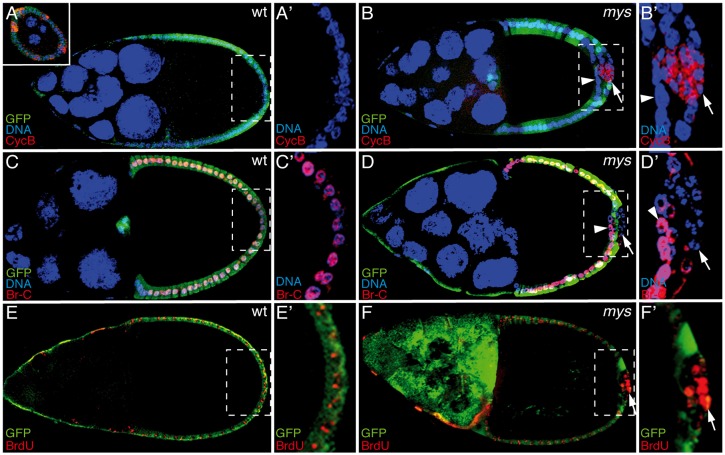
We show here that mys PFCs display differentiation defects. This could reflect a failure to exit the mitotic cycle or a problem with differentiation. To test between these possibilities, we analyzed the expression of the cell cycle regulator Cyclin B (CycB). CycB is normally expressed in mitotic follicle cells from S4 to S6, when it is downregulated (Lehman et al., 1999; inset in Fig. 2A). However, we found that mys PFCs located in ectopic layers showed prolonged expression of CycB after S6 (Fig. 2B, 62%, n = 34, arrow). We next analyzed the expression of the Broad-complex (BR-C) gene, an early ecdysone-response gene that is essential for endoreplication and gene amplification. In wild-type egg chambers, the expression of the BR-C protein is activated by ecdysone in all follicle cells from S6 onwards (Tzolovsky et al., 1999; Fig. 2C), where it drives gene amplification, as visualised by the incorporation of BrdU in four discrete spots that represent amplification of the chorion genes in S10B follicle cells (Fig. 2E). However, we did not detect BR-C expression in mys PFCs located in ectopic layers after S6 (Fig. 2D, 100%, n = 39, arrow). As expected, these mys PFCs also fail to undergo gene amplification, as shown by BrdU incorporation (Fig. 2F, 75%, n = 38, arrow). Thus, instead of the foci-like pattern of BrdU incorporation typical of follicle cells undergoing gene amplification (Fig. 2E), some S10B mys PFCs show a pattern characteristic of cells in mitosis, where BrdU labels the whole nucleus (Fig. 2F, arrow). CycB and BR-C expression patterns were not affected in mys PFCs in contact with the germline (arrowheads in Fig. 2B,D).
Fig. 2.
Defective endocycle and CycB expression in integrin-mutant PFCs located in ectopic layers. (A–D) S9–S10 wild-type (wt) and mosaic egg chambers were labeled with anti-GFP (green), anti-CycB (A,B; red), anti-Br-C (C,D; red) and TO-PRO-3 (blue). (A) In wild-type egg chambers, CycB is downregulated at S6 (upper-left panel shows CycB expression in an S4 egg chamber). (B) However, this expression is maintained at S10 in mys PFCs located in ectopic layers (GFP−, arrow). (C) In wild-type follicle cells, Br-C is upregulated after S6. (D) This upregulation fails to occur in mys PFCs located in ectopic layers (GFP−, arrow). Note that in both cases mys PFCs in contact with the germline (GFP−, arrowhead in B and D) behave as wild-type follicle cells. (E,F) S11 egg chambers stained with anti-GFP (green) and BrdU (red). Whereas in wild-type S11 egg chambers, BrdU incorporates in the four spots of amplification (E), genomic BrdU incorporation was observed in mys PFCs located in ectopic layers (F). (A′–F′) Magnifications of the white boxes in A–F, respectively.
Taken together, our results show that mys PFCs located in ectopic layers fail to undergo proper mitotic-to-endocycle switch and cell differentiation. As mentioned in the Introduction, a key pathway regulating cell proliferation and differentiation in follicle cells is the Notch pathway. Thus, we next decided to analyze whether elimination of integrins could affect the Notch pathway.
Notch signaling is disrupted in mys PFCs
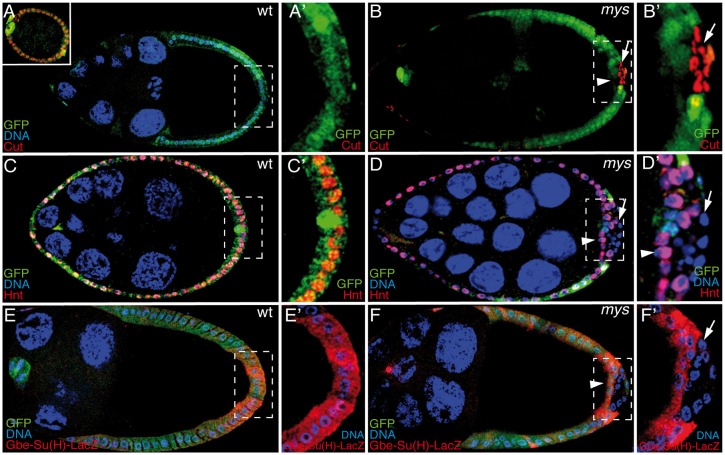
To examine whether Notch signaling was affected in mys PFCs, we analyzed the expression of Cut, a homeobox protein that is downregulated by Notch signaling in follicle cells after S6 (Sun and Deng, 2005; Fig. 3A), and that of Hindsight (Hnt, also known as Pebbled), a zinc-finger protein that is induced by Notch after S6 (Sun and Deng, 2007; Fig. 3C). We found that, in contrast to the wild-type PFCs, mys PFCs that had lost contact with the germline showed continued expression of Cut (Fig. 3B, n = 27, arrow) and failed to activate Hnt (Fig. 3D, n = 29, arrow) in 82% and 80% of S6 and older egg chambers analyzed, respectively. These results suggest that Notch signaling is affected in the absence of integrin function. To further confirm this, we measured Notch activity using the Gbe-Su(H)-lacZ reporter, which yields high levels of expression in cells where Notch is active (Furriols and Bray, 2001; Fig. 3E). Normally, the activity of this reporter is increased in follicle cells upon Notch activation during S7–S10A, although its expression is not uniform along the follicular epithelium, being highest in PFCs (Fig. 3E). We found that the expression of this reporter was significantly reduced in mys PFCs located in ectopic layers in 75% of the egg chambers analyzed (Fig. 3F, n = 28, arrow). These results show that Notch signaling is indeed defective in mys PFCs that have lost contact with the germline.
Fig. 3.
The Notch pathway is disrupted in integrin-mutant PFCs. Egg chambers were stained with TO-PRO-3 (A–F; blue), anti-GFP (A–F; green), anti-Cut (A,B; red), anti-Hnt (C,D; red) and anti-β-galactosidase (E,F; red) to detect the reporter of Notch activity, Gbe-Su(H)-lacZ. In wild-type (wt) egg chambers, Notch signaling induces downregulation of Cut (A) and upregulation of Hnt (C) from S6 onwards (upper-left panel in A shows the expression of Cut in an S4 egg chamber). By contrast, mys PFCs located in ectopic layers (GFP−, arrow) show prolonged Cut expression after S6 (B) and fail to upregulate Hnt at S7 (D). (E) In wild-type egg chambers, expression of the reporter of Notch activity, Gbe-Su(H)-lacZ is upregulated after S6 in PFCs. (F) This upregulation fails to take place in mys PFCs in ectopic layers (GFP−, arrow). mys PFCs in contact with the germline (GFP−, arrowhead in D and F) behave as wild-type follicle cells. (A′–F′) Magnifications of the white boxes in A–F, respectively.
Integrins are required to strengthen Notch signaling
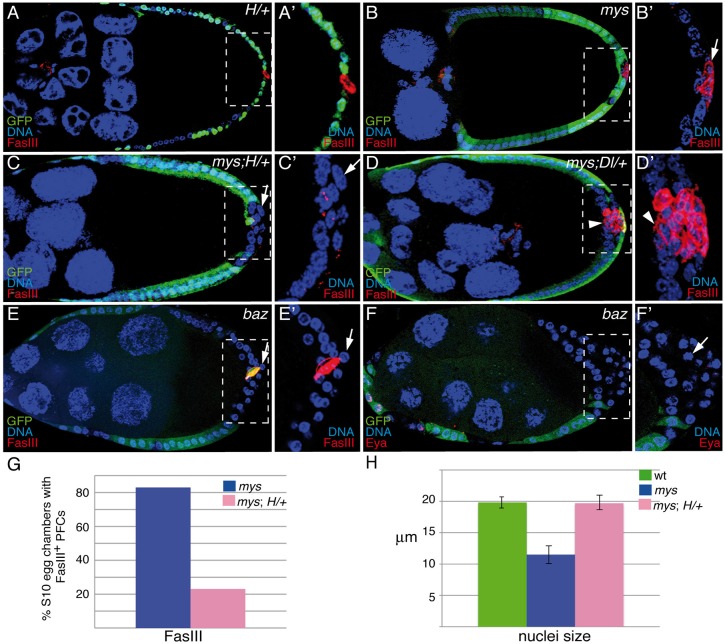
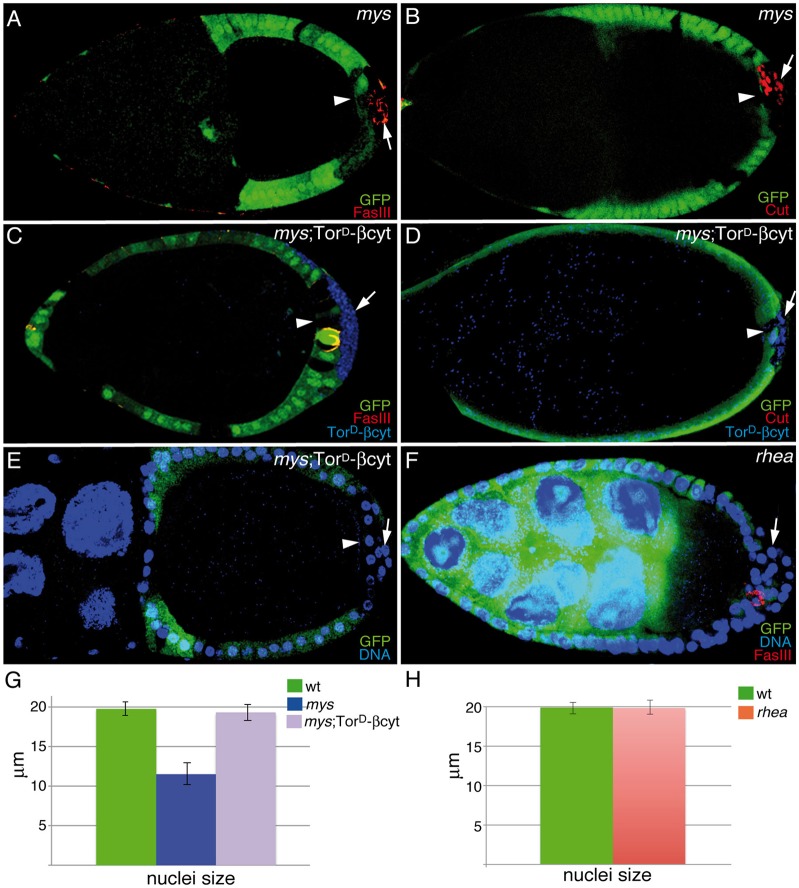
An intriguing aspect of the mys phenotype is the fact that the defects in Notch signaling are only observed in mutant PFCs located in ectopic layers, whereas those mys mutant cells in contact with the germline develop normally. In this scenario, because the ligand for the Notch receptor in the ovary, Dl, is produced in the germline (Deng et al., 2001), the mutant cells adjacent to the germline could receive a normal signal, activating the Notch pathway properly. By contrast, those mutant cells in the ectopic layers, placed away from the source of ligand, would receive much less ligand input and would consequently develop Notch-mutant phenotypes. Therefore, the Notch-like defects could just be a secondary consequence of the multilayering phenotype. Alternatively, integrins might themselves be required to strengthen Notch signaling. This hypothetical integrin modulation of Notch signaling would become more crucial in a sensitized background, such as cells located in the ectopic layers. To test between these two possibilities, we performed four sets of experiments. First, we tested whether increasing Notch signaling in mys PFCs could rescue the phenotype. In order to do this, we removed one copy of Hairless (H), the major antagonist of Notch signaling in Drosophila (reviewed in Maier, 2006), in mys mosaic egg chambers. Removing one copy of H in a wild-type background did not affect follicle cell differentiation (Fig. 4A). In addition, halving the dose of H in a mys background did not have any effect on the onset or formation of the multilayering (Fig. 4C). However, interestingly, we found that it rescued the differentiation (Fig. 4C,G, n = 32) and nuclear size defects (arrow in Fig. 4C′,H, n = 28) of mys PFCs, as now only 23% of mosaic egg chambers containing mys PFCs and heterozygous for H (mys; H/+) showed FasIII expression after S6, in contrast to the 83% observed in mosaic egg chambers containing mys PFCs (Fig. 4B,G). Second, we analyzed whether halving the amount of Dl, by heterozygosity of a Dl loss-of-function allele, which on its own did not cause any effect, would enhance the integrin phenotype. Indeed, we found that in 87% of mosaic egg chambers containing mys PFCs and heterozygous for Dl (mys; Dl/+, n = 23), the mutant PFCs in contact with the germline display differentiation defects, as monitored by FasIII expression (Fig. 4D, arrowhead). Third, we tested whether failure to maintain contact with the germ line was, on its own, sufficient to affect proper differentiation. To this end, we analyzed the expression of FasIII and Eya in mosaic egg chambers containing bazooka (baz)-mutant follicle cells, which very often grew extra layers at both poles of the egg chamber. We did not find any alteration in the expression of FasIII or Eya in the mutant cells located on the ectopic layers (Fig. 4E,F, arrows). And fourth, we tested whether increasing integrin signaling without rescuing the multilayer phenotype would rescue the differentiation defects. To this end, we made use of a chimeric integrin that allows constitutive integrin signaling in absence of adhesion, the TorD/βcyt (Martin-Bermudo and Brown, 1999). We have previously shown that expression of this construct in mosaic egg chambers containing mys PFCs results in a partial rescue of the multilayer phenotype (Fernández-Miñán et al., 2007). Here, we found that it rescued the differentiation (Fig. 5C,D, n = 21) and nuclear size defects (Fig. 5E,G, n = 26) in 100% of the cases, including those in which the stratification phenotype was not rescued. Taken together, these results strongly suggest that the cell differentiation phenotype is not just a consequence of the multilayering and that integrin-mediated signaling is required to enhance Notch signaling in PFCs.
Fig. 4.
Integrins are required to strengthen Notch signaling. S9–S10 egg chambers were labeled with anti-GFP (green), anti-FasIII (A–E; red), anti-Eya (F; red) and TO-PRO-3 (blue). (A) In an H/+ control egg chamber, FasIII (red) is restricted to polar cells, as is the case in wild-type egg chambers. (B) S10 mosaic egg chambers showing the abnormal expression of FasIII (red) in mys PFCs located in ectopic layers. (C) Reducing the amount of H restores FasIII expression and nuclear size in mys PFCs located in ectopic layers (GFP−, arrow). This is quantified in G and H, respectively. (D) mys PFCs in contact with the oocyte (arrowhead) show high levels of FasIII when Dl function is reduced. (E,F) FasIII (E) and Eya (F) expression patterns are not affected in baz PFCs located in ectopic layers. (A′–F′) Magnifications of the white boxes in A–F, respectively. Data in G and H show the mean±s.d. wt, wild type.
Fig. 5.
Integrin-mediated signaling is required for PFC maturation. Egg chambers were stained with TO-PRO-3 (E,F; blue), anti-GFP (A–F; green), anti-FasIII (A,C,F; red), anti-Cut (B,D; red) and anti-Myc (C,D; blue) to detect the TorD/βcyt chimeric integrin. (A,B) S9–S10 mosaic egg chambers showing abnormal expression of FasIII (A; red) and Cut (B; red) in mys PFCs located in ectopic layers (GFP−, arrow). (C–E) Ectopic expression of TorD/βcyt (blue) in these mys PFCs restores the normal expression of FasIII (C; red), Cut (D; red) and nuclear size (E,G). (F) FasIII expression is not affected in rhea PFCs located in ectopic layers (GFP−, arrow). (G,H) Quantification of the nuclear size of the indicated genotypes. The nuclear size of rhea PFCs is similar to that of wild-type (wt) follicle cells (H). In all cases, mys PFCs in contact with the germline (GFP−, arrowheads in A–E) behave as wild-type follicle cells. Data show the mean±s.d.
Finally, we tested whether elimination of other focal adhesion components, such as Talin, would also affect proper follicle cell differentiation. In order to do this, we generated mosaic egg chambers containing follicle cells mutant for rhea, the gene encoding Talin in Drosophila (Brown et al., 2002). We have previously shown that removal of rhea in follicle cells causes polarity and stratification defects identical to those caused by loss of mys (Fernández-Miñán et al., 2008; Fig. 5F). However, we found that both the expression of FasIII and the nuclear size of all rhea PFCs, including those located in ectopic layers, were similar to those of wild-type PFCs (Fig. 5F,H). These results argue against the view that the differentiation defects observed in mys PFCs are mainly due to the multilayering and polarity phenotypes and strongly support our conclusion that integrins strengthen Notch signaling.
The Notch intracellular domain can rescue the Notch signaling phenotype of mys mutant PFCs
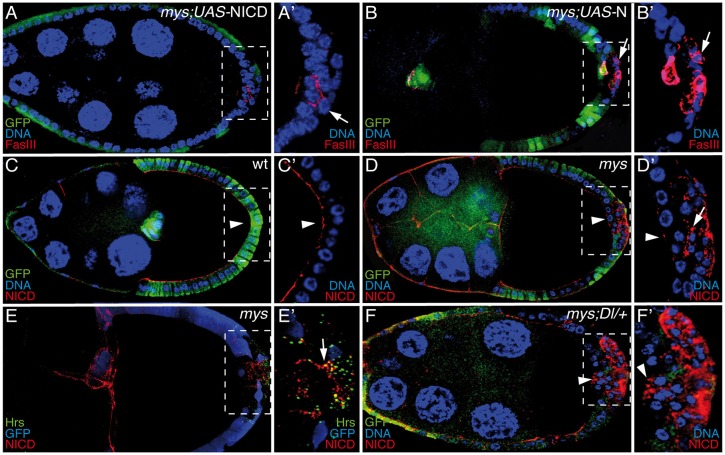
To investigate how integrin function regulates Notch signaling, we tested whether the expression of various Notch constructs in mys PFCs could rescue the differentiation defects. As a result of ligand binding, Notch is proteolytically processed, and the extracellular domain (NECD) is transendocytosed into the ligand-sending cell. The remaining receptor undergoes two successive proteolytic cleavages, first by ADAM and then by γ-secretase, the second step requiring Notch endocytosis. This is followed by the release of the Notch intracellular domain (NICD) and its translocation to the nucleus, where it regulates the transcription of its downstream targets (reviewed in Fortini and Bilder, 2009). We found that when the NICD was expressed in mys PFCs, both the nuclear size defect and the differentiation phenotype were rescued, as FasIII expression after S6 was only detected in 13% of the egg chambers carrying mys PFCs in ectopic layers (Fig. 6A, arrow), in contrast to the 83% observed in mosaic egg chambers containing mys PFCs without NICD (Fig. 1E). Interestingly, neither full-length Notch nor NECD were able to rescue the differentiation phenotype (Fig. 6B; data not shown). This result suggests that elimination of integrins disrupts Notch signaling probably at the level of the final Notch cleavage, as this generates the functional NICD. This cleavage is regulated at the level of endosomal trafficking. Thus, to further investigate the consequences of eliminating integrin function on the Notch pathway, we compared the expression and localization pattern of the Notch receptor itself in wild-type and mys PFCs. In wild-type follicle cells up to S6, the Notch receptor accumulates at high levels on the apical side. From this stage onwards, upon Notch activation by Dl from the germ line, the levels of Notch in the membrane are strongly reduced (López-Schier and St Johnston, 2001; Fig. 6C, arrowhead). In mys PFCs located in ectopic layers, we found significant accumulations of both NICD and NECD at or below the plasma membrane (Fig. 6D, n = 32; supplementary material Fig. S3B,C, arrows), suggesting that full-length Notch is present in excess amounts in these mutant cells. Furthermore, part of the ectopic Notch protein was visible in a punctate cytoplasmic pattern, indicating that some of the Notch protein was accumulating in vesicles (white arrows in Fig. 6D′; supplementary material Fig. S3B,C). In fact, we found that several of the ectopic Notch-positive vesicles present in mys PFCs were also positive for the early endosomal marker Hrs (hepatocyte growth factor-regulated tyrosine kinase substrate, Fig. 6E, arrow). This result suggested a potential defect in the processing and/or endocytic trafficking of Notch in mys PFCs. The aberrant Notch distribution observed in mys PFCs could just be a consequence of the multilayering and/or polarity defects found in mys PFCs. Alternatively, integrins might be required for proper endosomal trafficking of Notch. To distinguish between these two possibilities, we performed two sets of experiments. First, we tested whether halving the amount of Dl, which, on its own, does not affect Notch distribution (data not shown), affected Notch trafficking in mys PFCs that contacted the germline. Indeed, we found a punctate distribution of Notch in the cytoplasm of mys; Dl/+ PFCs in contact with the germline (Fig. 6F, arrowhead), which resembled that observed in mys PFCs located in ectopic layers (Fig. 6D, arrow). And second, we analyzed Notch distribution in baz follicle cells, which very often show both multilayering and polarity defects. We found that, in contrast to what happens in mys PFCs, there is no increased accumulation of Notch at or below the plasma membrane in baz PFCs located in ectopic layers (supplementary material Fig. S3D). These results strongly suggest that the changes in Notch distribution found in mys PFCs are not just a consequence of the multilayering or polarity defects and that integrins contribute to the proper endosomal trafficking and/or processing of the Notch receptor.
Fig. 6.
Integrins modulate the Notch pathway by regulating its intracellular trafficking and/or processing. Egg chambers were stained with anti-GFP (A–F; green), anti-FasIII (A,B; red), anti-NICD (C–F; red), anti-Hrs (E; green) and TO-PRO-3 (blue). Expression of NICD (A) but not full-length Notch (‘N’; B) restores the normal expression of FasIII and nuclear size of mys PFCs in ectopic layers (GFP−, arrow). (C) NICD expression in wild-type (wt) egg chambers (arrowhead). (D) mys PFCs located in ectopic layers (GFP−, arrow) contain discrete cytoplasmic and membrane-associated accumulations of NICD. (E) Some of the ectopic NICD is found to colocalize with Hrs (arrow). Note that mys PFCs in contact with the germline show normal NICD localization (GFP−, arrowhead in D). (F) However, reduction of Dl function in these mutant PFCs (arrowhead) leads to an accumulation of NICD similar to that found in mys PFCs in ectopic layers (arrow in D). (A′–F′) Magnifications of the white boxes in A–F, respectively.
Integrins are required for proper downregulation of the expression of dacapo but not that of string
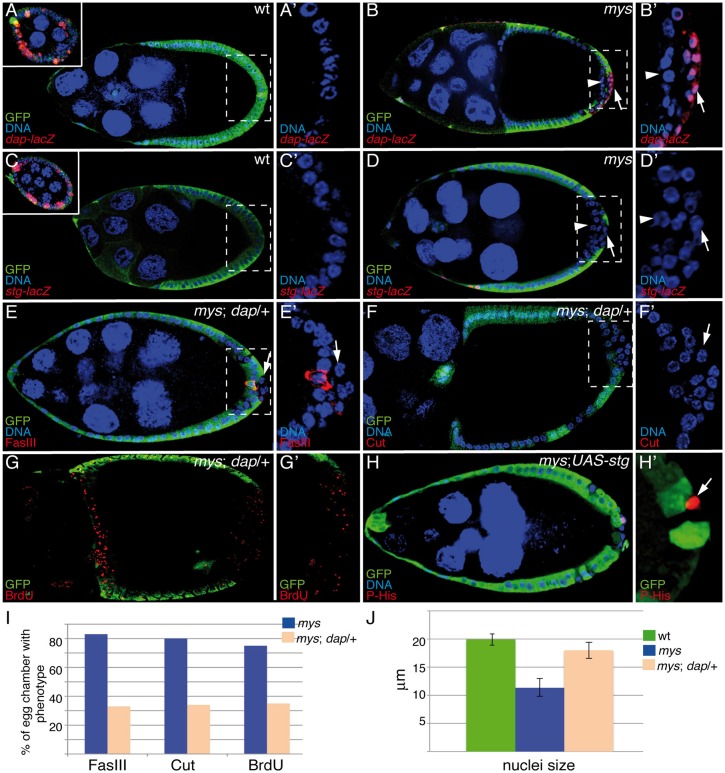
An intriguing aspect of the ovarian phenotype of mys when compared with that of Notch is that even though, like Notch, integrin mutant PFCs fail to enter endocycle and to differentiate, in contrast to Notch mutants, they do not continue proliferating. The Notch pathway has been shown to regulate the mitotic-to-endocycle transition by independently regulating three mitotic regulators: it downregulates the G2 phosphatase String (Stg) and the G1 CKI p21CIP/Dacapo (Dap) and upregulates the APC activator Fzr (Shcherbata et al., 2004). To investigate what lies behind the difference between the Notch and mys phenotypes, we analyzed the expression of these cell cycle regulators in mys PFCs using lacZ reporters (Lehman et al., 1999; Shcherbata et al., 2004). dap-lacZ and stg-lacZ are normally expressed in mitotic follicle cells from S4 to S6, when they are downregulated (Lehman et al., 1999; Shcherbata et al., 2004; Fig. 7A,C). However, we found that even though mys PFCs in contact with the germline showed no alteration of the dap-lacZ pattern of expression (arrowheads in Fig. 7B), those located in ectopic layers showed prolonged expression of dap-lacZ (arrow in Fig. 7B, 86%, n = 39) after S6. By contrast, all mys PFCs were able to downregulate stg-lacZ (Fig. 7D, n = 30).
Fig. 7.
Integrins regulate PFC maturation by regulating dap expression. Egg chambers were stained with anti-GFP (A–H; green), anti-FasIII (E; red), anti-Cut (F; red), anti-BrdU (G; red), anti-PH3 (H; red), TO-PRO-3 (A–F,H; blue) and anti-β-galactosidase (A–D; red) to monitor the expression of the dap-lacZ (A,B) and stg-lacZ (C,D) reporters. (A,C) In wild-type (wt) egg chambers, dap (A) and stg (C) are downregulated at S6. Upper-left panels show the expression of these markers in S4 egg chambers from the same ovarioles. (B,D) In mys PFCs located in ectopic layers (GFP−, arrow), dap expression is maintained after S6 (B) whereas stg is properly downregulated (D). mys PFCs in contact with the germline show normal dap and stg expression (GFP−, arrowheads in B and D). (E–G) Reducing dap levels in mosaic egg chambers carrying mys PFCs restores normal expression of FasIII (E,I) and Cut (F,I), BrdU incorporation pattern (G,I) and nuclear size (J). (H) Ectopic expression of stg in mys PFCs located in ectopic layers (GFP−, arrow) is sufficient to maintain these mutant cells within the cell cycle. (A′–H′) Magnifications of the white boxes in A–H, respectively. Data in I and J show the mean±s.d.
Taken together, our results show that, when contact with the germline is lost, integrins are required in PFCs to downregulate the expression of the CKI dap but not that of string. This might explain why mys PFCs do not differentiate properly, yet they do not continue dividing after S6. We next decided to test this by performing two sets of experiments. First, we tested whether a reduction in the amount of Dap would rescue the differentiation and cell cycle defects found in mys PFCs located in ectopic layers. Indeed, we found that removing one copy of dap partially rescued the differentiation and Notch signaling defects of mys PFCs, as now only 33% (n = 26) and 35% (n = 24) of mosaic egg chambers containing mys PFCs and heterozygous for dap (mys; dap/+) showed expression of FasIII and Cut, respectively, after S6 (arrows in Fig. 7E,F,I), in contrast to the 83% and 82% found in mosaic egg chambers containing mys PFCs (Fig. 1E; Fig. 3B; Fig. 7I). In addition, the aberrant pattern of BrdU incorporation and nuclear size of mys PFCs were also partially rescued (Fig. 7G,I,J), as now only 35% (n = 26) of mys; dap/+ egg chambers carrying mys PFCs showed an aberrant BrdU incorporation pattern, in contrast to the 75% observed in egg chambers containing mys PFCs (Fig. 7G,I). Second, we analyzed whether the expression of stg was sufficient to maintain mys PFCs located in ectopic layers within the cell cycle after S6. Using the anti-PH3 antibody, we found that indeed this was the case (Fig. 7H, arrow).
DISCUSSION
The coordination of cell proliferation with the gradual differentiation of different cell types is essential for proper development and tissue homeostasis. However, little is known about the signals that couple cell cycle exit to differentiation switch during development. Here, we show that signaling mediated by integrins, the major cell–ECM receptors, contributes to the regulation of this switch in the posterior follicle cells of the Drosophila ovary. Furthermore, our experiments strongly suggest that one of the mechanisms by which integrins regulate epithelial cell differentiation is by modulating the activity of the Notch pathway through promoting the proper endosomal trafficking and/or processing of Notch.
Integrins are known to regulate cell differentiation and proliferation in other systems. The effects of particular integrins in regulating differentiation vary depending on the epithelial cell type. Thus, although β1 integrin signaling is inhibitory for differentiation in the epidermis (reviewed in Watt, 2002), it is crucial for proper differentiation in the mammary gland (Naylor et al., 2005). Interestingly, as we show here for Drosophila PFCs, loss of β1 integrin in the mammary gland leads to an upregulation in the expression of the CKI p21Cip1 (also known as CDKN1A) (Li et al., 2005). Furthermore, as is the case for PFCs, this upregulation is responsible for the proliferation defects observed in β1-integrin-mutant mammary glands, because knockdown of p21Cip1 rescues the effect of β1 integrin loss. Integrins have been shown to control CKI levels in other cell types. Thus, β1-integrin-mutant chondrocytes show a defect in the G1–S transition, which is accompanied by upregulation of the CKIs p16Ink4a (also known as CDKN2A) and p21Cip1 (Aszodi et al., 2003). Ablation of β1 integrin in developing cerebellum results in a failure in the expansion of the cerebellar granule cell precursors due to upregulation of p27Kip1 (also known as CDKN1B) (Blaess et al., 2004). However, the mechanisms underlying this regulation remain to be determined. In summary, our work extends previous studies demonstrating a key role for integrin signaling in controlling growth and differentiation by regulating CKI levels, which suggests that this role is conserved throughout evolution. In addition, our studies unravel one of the mechanisms underlying this regulation – modulation of Notch signaling.
We have found that elimination of integrins results in defective Notch signaling in follicle cells. However, one puzzling aspect of the integrin phenotype is the fact that the phenotypes of defective Notch are partial and position-dependent. Similar to Notch-mutant PFCs, integrin-mutant PFCs fail to enter endocycle and to differentiate; however, unlike Notch-mutant cells, they do not continue proliferating. In addition, and in contrast to Notch mutants, the defects due to absence of integrin function are restricted to PFCs. Position-dependent phenotypes have also been observed in follicle cells in mutants of the SWH pathway or mutants with excessive myosin activity (Meignin et al., 2007; Polesello and Tapon, 2007; Sun et al., 2011; Yu et al., 2008). These position-dependent responses could be due to the involvement of signaling pathways specific to these follicle cell subpopulations, such as the EGFR and the JAK–STAT pathways. Alternatively, they could reflect an intrinsic difference in Notch signaling levels between the PFCs and other follicle cells, as it has been proposed that the expression of Dl in the oocyte is lower than that in the nurse cells (Yu et al., 2008). Therefore, the intensity of Notch signaling might be weaker in PFCs with respect to other follicle cells and might depend more on positive modulators such as integrins to achieve adequate levels of activity. In this context, integrin signaling might play a general role in reinforcing Notch activity, with this regulation being more important on a sensitized background. This could explain the restriction of the Notch defects to integrin-mutant PFCs located in the ectopic layers, as Notch signaling in these cells could be relatively low, given that they are at a greater distance from the source of ligand. In addition, a role for integrins as positive modulators of Notch signaling could also explain why dacapo but not string expression is affected in integrin-mutant PFCs. The reduced Notch activity present in the integrin-mutant PFCs might be sufficient to induce normal levels of string expression but not of dacapo. In fact, Notch has been shown to independently control these two cell cycle regulators in follicle cells (Shcherbata et al., 2004).
There are different mechanisms by which integrins could modulate Notch signaling. We have shown that defective Notch signaling in mys PFCs can be rescued by expression of NICD, but not by full-length Notch or NECD. This suggests that elimination of integrins disrupts Notch signaling, most likely at the level of the last Notch cleavage, which generates the functional NICD. This step is subject to regulation at the level of endosomal trafficking (reviewed in Fortini and Bilder, 2009). In fact, we have observed increased punctate distribution of Notch in the cytoplasm of integrin-mutant PFCs. Therefore, we propose that a way by which integrins could modulate Notch activity in PFCs could be through the regulation of its intracellular trafficking and/or processing. At what level could integrins and Notch interact? A level of intersection between Notch and integrins could be at the plasma membrane lipid rafts, specialized membrane microdomains involved in endocytosis and signaling (Lajoie and Nabi, 2010). Interestingly, mutations in genes required for the biogenesis of sphingolipids, major constituents of lipid rafts, such as lace and acc, cause abnormal accumulation of Notch in endosomes and significant alterations in the pathway (Sasamura et al., 2013). These results have led to the suggestion that genes involved in the formation and/or function of lipid rafts might influence tissue growth and differentiation by regulating the intracellular trafficking of signaling molecules such as Notch. Integrin signaling has been shown to regulate the location and behavior of lipid rafts (del Pozo et al., 2004; Norambuena and Schwartz, 2011). Furthermore, the local regulation of these membrane domains by integrins has been proposed to locally regulate signaling proteins thought to associate with these domains, such as the PDGFR or Rac (Baron et al., 2003). Thus, lipid rafts might be a cellular platform to allow for Notch–integrin interactions. Finally, the increased levels of Hrs in cytoplasmic vesicles observed in mys PFCs suggest that the endocytosis defects might not be specific to the Notch receptor, but rather might indicate a more generalized defect in endocytosis.
A temporal coupling of cell-cycle arrest and terminal differentiation is common during development and homeostasis. Furthermore, a loss of coordination between these two processes can lead to aberrant tissue development and tumorigenesis. Here, we have shown that, in Drosophila, as is the case in mammals, integrins can regulate the switch between cell cycle and differentiation in epithelial cells by regulating CKI levels, suggesting that this role of integrins is conserved. Furthermore, we show that one of the mechanisms by which integrins regulate the switch between cell cycle and differentiation in follicle cells is by modulating the activity of the Notch pathway, and that this regulation might be achieved by promoting proper endosomal trafficking of Notch. As integrins transmit signals from the ECM inside the cell, our study demonstrates that the ECM environment can fine-tune the cellular response to pathways regulating cell proliferation and differentiation, such as the Notch pathway, and ultimately can determine the cellular state of a specific cell type. In the future, it will be interesting to test whether integrins could interact with other signals known to regulate follicle cell differentiation, such as those downstream of the SWH pathway or myosin activity.
MATERIALS AND METHODS
Fly stocks
To generate follicle cell mutant clones we used the FRT/FLP technique (Chou and Perrimon, 1992). Mutant clones were marked by the absence of GFP. The following mutant alleles and chromosomes were used: mys11 [also known as mysXG43 (Bunch et al., 1992)], e22c-Gal4 UAS-flipase (Duffy et al., 1998), H2 (Bang and Posakony, 1992), dap04454-LacZ, DlB2 and Notch55e11 (Bloomington Stock Center), Gbe-Su(H)-lacZ (Furriols and Bray, 2001), pnt-LacZ (Morimoto et al., 1996), UAS-TorD/βcyt (Martin-Bermudo and Brown, 1999), baz815 (Djiane et al., 2005), rhea79 (Brown et al., 2002) and ilk1 (Zervas et al., 2001). The e22c-Gal4 driver is expressed in the follicle stem cells in the germarium and was therefore used in combination with UAS-flp to generate large mys and Notch follicle cell clones. The GR1Gal4 is expressed in all follicle cells (Gupta and Schüpbach, 2003) and it was used to express N full-length, NEXT, NICD (Rauskolb et al., 1999) or string (Bloomington Stock Center) in mys PFCs in combination with the HS flipase system. Thus, mys11FRT101/FMZ; UAS-N full length (or UAS-NICD or UAS-NEXT) were crossed to y w hs-Flipase 122 ubiquitin-GFPFRT101;; GR1Gal4 males. Newly hatched females from this cross were heat-shocked at 37°C for 20 minutes and kept at 25°C for 2 days prior to ovary dissection. To generate mys mutant cells expressing TorD/βcyt (Martin-Bermudo and Brown, 1999) in the follicular epithelium, we grew females of the genotype mys11FRT101/ubiquitin-GFPFRT101;e22c-Gal4/UAS-TorD/βcyt; tubulin-Gal80ts/+ at 18°C until eclosion. Adult females were kept at 31°C for 3–5 days prior to ovary dissection. To generate clones of cells expressing CycE, Stg, DP and E2F2 in the follicular epithelium, we used the ‘flip-out’ technique (Struhl et al., 1993). y w hs-Flipase 122, Act <CD2 >Gal4;;UAS-GFP females were crossed to UAS-CycE,UAS-Stg;UAS-DP,UAS-E2F2 (Buttitta et al., 2007). Newly hatched females from this cross were heat-shocked at 37°C for 20 minutes and kept at 25°C for 2 days prior to ovary dissection.
Immunohistochemistry and microscopy
Drosophila females were yeasted for 1–2 days before dissection. Staining was performed at room temperature according to standard procedures. The DNA dye TO-PRO-3 (Molecular Probes™, 1∶1000) was added for 10 minutes in PBT (PBS+0.1% Tween) after the secondary antibody. The following primary antibodies were used: mouse anti-FasIII (1∶20), mouse anti-Eya (1∶20), mouse anti-Cut (1∶25), mouse anti-NICD (1∶100), mouse anti-NECD (1∶100), mouse anti-Hnt (1∶15), mouse anti-CycB (1∶20) and mouse anti-Br-C (1∶100; all from Developmental Studies Hybridoma Bank; DSHB); rabbit anti-Staufen (1∶1000) (St Johnston et al., 1991); mouse anti-BrdU (1∶20; Roche); mouse anti-β-gal (1∶10,000; Promega); rabbit anti-β-gal (1∶10,000; CAPPEL™); rabbit anti-GFP (1∶10,000; Molecular Probes™); mouse anti-GFP (1∶100; Molecular Probes™); mouse anti-Myc (1∶100; Oncogen Science) and guinea-pig anti-Hrs (1∶1000; Lloyd et al., 2002). The secondary antibodies were conjugated to Alexa Fluor 488 (Molecular Probes™), Cy3 or Cy5 (Jackson ImmunoReseach Laboratories) and were used at 1∶200. Images were captured with a Leica TCS-SPE confocal microscope and processed with ImageJ.
For the BrdU incorporation assay, the experiment was performed using the 5-bromo-2′-deoxy-uridine (BrdU) Labeling and Detection Kit I from Roche (Cat. 11296736001). Ovaries were dissected in PBS and incubated in BrdU incorporation medium (PBS+10 µM BrdU, Roche) for 30 minutes and washed for 5 minutes with PBS. After fixing the ovaries for 20 minutes in 4% paraformaldehyde (PFA), they were blocked with PBT-10 (PBS+0.1% Tween, 10% BSA) for 1 hour. Ovaries were then incubated overnight with anti-GFP antibody in PBT-1 (PBS+0.1% Tween, 1% BSA) to label the clones, and were washed with PBT-1 for 1 hour. Ovaries were re-fixed in 4% PFA for 20 minutes, treated with 4 M HCl in PBS for 7 minutes and washed several times for 30 minutes in PBS, until the pH reached 7. Then, ovaries were blocked again with PBT-10 and incubated overnight with mouse anti-BrdU (Roche) diluted in incubation buffer. After washes with PBT-1 for 1–2 hours, egg chambers were incubated with the secondary antibody in PBT-0.1 (0.1% Tween+0.1% BSA) for 2–4 hours in the dark. Egg chambers were then washed with PBT for 1–2 hours and finally mounted in Vectashield (Vector).
Supplementary Material
Acknowledgments
We thank Sarah Bray, David Bilder, Sol Sotillos and Bloomington Stock Center for fly stocks and for sharing valuable reagents. Special thanks to Acaimo González-Reyes and Sarah Bray for helpful comments on the manuscript.
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
M.D.M.-B. conceived of the project, performed experiments, analyzed the data and wrote the paper. M.J.G.-L., L.C.-R. and B.I.-J. performed experiments, analyzed the data and contributed to the writing of the paper. I.M.P. contributed to the writing of the paper.
Funding
This work was supported by the Spanish Ministerio de Ciencia y Tecnología [grant numbers BMC2010-16669, CSD-2007-00008 to M.D.M.-B.]; the Junta de Andalucía (JA) [grant numbers CVI-280 and P09-CVI-5058 to M.D.M.-B.]; and the UK Medical Research Council (MRC) [grant number 200937 to I.M.P.]. M.J.G-L is supported by a FPI studentship from the Ministerio de Ciencia y TecnologÚa. Institutional support from JA to C.A.B.D. is acknowledged. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.153122/-/DC1
References
- Andersson E. R., Sandberg R., Lendahl U. (2011). Notch signaling: simplicity in design, versatility in function. Development 138, 3593–3612. 10.1242/dev.063610 [DOI] [PubMed] [Google Scholar]
- Aszodi A., Hunziker E. B., Brakebusch C., Fässler R. (2003). Beta1 integrins regulate chondrocyte rotation, G1 progression, and cytokinesis. Genes Dev. 17, 2465–2479. 10.1101/gad.277003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J., Montell D. (2002). Eyes absent, a key repressor of polar cell fate during Drosophila oogenesis. Development 129, 5377–5388. 10.1242/dev.00115 [DOI] [PubMed] [Google Scholar]
- Bang A. G., Posakony J. W. (1992). The Drosophila gene Hairless encodes a novel basic protein that controls alternative cell fates in adult sensory organ development. Genes Dev. 6, 1752–1769. 10.1101/gad.6.9.1752 [DOI] [PubMed] [Google Scholar]
- Baron W., Decker L., Colognato H., ffrench-Constant C. (2003). Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr. Biol. 13, 151–155. 10.1016/S0960-9822(02)01437-9 [DOI] [PubMed] [Google Scholar]
- Blaess S., Graus-Porta D., Belvindrah R., Radakovits R., Pons S., Littlewood-Evans A., Senften M., Guo H., Li Y., Miner J. H. et al. (2004). Beta1-integrins are critical for cerebellar granule cell precursor proliferation. J. Neurosci. 24, 3402–3412. 10.1523/JNEUROSCI.5241-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramsiepe J., Wester K., Weinl C., Roodbarkelari F., Kasili R., Larkin J. C., Hülskamp M., Schnittger A. (2010). Endoreplication controls cell fate maintenance. PLoS Genet. 6, e1000996. 10.1371/journal.pgen.1000996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S. J. (2006). Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–689. 10.1038/nrm2009 [DOI] [PubMed] [Google Scholar]
- Brown N. H., Gregory S. L., Rickoll W. L., Fessler L. I., Prout M., White R. A., Fristrom J. W. (2002). Talin is essential for integrin function in Drosophila. Dev. Cell 3, 569–579. 10.1016/S1534-5807(02)00290-3 [DOI] [PubMed] [Google Scholar]
- Bunch T. A., Salatino R., Engelsgjerd M. C., Mukai L., West R. F., Brower D. L. (1992). Characterization of mutant alleles of myospheroid, the gene encoding the beta subunit of the Drosophila PS integrins. Genetics 132, 519–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttitta L. A., Katzaroff A. J., Perez C. L., de la Cruz A., Edgar B. A. (2007). A double-assurance mechanism controls cell cycle exit upon terminal differentiation in Drosophila. Dev. Cell 12, 631–643. 10.1016/j.devcel.2007.02.020 [DOI] [PubMed] [Google Scholar]
- Calvi B. R., Lilly M. A., Spradling A. C. (1998). Cell cycle control of chorion gene amplification. Genes Dev. 12, 734–744. 10.1101/gad.12.5.734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou T. B., Perrimon N. (1992). Use of a yeast site-specific recombinase to produce female germline chimeras in Drosophila. Genetics 131, 643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo M. A., Alderson N. B., Kiosses W. B., Chiang H. H., Anderson R. G., Schwartz M. A. (2004). Integrins regulate Rac targeting by internalization of membrane domains. Science 303, 839–842. 10.1126/science.1092571 [DOI] [PubMed] [Google Scholar]
- Deng W. M., Althauser C., Ruohola-Baker H. (2001). Notch-Delta signaling induces a transition from mitotic cell cycle to endocycle in Drosophila follicle cells. Development 128, 4737–4746 [DOI] [PubMed] [Google Scholar]
- Djiane A., Yogev S., Mlodzik M. (2005). The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell 121, 621–631. 10.1016/j.cell.2005.03.014 [DOI] [PubMed] [Google Scholar]
- Duffy J. B., Harrison D. A., Perrimon N. (1998). Identifying loci required for follicular patterning using directed mosaics. Development 125, 2263–2271 [DOI] [PubMed] [Google Scholar]
- Fernández-Miñán A., Martín-Bermudo M. D., González-Reyes A. (2007). Integrin signaling regulates spindle orientation in Drosophila to preserve the follicular-epithelium monolayer. Curr. Biol. 17, 683–688. 10.1016/j.cub.2007.02.052 [DOI] [PubMed] [Google Scholar]
- Fernández-Miñán A., Cobreros L., González-Reyes A., Martín-Bermudo M. D. (2008). Integrins contribute to the establishment and maintenance of cell polarity in the follicular epithelium of the Drosophila ovary. Int. J. Dev. Biol. 52, 925–932. 10.1387/ijdb.072418af [DOI] [PubMed] [Google Scholar]
- Fortini M. E., Bilder D. (2009). Endocytic regulation of Notch signaling. Curr. Opin. Genet. Dev. 19, 323–328. 10.1016/j.gde.2009.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furriols M., Bray S. (2001). A model Notch response element detects suppressor of Hairless-dependent molecular switch. Curr. Biol. 11, 60–64. 10.1016/S0960-9822(00)00044-0 [DOI] [PubMed] [Google Scholar]
- González-Reyes A., St Johnston D. (1998). Patterning of the follicle cell epithelium along the anterior-posterior axis during Drosophila oogenesis. Development 125, 2837–2846 [DOI] [PubMed] [Google Scholar]
- Gupta T., Schüpbach T. (2003). Cct1, a phosphatidylcholine biosynthesis enzyme, is required for Drosophila oogenesis and ovarian morphogenesis. Development 130, 6075–6087. 10.1242/dev.00817 [DOI] [PubMed] [Google Scholar]
- Guruharsha K. G., Kankel M. W., Artavanis-Tsakonas S. (2012). The Notch signalling system: recent insights into the complexity of a conserved pathway. Nat. Rev. Genet. 13, 654–666. 10.1038/nrg3272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687. 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- King R. C. (1970). Ovarian Development in Drosophila Melanogaster New York, NY: Academic Press [Google Scholar]
- Klusza S., Deng W. M. (2011). At the crossroads of differentiation and proliferation: precise control of cell-cycle changes by multiple signaling pathways in Drosophila follicle cells. BioEssays 33, 124–134. 10.1002/bies.201000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie P., Nabi I. R. (2010). Lipid rafts, caveolae, and their endocytosis. Int. Rev. Cell Mol. Biol. 282, 135–163. 10.1016/S1937-6448(10)82003-9 [DOI] [PubMed] [Google Scholar]
- Lehman D. A., Patterson B., Johnston L. A., Balzer T., Britton J. S., Saint R., Edgar B. A. (1999). Cis-regulatory elements of the mitotic regulator, string/Cdc25. Development 126, 1793–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N., Zhang Y., Naylor M. J., Schatzmann F., Maurer F., Wintermantel T., Schuetz G., Mueller U., Streuli C. H., Hynes N. E. (2005). Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J. 24, 1942–1953. 10.1038/sj.emboj.7600674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd T. E., Atkinson R., Wu M. N., Zhou Y., Pennetta G., Bellen H. J. (2002). Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108, 261–269. 10.1016/S0092-8674(02)00611-6 [DOI] [PubMed] [Google Scholar]
- López-Schier H., St Johnston D. (2001). Delta signaling from the germ line controls the proliferation and differentiation of the somatic follicle cells during Drosophila oogenesis. Genes Dev. 15, 1393–1405. 10.1101/gad.200901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier D. (2006). Hairless: the ignored antagonist of the Notch signalling pathway. Hereditas 143, 212–221. 10.1111/j.2007.0018-0661.01971.x [DOI] [PubMed] [Google Scholar]
- Margolis J., Spradling A. (1995). Identification and behavior of epithelial stem cells in the Drosophila ovary. Development 121, 3797–3807 [DOI] [PubMed] [Google Scholar]
- Martin-Bermudo M. D., Brown N. H. (1999). Uncoupling integrin adhesion and signaling: the betaPS cytoplasmic domain is sufficient to regulate gene expression in the Drosophila embryo. Genes Dev. 13, 729–739. 10.1101/gad.13.6.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meignin C., Alvarez-Garcia I., Davis I., Palacios I. M. (2007). The salvador-warts-hippo pathway is required for epithelial proliferation and axis specification in Drosophila. Curr. Biol. 17, 1871–1878. 10.1016/j.cub.2007.09.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto A. M., Jordan K. C., Tietze K., Britton J. S., O'Neill E. M., Ruohola-Baker H. (1996). Pointed, an ETS domain transcription factor, negatively regulates the EGF receptor pathway in Drosophila oogenesis. Development 122, 3745–3754 [DOI] [PubMed] [Google Scholar]
- Naylor M. J., Li N., Cheung J., Lowe E. T., Lambert E., Marlow R., Wang P., Schatzmann F., Wintermantel T., Schüetz G. et al. (2005). Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J. Cell Biol. 171, 717–728. 10.1083/jcb.200503144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norambuena A., Schwartz M. A. (2011). Effects of integrin-mediated cell adhesion on plasma membrane lipid raft components and signaling. Mol. Biol. Cell 22, 3456–3464. 10.1091/mbc.E11-04-0361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesello C., Tapon N. (2007). Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Curr. Biol. 17, 1864–1870. 10.1016/j.cub.2007.09.049 [DOI] [PubMed] [Google Scholar]
- Rauskolb C., Correia T., Irvine K. D. (1999). Fringe-dependent separation of dorsal and ventral cells in the Drosophila wing. Nature 401, 476–480. 10.1038/46786 [DOI] [PubMed] [Google Scholar]
- Sasamura T., Matsuno K., Fortini M. E. (2013). Disruption of Drosophila melanogaster lipid metabolism genes causes tissue overgrowth associated with altered developmental signaling. PLoS Genet. 9, e1003917. 10.1371/journal.pgen.1003917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer V., Althauser C., Shcherbata H. R., Deng W. M., Ruohola-Baker H. (2004). Notch-dependent Fizzy-related/Hec1/Cdh1 expression is required for the mitotic-to-endocycle transition in Drosophila follicle cells. Curr. Biol. 14, 630–636. 10.1016/j.cub.2004.03.040 [DOI] [PubMed] [Google Scholar]
- Shcherbata H. R., Althauser C., Findley S. D., Ruohola-Baker H. (2004). The mitotic-to-endocycle switch in Drosophila follicle cells is executed by Notch-dependent regulation of G1/S, G2/M and M/G1 cell-cycle transitions. Development 131, 3169–3181. 10.1242/dev.01172 [DOI] [PubMed] [Google Scholar]
- Snow P. M., Bieber A. J., Goodman C. S. (1989). Fasciclin III: a novel homophilic adhesion molecule in Drosophila. Cell 59, 313–323. 10.1016/0092-8674(89)90293-6 [DOI] [PubMed] [Google Scholar]
- St Johnston D., Beuchle D., Nüsslein-Volhard C. (1991). Staufen, a gene required to localize maternal RNAs in the Drosophila egg. Cell 66, 51–63. 10.1016/0092-8674(91)90138-O [DOI] [PubMed] [Google Scholar]
- Struhl G., Fitzgerald K., Greenwald I. (1993). Intrinsic activity of the Lin-12 and Notch intracellular domains in vivo. Cell 74, 331–345. 10.1016/0092-8674(93)90424-O [DOI] [PubMed] [Google Scholar]
- Sun J., Deng W. M. (2005). Notch-dependent downregulation of the homeodomain gene cut is required for the mitotic cycle/endocycle switch and cell differentiation in Drosophila follicle cells. Development 132, 4299–4308. 10.1242/dev.02015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Deng W. M. (2007). Hindsight mediates the role of notch in suppressing hedgehog signaling and cell proliferation. Dev. Cell 12, 431–442. 10.1016/j.devcel.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Yan Y., Denef N., Schüpbach T. (2011). Regulation of somatic myosin activity by protein phosphatase 1β controls Drosophila oocyte polarization. Development 138, 1991–2001. 10.1242/dev.062190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzolovsky G., Deng W. M., Schlitt T., Bownes M. (1999). The function of the broad-complex during Drosophila melanogaster oogenesis. Genetics 153, 1371–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. M. (2002). Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 21, 3919–3926. 10.1093/emboj/cdf399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Poulton J., Huang Y. C., Deng W. M. (2008). The hippo pathway promotes Notch signaling in regulation of cell differentiation, proliferation, and oocyte polarity. PLoS One 3, e1761. 10.1371/journal.pone.0001761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervas C. G., Gregory S. L., Brown N. H. (2001). Drosophila integrin-linked kinase is required at sites of integrin adhesion to link the cytoskeleton to the plasma membrane. J. Cell Biol. 152, 1007–1018. 10.1083/jcb.152.5.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.