Abstract
Acellular whole human lung scaffolds represent a unique opportunity for ex vivo tissue engineering. However, it remains unclear whether lungs from individuals with chronic lung diseases such as chronic obstructive pulmonary disease (COPD) can be appropriately decellularized and recellularized. To assess this, cadaveric human lungs from normal (non-smoking) patients and from patients with COPD (smoking history) were decellularized and found by histochemical and immunohistochemical staining, electron microscopy, and mass spectrometry to retain characteristic histological architecture and extracellular matrix components (ECM) reflecting either normal or COPD, particularly emphysematous, origin. Inoculation of human bronchial epithelial cells, endothelial progenitor cells, bone marrow-derived mesenchymal stem cells, and lung fibroblasts via airway or vascular routes into small, excised segments of the decellularized lungs demonstrated that normal lung scaffolds robustly supported initial engraftment and growth of each cell type for up to one month. In contrast, despite initial binding, all cell types inoculated into decellularized emphysematous lungs did not survive beyond one week. However, cell attachment and proliferation on solubilized ECM homogenates of decellularized normal and emphysematous lungs coated onto tissue culture plates was comparable and not impaired, suggesting that the 3-dimensional decellularized emphysematous scaffolds may lack the necessary ECM architecture to support sustained cell growth.
Keywords: Acellular matrix, emphysema, endothelial cell, epithelial cell, extracellular matrix (ECM), human lung fibroblast, mesenchymal stem cell
Introduction
Devastating lung diseases, such as pulmonary fibrosis and chronic obstructive pulmonary diseases (COPD), are increasing in prevalence and remain without a cure except for lung transplantation. However, there are not enough donor lungs to match the current clinical need and transplantation efficacy is further inhibited by acute and chronic rejection and by complications from immunosuppressive drugs. Alternative options need to be explored to increase the potential supply of donor lungs and the subsequent efficacy of transplantation. A rapidly growing body of literature suggests that decellularized (acellular) whole lung scaffolds recellularized with autologous stem or progenitor cells obtained from the intended transplant recipient may provide a potential means of utilizing failed donor or even cadaveric lungs in clinical transplantation approaches [1–22]. However, lungs available for producing acellular lung scaffolds may come from older individuals with a history of pre-existing pulmonary disorders, such as emphysema or pulmonary fibrosis. It is currently unknown whether these lungs could be used to produce suitable acellular scaffolds for ex vivo tissue regeneration. In previous studies, we found that murine alveolar epithelial cells (C10) had limited survival when inoculated into acellular mouse lung scaffolds obtained from mice with experimentally-induced emphysema compared to normal mice [13]. To assess whether these results translated to repopulation of acellular emphysematous human lung tissue, acellular scaffolds were generated from cadaveric lungs obtained from emphysematous and healthy individuals. Lung architecture and residual protein content were assessed by histology, immunohistochemistry, and mass spectrometry as well as the ability of the acellular scaffolds to support short and long term recellularization with a variety of human cell types including human bronchial epithelial cells (HBEs), endothelial progenitor cells (CBFs), lung fibroblasts (HLFs), and bone marrow-derived MSCs (hMSCs). Potential scaffold toxicity and the ability of matrix components to support cell growth was also assessed on tissue culture plates coated with solubilized extracellular matrix from decellularized normal and emphysematous lungs.
Materials & Methods
Human lungs
Human lungs were obtained from autopsy services at Fletcher Allen Hospital in Burlington, Vermont. Classification of normal versus COPD (emphysematous) was based on review of available clinical records including known previous COPD or other lung diseases, smoking history, chest radiographs, pulmonary function testing, and use of respiratory medications. Subsequent confirmation of emphysematous changes on histologic sections were utilized to corroborate clinical data. A total of 18 lungs (7 normal and 11 COPD) were assessed for the current study. Clinical characteristics are depicted in Table 1.
Table 1.
Clinical Characteristics of Lungs Obtained from Autopsy by Smoking Status
| Smoker/Former Smoker | Non-Smoker | Total | P Value | |
|---|---|---|---|---|
| (n = 11) | (n = 7) | (n = 18) | ||
| Patient Age at Time of Death (years) | 71.1 ± 13.8 | 61.3 ± 14.6 | 65.7 (Range 38 – 89) | 0.170 |
| Sex (M:F) | 9:2 (82%/18%) | 3:4 (43%/57%) | 12:6 (67%/33%) | 0.141 |
| Mean Time from Death to Autopsy (hours) | 32.8 ± 23.9 | 35.0 ± 22.6 | 33.7 (Range 4 – 89) | 0.850 |
| Cause of Death | ||||
| Pulmonary* | 3 (27%) | 3 (43%) | 6 (33%) | 0.627 |
| Cardiac | 5 (46%) | 2 (28.5%) | 7 (39%) | 0.637 |
| Neurological | 1 (9%) | 2 (28.5%) | 3 (17%) | 0.528 |
| Other** | 2 (18%) | 0(0%) | 2 (11%) | 0.497 |
| Pulmonary History | ||||
| COPD | 5 (46%) | 0(0%) | 5 (28%) | 0.101 |
| Emphysema*** | 3 (27%) | 0(0%) | 3 (17%) | 0.245 |
| Emphysema + COPD | 2 (18%) | 0(0%) | 2 (11%) | 0.497 |
| Additional Findings at Autopsy | ||||
| Gastric Aspiration | 3 (27%) | 2 (28.5%) | 5 (28%) | 1.000 |
| Bronchopneumonia | 4 (36%) | 3 (43%) | 7 (39%) | 1.000 |
| Pleural Effusions | 4 (36%) | 2 (28.5%) | 6 (33%) | 1.000 |
| Pulmonary Congestion | 5 (46%) | 3 (43%) | 8 (44%) | 1.000 |
Data are given as mean ± SD or as number of patients (%)
P values calculated using Student’s t-test or Fisher’s Exact Test with 95% confidence interval
Pulmonary Causes of Death include: Acute bronchopneumonia, Aspiration pneumonia and Pulmonary embolism
Other Causes of Death include: Metastatic breast cancer and Rhabdomyolysis from trauma
Emphysema includes: Centrilobar, Paraseptal and Centriacinar
Lung decellularization
Human lungs were decellularized using a combined perfusion and physical approach we have developed after scaling up and optimizing methods similarly developed for rodent and small non-human primate models [8, 9, 11, 13, 15, 22]. Individual lobes were separated from one another prior to decellularization. Decellularization fluids were instilled into lobes at a flow rate of 2L/minute which we have determined to maximize instillation of decellularization fluids and minimize tissue damage (unpublished data). On day 1 of the decellularization protocol, the lungs were rinsed with 8L of de-ionized (DI) water containing 500IU/mL Penicillin/500ug/mL Streptomycin (5X pen/strep) (Lonza) through both the lobar main stem bronchus and the major vascular arterial supplies to each respective lobe. Next, 4L of 0.1% Triton-X (Sigma) and 5X pen/strep in DI water was infused through airway and vascular ports. Lobes were submerged in Triton-X solution and incubated on a shaker table for 24 hours at 4°C. On day 2, the lungs were removed from Triton-X and rinsed with 8L of DI water and 5X pen/strep as previously described. 4L of 2% sodium deoxycholate (SDC, Sigma) and 100IU/mL Penicillin/100ug/mL Streptomycin (1X pen/strep) in DI water was then instilled as previously described for day 1. Lungs were incubated in SDC and placed on a shaker table for 24 hours at 4°C. The next day, lungs were removed from the SDC solution and rinsed with 8L of DI water and pen/strep. 4L of 1M sodium chloride (NaCl) (USB) and 5X pen/strep in DI water was instilled into the lung. The lungs were then incubated in the NaCl solution and placed on a shaker table for 1 hour at room temperature (~25°C). Lungs were removed from the NaCl solution, rinsed with 8L of DI water and penstrep, and then instilled with 4L of 30μg/mL porcine pancreatic DNase (Sigma), 2mM calcium chloride (CaCl2) (Sigma), 1.3mM magnesium sulfate (MgSO4) (Sigma), and 5X pen/strep in DI water and incubated on a shaker table for 1 hour at room temperature. The lungs were removed from the DNase solution, rinsed with 8L of DI water and pen/strep, and then infused with 4L of 0.1% peracetic acid (Sigma) in 4% ethanol solution and incubated on a shaker table for 2 hours at room temperature. Finally, lungs were removed from the peracetic acid solution and rinsed with 12–15L of a 5X pen/strep, 50mg/L gentamicin (Cellgro), 2.5ug/mL Amphotericin B (Cellgro) in 1X PBS solution (storage solution) as described for the pen/strep DI water rinses. Lungs were stored in storage solution at 4°C until needed.
Lung histology
Native and decellularized human lungs were fixed with 4% paraformaldehyde for 10 minutes at room temperature and embedded in paraffin. Five μm tissue sections were cut and mounted on glass slides. Following deparaffinization, sections were histologically stained with hematoxylin & eosin (H&E), Verhoeff’s Van Gieson (EVG), Masson’s Trichrome, or Alcian Blue, and were assessed by standard light microscopy [8, 9, 13, 15, 22]. Recellularized slices of decellularized tissue were also fixed for 10 minutes at room temperature in 4% paraformaldehyde. Harvested samples were embedded in paraffin, cut and mounted as 5μm sections, and then assessed by H&E staining for the presence and distribution of the inoculated cells.
For electron microscopic analyses, excised segments of decellularized emphysematous and normal human lung were fixed overnight at 4°C in Karnovsky’s fixative (2.5% glutaraldehyde, 1.0% paraformaldehyde in 0.1M Cacodylate buffer, pH 7.2). After rinsing in Cacodylate buffer, the tissue was minced into 1mm3 pieces and then fixed in 1% osmium tetroxide for 2 hours at 4°C. Subsequently, the pieces were rinsed again in Cacodylate buffer, dehydrated through graded ethanols, and then cleared in propylene oxide and embedded in Spurr’s epoxy resin. Semithin sections (1μm) were cut with glass knives on a Reichert ultracut microtome, stained with methylene blue–azure II and then evaluated for areas of interest (proximal and distal alveolar septae, large/small airways, blood vessels). Ultrathin sections (60–80nm) were cut with a diamond knife, retrieved onto 200 mesh thin bar nickel grids, contrasted with uranyl acetate (2% in 50% ethanol) and lead citrate, and examined with a JEOL 1400 TEM (JEOL USA, Inc, Peabody, Ma) operating at 60kV [8].
Immunohistochemical (IHC) staining
Deparaffinization was performed with three xylene incubations for 10 min each, followed by rehydration in descending ethanol series and water. Antigen retrieval was performed for 20 minutes at 98°C in 10mM sodium citrate buffer (Dako, Carpentaria, CA). Tissue sections were then cooled down at room temp for 30 min. Tissue sections were permeabilized with a 0.1% Triton-X solution (Sigma Aldrich, St. Louis, MO) for 15 min. Triton-X was removed with two 5 min washes of 1% bovine serum albumin BSA solution. Blocking was performed using 10% goat serum for 60 min at room temp. After aspirating the blocking solution, primary antibodies were applied in their appropriate concentrations. Primary antibodies that were used include: Purified Mouse Anti-Fibronectin monoclonal (610077 Fibronectin – 1:100, BD Transduction Laboratories), Laminin antibody polyclonal (ab11575 – 1:100, Abcam), Smooth muscle myosin heavy chain 2 polyclonal (ab53219 – 1:100, Abcam), Collagen I polyclonal (ab292 – 1:100, Abcam), Collagen 4 polyclonal (ab6586 – 1:100, Abcam), Rabbit polyclonal to alpha elastin (ab21607 – 1:100, Abcam), Ki67 Proliferation marker polyclonal (ab16667 - 1:50, Abcam), Cleaved Caspase-3 polyclonal (Asp175 – 1:100, Cell Signaling Technology), Mouse clone anti-human Actin polyclonal (1A4 - 1:10,000, Dako).
After the primary antibodies were applied, the tissue sections were incubated overnight at 4° C inside a humidified chamber. The tissue slides were then washed three times with 1% BSA solution for 5 min each, and an appropriate secondary antibody was added. Sections were incubated for 60 min at room temp in a humidified chamber in the dark. Two secondary antibodies were used in this experiment: Alexa Fluor 568 goat anti-rabbit IgG (H+L) (1:500, Invitrogen, Grand Isle, NY), and Alexa Fluor 568 F(ab′)2 fragment of goat anti-mouse IgG (H+L) (1:500, Invitrogen, Grand Isle, NY). After the incubation, the tissue sections were washed three times with a 1% BSA solution for 5 min each in the dark at room temp. A DAPI (Invitrogen, Grand Isle, NY) nuclear stain was added for 5 min in the dark, and was followed by 2 washes in 1% BSA solution for 5 min each. Finally, tissue sections were mounted in Aqua Polymount (Lerner Laboratories, Pittsburgh, PA) [8, 9, 13, 15, 22].
Mass spectrometric assessment of decellularized emphysematous and normal human lung tissue
Samples of the same approximate weight and volume, (approximately 125 mg and 1 cm3, respectively for each sample), were obtained from similar parenchymal regions of lungs and subjected to mass spectrometry as previously described [8, 9, 13, 15, 22]. Each sample was dried separately in a SpeedVac and suspended in 40 μl of 100 mM ammonium bicarbonate (NH4HCO3) and 50 mM dithiothreitol (DTT) and stored at 56° C for 1 hour. After cooling, 5 μl of 500 mM iodoacetamide in 100 mM NH4HCO3 were added and the solution was incubated for 30 min at room temperature in the dark, and then dried in a SpeedVac. The dried tissue was suspended in 50 μl of trypsin solution (10 ng/μl) in 50 mM NH4HCO3 and incubated overnight at 37° C. Next, 5 μl of 10 % formic acid were added to stop the digestion. The sample was centrifuged at 14,000 × g. 15 μl of supernatant were drawn and desalted using a ZipTip C18 (P10, Millipore Corporation, Billerica, MA) according the manufacturer’s protocol, and then dried in a SpeedVac. The dried peptide samples were dissolved in 20 μl 0.1% formic acid and 2% acetonitrile, and 6 μl were loaded onto a fused silica microcapillary LC column (12 cm × 100 μm inner diameter) packed with C18 reversed-phase resin (5 μm particle size; 20 nm pore size; Magic C18AQ, Michrom Bioresources Inc.). Peptides were separated by applying a gradient of 3–60% acetonitrile in 0.1% formic acid at a flow rate of 500 nL/min for 45 min. Nanospray ESI was used to introduce peptides into a liner ion trap (LTQ)-Orbitrap mass spectrometer (Thermo Fisher Scientific) via a nanospray ionization source. Mass spectrometry data was acquired in a data-dependent acquisition mode, in which an Orbitrap survey scan from m/z 400–2000 (resolution: 30,000 FWHM at m/z 400) was paralleled by 10 LTQ MS/MS scans of the most abundant ions. After an LC-MS run was completed and spectra were obtained, the spectra were searched against the IPI Human protein sequence databases (V 3.87) using SEQUEST (Bioworks software, version 3.3.1; Thermo Electron, San Jose, CA). The search parameters permitted a 20 ppm precursor MS tolerance and a 1.0 Da MS/MS tolerance. Oxidation of methionine (M) and carboxymethylation of cysteines (C) were allowed as variable modifications. Up to two missed tryptic cleavages of peptides were considered. The cutoffs for SEQUEST assignments were: cross-correlation (Xcorr) scores greater than 1.9, 2.5, and 3.8 for peptide charge states of +1, +2, and +3, respectively; and a delta-correlation (ΔCn) score > 0.1. Then, all SRF files for each sample were inputted into Scaffold (version Scaffold_4.0.5, Proteome Software Inc., Portland, OR) for the calculations of total spectrum counts. Peptides were accepted and grouped if they could be established at greater than 99.0% probability [23] and peptide prophet FDR<0.5%. Proteins that contained similar peptides and could not be differentiated based on MS/MS analysis alone were grouped to satisfy the principles of parsimony.
Proteins presented are broadly categorized and ranked by the average number of peptides identified from the replicate samples. Proteins positively identified with two or more unique peptide hits were assigned to one of six groups: ECM, cytosolic, cytoskeletal, nuclear, membrane-associated, and secreted by manually entering IPI accession numbers into the UniProtKB/Swiss Prot database (http://www.uniprot.org). Heatmaps were generated with the log normal transformation of unique peptide hits from each positively identified protein [13, 15, 22]. If any of the proteins were matched to more than one category, we chose its predominant subcellular location for functional grouping. Positive protein identification was assigned based on two or more unique peptide hits within each individual sample, not across all samples.
Cell culture
Human bronchial epithelial cells (HBE) (courtesy of Albert van der Vliet, University of Vermont, originally from Drs. J. Yankaskas and R. Wu [24] were cultured on cell-culture treated plastic at 37°C and 5% CO2 in serum free cu lture medium consisting of DMEM/F-12 50/50 mix (Corning), 10 ng/ml Cholera toxin (Sigma), 10 ng/ml epidermal growth factor (Sigma), 5 ug/ml insulin (Gemini Bio-Products), 5 ug/ml transferrin (Sigma), 0.1uM dexamethasone (Sigma), 15 ug/ml bovine pituitary extract (Lonza), 0.5 mg/ml bovine serum albumin (Life Technologies), and 100 IU/ml penicillin/100 μg/ml streptomycin (Corning). Human Lung Fibroblasts (HLF) (ATCC, CCL 171) and grown in media consisting of DMEM/F-12 50/50 mix (Corning), 10% fetal bovine serum (Hyclone), 100 IU/ml penicillin/100 μg/ml streptomycin (Corning), 2mM L-glutamine (Hyclone). CBF (endothelial colony forming cells) cells were obtained from Mervin Yoder (Indiana University – Purdue University Indianapolis) and grown in cEGM-2 (Lonza) supplemented with 10% fetal bovine serum (Hyclone), and 100 IU/ml penicillin/100 μg/ml streptomycin (Corning). These cells were expanded on collagen type I coated tissue culture surfaces. Human bone marrow-derived mesenchymal stromal cells (hMSCs) were obtained from the Texas A&M Stem Cell Core facility. These cells have previously been extensively characterized for cell-surface marker expression and differentiation capacity [25]. hMSCs were expanded in culture using media consisting of Modification of Eagle Medium-Earls Balanced Salt Solution (MEM-EBSS) (Hyclone), 20% fetal bovine serum (Hyclone), 100 IU/ml penicillin/100 μg/ml streptomycin (Corning), 2mM L-glutamine (Hyclone), and used only at passages 7 or 8.
Preparation and culture of recellularized segments of decellularized normal and emphysematous human lungs
Small, approximately 2cm3 segments were excised from decellularized normal and emphysematous lung and coated with a 2.5% sodium alginate (Manugel GMB, FMC Biopolymer) solution in DI water and immediately cross-linked with a 3% calcium chloride solution, leading to the segments being uniformly coated in a calcium alginate hydrogel. Small airways and/or blood vessels were identified and cannulated with a 25g cannula and secured with surgical clips. The segments were then inoculated with the various cell lines through their biologically relevant compartment (HBEs, HMSCs, and HLFs through small airways; CBFs through small vessels) and allowed to incubate at 37°C overnight. Segments were then sliced with a sterile razor blade and a single slice was placed in a well of a 24-well non-tissue culture treated dish, covered with 2mL of sterile cell media, and placed in a standard tissue culture incubator at 37°C with 5% CO2 [8, 9, 13, 15, 22].
ECM coated tissue culture wells from acellular human lungs
Small segments were excised from decellularized normal and emphysematous lung. Samples were mechanically minced in to small pieces with sterile scissors and a sterile razor blade and placed into 50 mL conical tubes. Samples were then snap frozen in liquid nitrogen for three minutes and then lyophilized. The lyophilized samples of normal and emphysematous lung were resuspended in 0.01M HCl to produce a 0.01 g/mL solution. These solutions were agitated using a Roto-Shake Genie (Scientific Industries, Inc. SI-1100) for at least 48 hours until no large particles were visible. Solubilized acellular homogenates were diluted with sterile PBS to create 0.05 mg/mL solutions. Each solution was pipetted directly onto the well-plates using 700 μL per well. The wells were then incubated overnight at 4 °C to allow for ECM attachment and washed with PBS 3X prior to cell seeding.
CBFs, HLFs, and hMSCs were separately seeded at 5,000/well and HBEs seeded at 20,000/well onto normal and emphysematous lung-coated 24 well plates or on standard tissue culture treated plastic (HBE, HLF, hMSC) or rat-tail collagen-1 coated (CBF) 24 well plates as controls. Cells were allowed to grow for 7 days with media changes occurring every other day. Images of cells were taken at D1 and D5. Cytotox96 Non-Radioactive Cytotoxicity Assay (Promega, G1782), a measure of cell death, was performed at D1, D2, and D7 according to the manufacturer’s instructions. CellTiter 96 Non-Radioactive Cell Proliferation Assay (Promega, G4001), a variant of an MTT assay, was used to determine cell viability on D7 according to the manufacturer’s instructions.
Recellularization of excised segments of acellular human lungs
Small pieces of decellularized human lung were excised from larger lobes and blood vessels and/or airways were cannulated. Lung segments were then coated with a calcium alginate hydrogel and inoculated with cell suspensions of human bronchial epithelial cells (HBEs), mesenchymal stem cells (MSCs), endothelial progenitor cells (CBFs), or lung fibroblasts (HLFs) and sliced into roughly 1mm sections [22]. (Full details of coating method and cell culture conditions are given in the online supplemental methods section). Slices were harvested at 1, 3, 7, 14, 21, and 28 days post-inoculation.
Statistical analyses
The natural log of unique peptide hits for each positively identified protein in the mass spectrometric analyses of lungs decellularized under each experimental condition were generated as heat maps using the ‘pheatmap’ package for ‘R’ statistical software (version 2.15.1). Due to the non-normality of the data and the small sample sizes per group, two group comparisons were done using the non-parametric exact permutation test with p<0.05 considered statistically significant [26].
Cytotoxicity (LDH) and viability (MTS) analysis of each cell type on control or tissue culture plastic treated with normal or emphysematous ECM from solubilized acellular lung scaffolds was performed using one way ANOVA. All data are represented as percent of control (cells cultured in tissue culture plastic at the same time point) +/− standard deviation. N=4 for controls and N=3 for acellular normal or emphysematous coated wells. Post-analysis multiple comparisons were conducted using the Tukey test with a 95% confidence level and p<0.05 considered statistically significant [26]. All statistical analyses were performed using GraphPad Prism 6.
Results
Histologic and Proteomic Characterization of Acellular Emphysematous and Normal Lungs
Whole cadaveric lungs or isolated pulmonary lobes were decellularized using a protocol we have developed for lungs from larger animals and humans (Figure 1A) [22]. The lungs were predominantly from an aged population (mean age at death 65.7 years, range 38–89 years) with no statistically significant differences (p<0.015) between time from death until autopsy, cause of death, or additional findings at autopsy. Relevant clinical characteristics are detailed in Table 1.
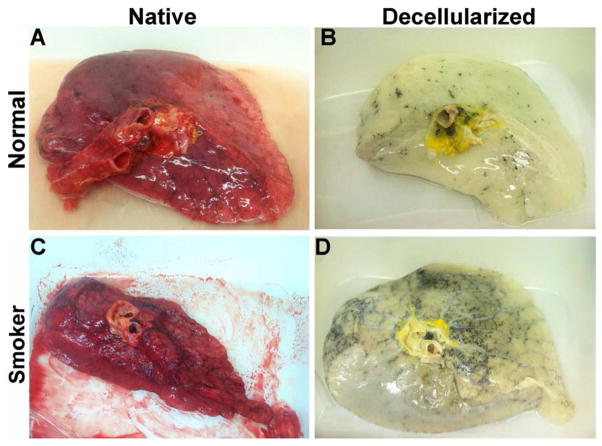
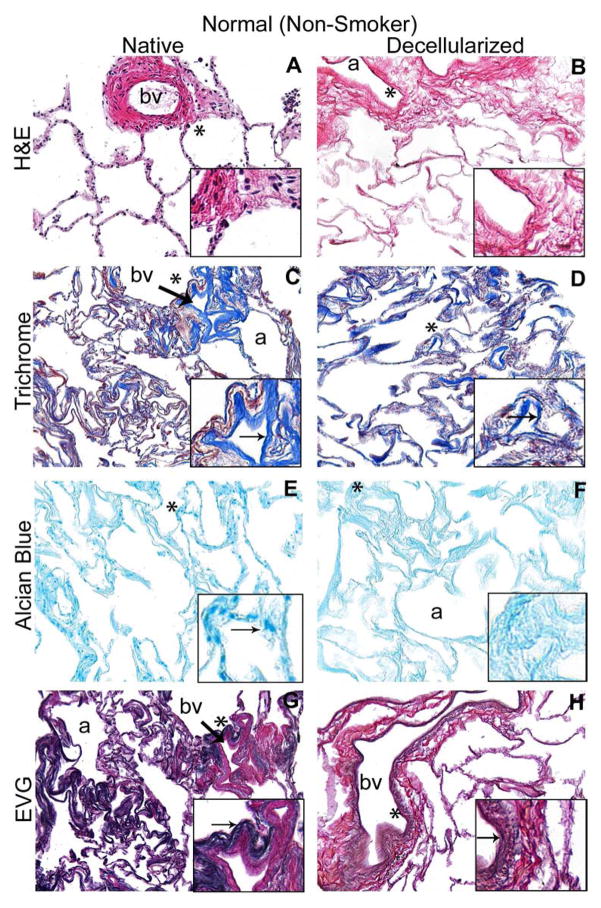
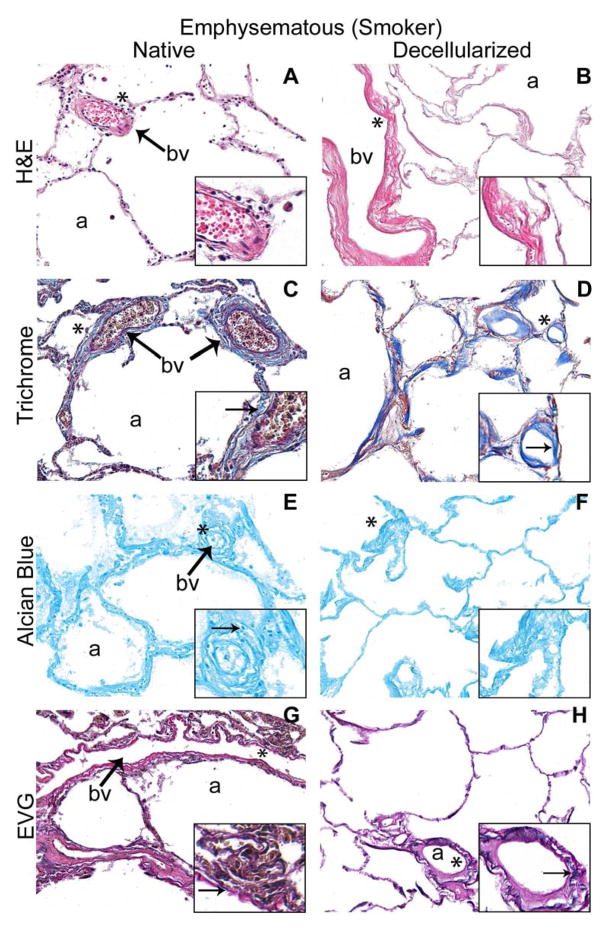
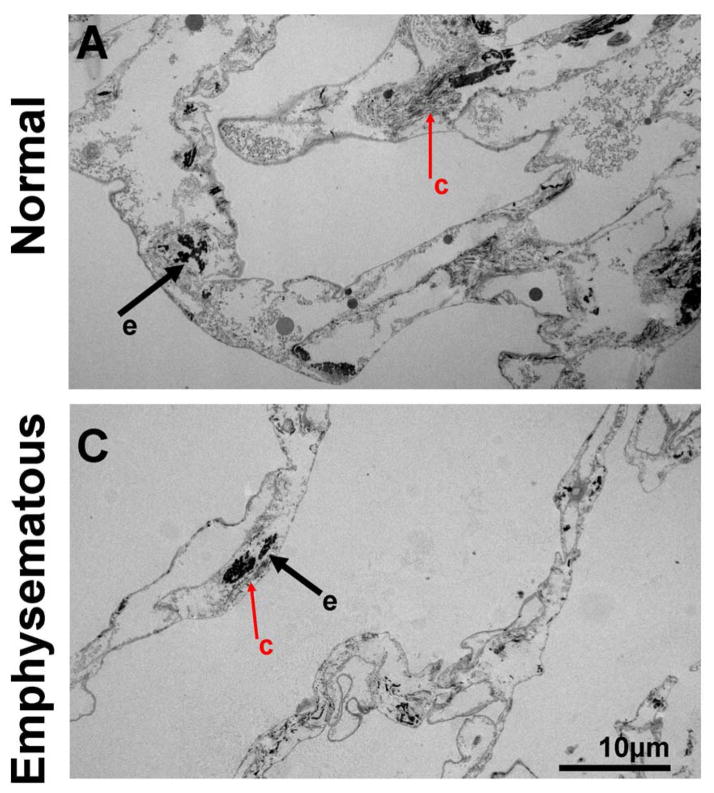
Figure 1. Decellularized normal and emphysematous human lungs retain characteristic gross and histologic appearances.
1A) Representative images of naïve and decellularized normal and emphysematous human lungs. Anthracotic pigment (black, dense deposits) is grossly retained following decellularization (panels B,D). 1B,C) Representative photomicrographs demonstrate maintenance of characteristic histologic and collagen content but loss of elastin and glycosaminoglycans following decellularization of both normal (1B) and emphysematous human lungs (1C) a = airways, bv = blood vessels. Arrows highlight individual blood vessels. Original magnifications 100X. Inserts indicate higher power images (200X) of areas indicated by *. 1D) Electron micrographs demonstrate retention of characteristic normal and emphysematous alveolar septa following decellularization with alveolar wall thinning observed in emphysematous samples. Collagen (labeled c) and elastin (labeled e) are indicated with black and red arrows, respectively. All scale bars are 10μm.
Histological assessment demonstrated that characteristic architecture, either normal or reflecting emphysematous changes, was preserved in decellularized lungs, similar to what we have previously observed in decellularized mouse lungs, including elastase-induced emphysema (Figures 1B–D) [13]. Notably, acellular lungs derived from emphysematous native lung demonstrated heterogeneously distributed regions of alveolar septal destruction and enlarged alveolar air sacs in addition to scattered areas of fibrosis (Figure 1C Panels B,D,F,H). The overall GAG content seemed to be comparably qualitatively decreased following decellularization of normal or emphysematous human lungs (Figures 1B,1C Panels E,F). Collagen architecture was qualitatively preserved (Figures 1B,1C Panels C,D) but less elastin was evident in all decellularized lungs (Figures 1B,1C Panels G,H). Electron microscopy confirmed removal of cellular bodies and maintenance of alveolar septa, with retention of collagen and elastin. Further, characteristic alveolar wall thinning and airspace enlargement was evident in acellular emphysematous lungs (Figure 1D). As an incidental observation, anthracotic pigment deposition in the ECM was observed in most decellularized lungs, including from non-smoking individuals but was markedly increased in lungs from smokers (Figures 1A, 1D).
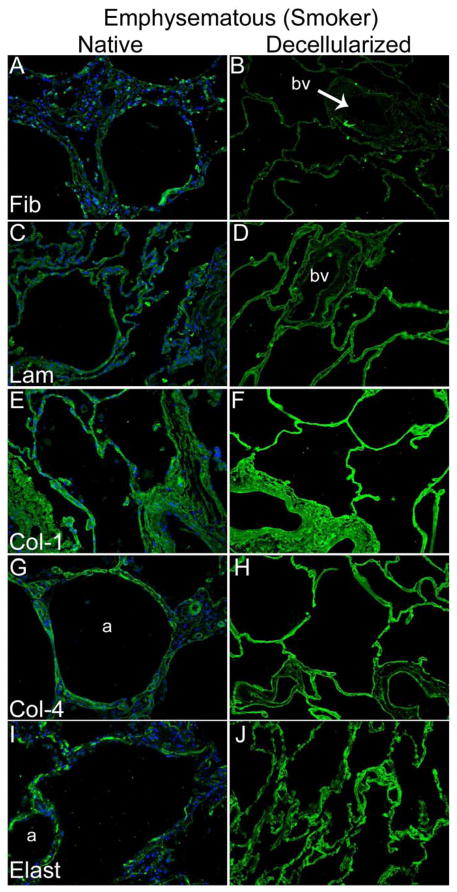
As we and others have previously observed in both rodent and in non-human primate models [8, 9, 11] as well as in acellular pig and human lungs [22], immunohistochemical staining (Figures 2A,B) demonstrated that fibronectin (panels A and B), laminin (panels C and D), type I collagen (panels E and F), and type IV collagen (panels G and H), were largely qualitatively retained in decellularized human lungs. Additionally, as we have previously observed in normal and emphysematous mouse lungs [8, 9, 13, 15] and in normal human lungs [22], smooth muscle myosin (SMM) and smooth muscle actin (SMA) remain following decellularization of human lungs (Figure 2C). Overall, there were no significant qualitative differences between the pattern of immunofluorescence staining of ECM proteins or for SMM and SMA between normal and emphysematous decellularized lungs. No primary antibody controls are shown in Supplemental Figure 1.
Figure 2. Decellularization preserves major ECM proteins in normal and emphysematous human lungs.
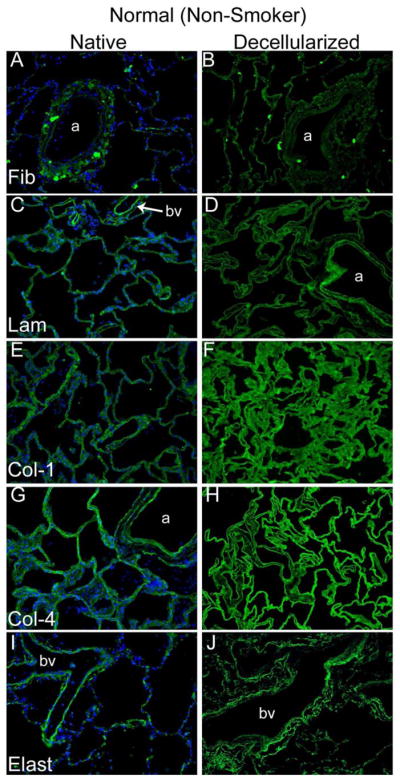
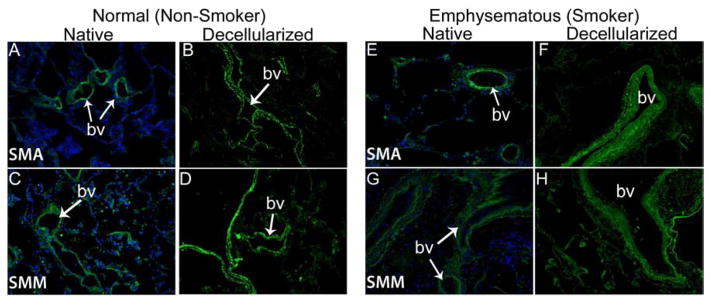
Representative photomicrographs comparing native normal human lung and freshly decellularized human lung (2A) and native and freshly decellularized emphysematous lung (2B) are depicted. Smooth muscle actin and smooth muscle myosin similarly are retained following decellularization of both normal and emphysematous human lungs (2C). Nuclear DAPI staining is depicted in blue, and the stain(s) of interest are depicted in green in each respective panel. Lam = laminin, Col-1 = type I collagen, Col-4 = type 4 collagen, Elast = elastin, Fib = fibronectin. SMA = smooth muscle actin, SMM = smooth. a = airways, bv = blood vessels. Original magnifications 200x.
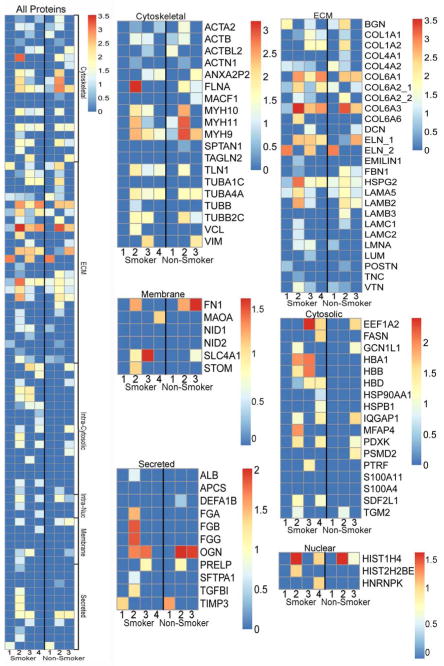
We have previously demonstrated that semi quantitative proteomics is a powerful tool for assessing residual proteins in decellularized mouse, pig, non-human primate, and normal human lung scaffolds [8, 9, 13, 15, 22]. Applying this approach, proteins positively identified with two or more unique peptide hits were assigned to one of six groups: ECM, cytosolic, cytoskeletal, nuclear, membrane-associated, and secreted. Heatmaps were generated from the log normal transformation of unique peptide hits (Supplemental Table 1 and Figure 3). Both groups retained major lung ECM components to comparable degrees (Figures 2A,B and Figure 3), as well as residual cytoskeletal (Figure 2C and Figure 3) and nuclear proteins. While there were no statistically significant differences between groups, there was a trend towards increased retention of cytosolic and secreted proteins in decellularized emphysematous lungs (Supplemental Table 2). All proteins positively identified by mass spectrometry are provided along with unique peptide hit counts (without any statistical conversions) (Supplemental Table 1), where the presence/absence of individual proteins can be compared in individual lungs between smokers and non-smokers.
Figure 3. Decellularized normal and emphysematous human lungs retain both ECM and non-ECM proteins.
Positively identified proteins (ie. those with unique peptide hits greater than or equal to two) were manually grouped according to their subcellular location. Heat maps for all proteins detected, grouped by category, as well as individually protein categories with identified proteins listed are shown. All unique peptide hit values were log normal transformed to more readily show the relative abundance of each protein. Individual heat maps are shown for scaffolds produced from lungs obtained from obtained from 4 normal (non-smoking) and 3 emphysematous (smoking) lungs. The key to protein identification is depicted in Supplemental Table 1.
Recellularization of Acellular Normal versus Emphysematous Lungs
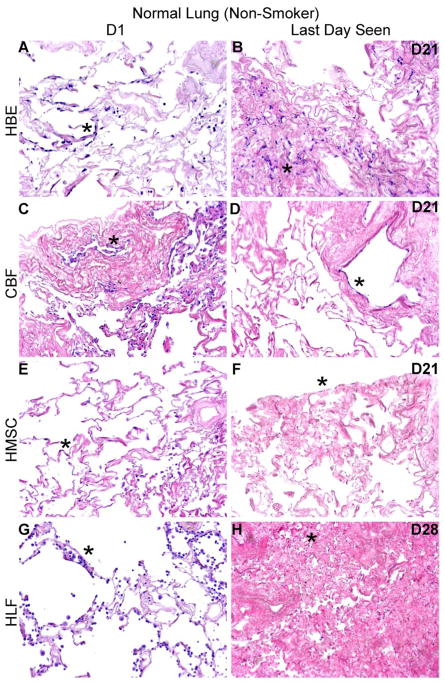
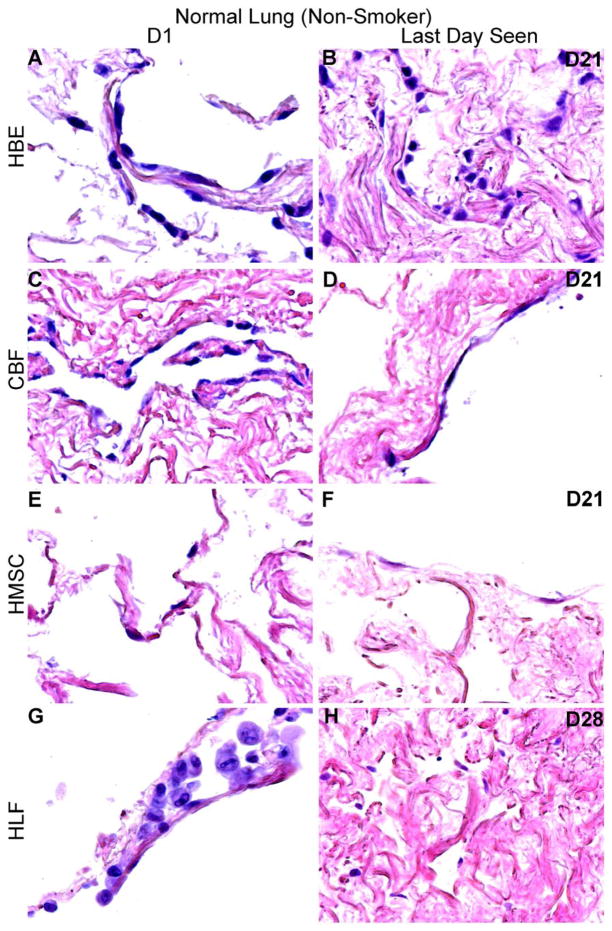
Using techniques we have developed to seed small segments of acellular human lungs by physiologically relevant inoculation through small airways or vessels and subsequent culturing of thin slices of inoculated lungs [8, 9, 13, 15, 22], initial cell binding, localization and cellular growth over one month was assessed for representative human lung cell types. One day following airway inoculation of HBEs, HLFs, or hMSCs, each cell type localized primarily in alveolar spaces and parenchymal regions in both normal and emphysematous acellular scaffolds (Figures 4A, 4B, 4C, and 4D, panels A, E, and G). Similarly, one day after vascular inoculation, CBFs appeared to either be predominantly localized in vessels or parenchymal regions in both normal and emphysematous lungs (Figures 4A and C, panel C). Initial distribution patterns were similar in both decellularized normal and emphysematous human lung scaffolds. Cells seeded into normal lungs appeared to readily adopt an adherent, flattened phenotype, indicative of successful engraftment. Despite the initial appearance of HLFs on D1 (Figure 4A and B, panel G), those persisting adopted an adherent phenotype by D3 (Supplemental Figure 2). In contrast, cells seeded into emphysematous lungs appeared less adherent and exhibited a more rounded phenotype, notably the HBEs and CBFs (Figure 4A–D, panel A and C, respectively). Over longer time periods, engrafted cells (cells adhered to either airway or vascular walls), acquired a variety of morphologies and there were no obvious phenotypic differences between cells seeded into normal or emphysematous decellularized lungs (Figures 4A, 4B, 4C and 4D). In contrast, unattached cells appeared to undergo anoikis in both normal and emphysematous acellular scaffolds at various time points (Figures 4A and 4C, panels B,D,F,H). This phenomenon was more pronounced and occurred more rapidly for cells seeded into emphysematous scaffolds.
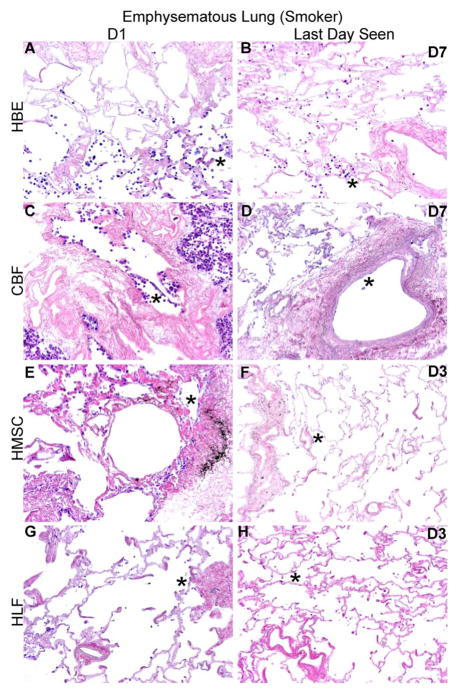
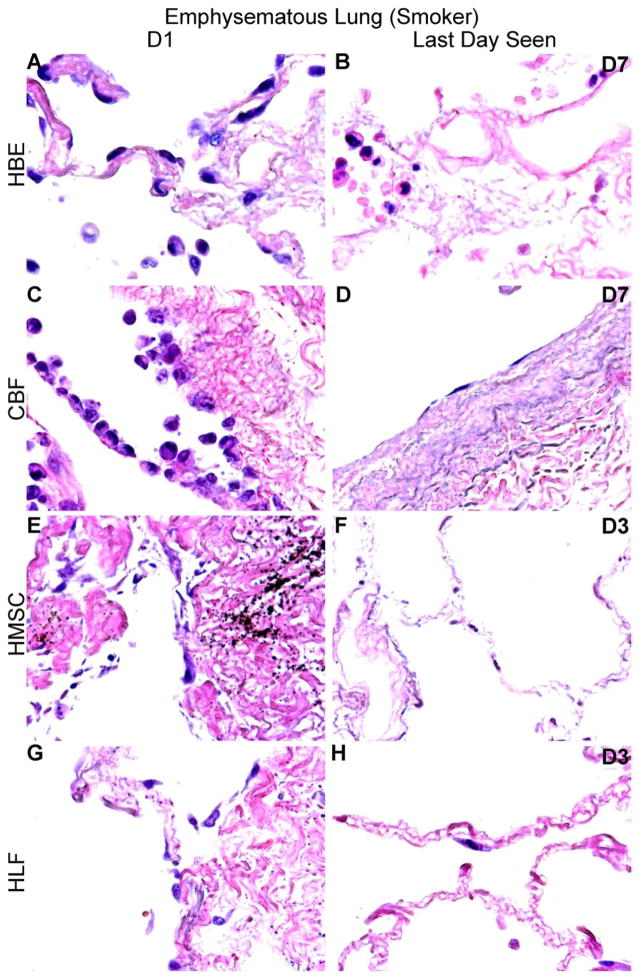
Figure 4. HBE, CBF, hMSC, and HLF cells engraft and proliferate in decellularized normal human lungs for up to 28 days, but fail to thrive in emphysematous lungs beyond 3–7 days.
Representative H and E photomicrographs depict the characteristic recellularization patterns one day post-inoculation of each cell type and the last day viable cells were observed. Engrafted cells in decellularized normal human lungs predominantly acquired characteristic adherent, flattened phenotypes, while those in emphysematous lungs do not appear to similarly engraft. All cell types were viable up to 21 days post-inoculation in acellular normal tissue, except for the HLFs which were viable until day 28 in normal acellular tissue. hMSCs and HLFs remained viable in acellular emphysematous lungs for three days whereas the HBEs and CBFs remained viable for seven days. Normal lungs: N = 3 for HBEs, N = 13 for CBFs, N = 3 for hMSCs, and N = 6 for HLFs. Emphysematous lungs: N = 7 for HBEs, N = 10 for CBFs, N = 5 for hMSCs, and N = 3 for HLFs. Asterisk indicates enlarged region shown in lower power images. Original magnification 100x (low power images) and 400x (high power images)
Viable HBEs, CBFs, and hMSCs could be found through 21 days of culture (Figures 4A and 4B, panels B, D, and F) and HLFs through 28 days (Figures 4A and 4B, panel H) following seeding into normal decellularized human lungs. There were no obvious distribution patterns with prolonged culture of HBEs and HLFs. In contrast, hMSCs were located along the periphery of the tissue at longer time points. CBFs were primarily observed lining major vessels after 21 days in normal acellular lung, and very few cells were found in parenchymal regions (data not shown).
In distinct contrast, cells seeded into emphysematous acellular scaffolds did not persist past day 7. hMSCs and HLFs were only viable through 3 days of culture (Figures 4C and 4D, panels F and H) and HBEs and CBFs were only viable through 7 days (Figures 4C and 4D, panels B and D). HBEs, hMSCs, and HLFs were observed predominantly in parenchymal regions at their last viable time point but were found lining alveolar septa and airways. CBFs were sparsely observed after 7 days in emphysematous acellular scaffolds, but those persisting were found lining major vessels. These striking results were observed in repeated inoculations of acellular scaffolds obtained from different emphysematous lungs.
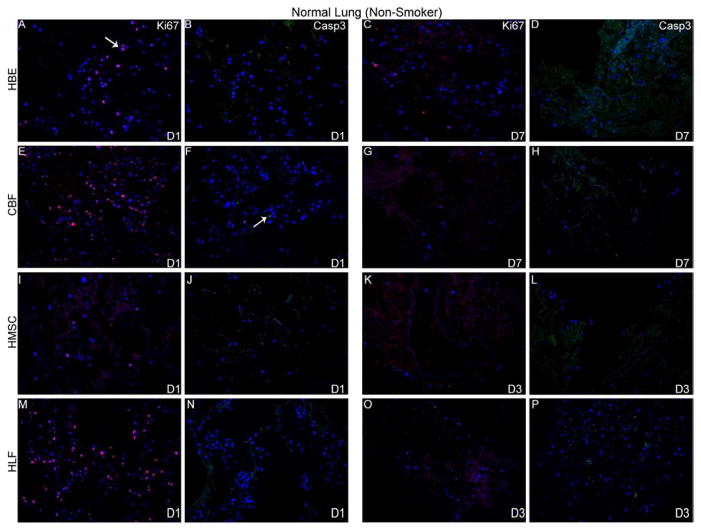
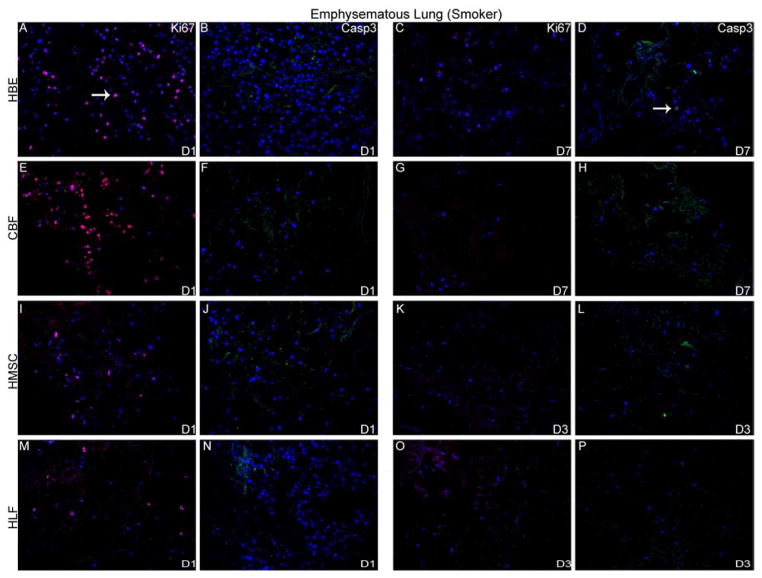
To further characterize cell behavior following cell inoculations into acellular scaffolds, qualitative assessment of proliferation and apoptosis was determined by immunohistochemical staining for Ki67 and caspase 3, respectively. Robust proliferation and no early apoptotic cellular death of HLFs, HBEs, CBFs, and MSCs was observed 1 day after initial inoculation into either normal or emphysematous human lungs (Figure 5). Proliferation decreased over time and minimal positive Ki67 staining was observed at the last viable time point in either acellular normal or emphysematous scaffolds (Figures 5A and 5B, panels C, G, K, O). Despite evidence of cell death by H and E staining, minimal caspase-3 expression was observed at any time point for each of the cell types seeded into either normal or emphysematous scaffolds (Figure 5A and 5B, panels D,H,L,P).
Figure 5. HBE, CBF, hMSC, and HLFs all demonstrate initial proliferation post-inoculation in decellularized normal and emphysematous human lungs by positive Ki67 staining.
Representative photomicrographs of Ki67 (red) or caspase-3 (green) staining are shown at 1 day post-inoculation and at the last viable time point cells were observed in acellular normal (Panel A) or emphysematous lung (Panel B). DAPI nuclear staining is depicted in blue. White arrow indicates positive staining. Original magnification 200x.
Cellular Viability Assessment of ECM Components from Acellular Lungs
To assess whether the acellular emphysematous scaffold might inhibit cellular viability or be cytotoxic, standard tissue culture plastic dishes were coated with solubilized acellular scaffolds produced from normal or emphysematous lungs and seeded with each cell type. After one, two or seven days of culture, hMSCs, CBFs, HBEs, and HLFs did not demonstrate any noticeable phenotypic differences nor was growth significantly inhibited (Figure 6A,B). There did not appear to be any significant cell death at any time point as demonstrated by LDH assay (Figure 7A) and there were no significant differences between cell viability in wells coated with acellular normal or emphysematous lungs (Figure 7B). This indicates that neither the normal or emphysematous acellular scaffolds are themselves cytotoxic and that the ECM components from both scaffolds can support cellular growth.
Figure 6. No significant differences in cell death or viability are detected when cells are cultured wells coated with ECM from normal or emphysematous decellularized human lungs.
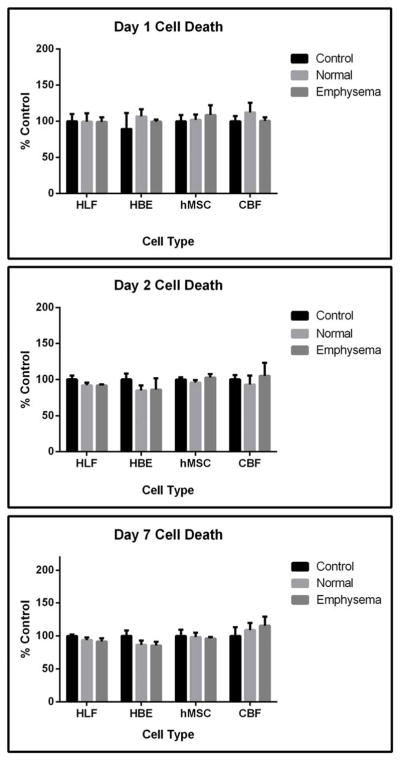
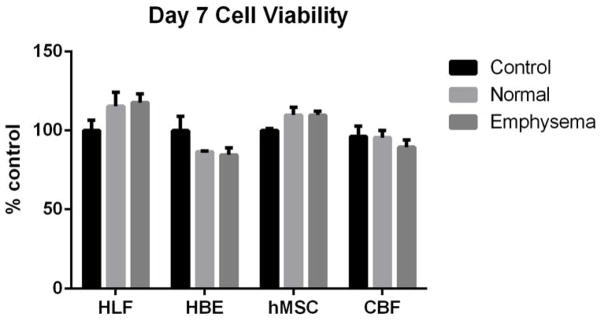
Cells grown on ECM derived from normal or emphysematous human lungs show no detectable differences in cell death between day 1 (6A) and day 7 (6B). Quantitatively, there were no differences in cell death at days 1, 2, and 7 as assessed by an LDH assay (6C) or in cell viability on day 7 as assessed by MTS assay (6D) indicating that the scaffolds are nontoxic and that the ECM components support cellular proliferation for 7 days. Data are presented as mean+/− standard deviation as percentage compared to control cells grown on standard tissue culture plastic (HBE, HLF, hMSC) or collagen I-coated tissue culture plastic (CBF). No statistically significant differences were found. N=4 for each cell grown on control wells, N=3 for each cell type grown on wells coated with either solubilized ECM lyophilates of normal or emphysematous decellularized human lungs. Data shown is representative of one of two similar experiments.
Discussion
Ex vivo lung bioengineering using decellularized whole lungs as scaffolds represents one potential new strategy for lung transplantation and in particular address the shortage of available donor lungs [1]. The acellular donor or cadaveric lungs can be inoculated with autologous stem or progenitor cells obtained from the transplant recipient and then clinically implanted after a suitable ex vivo regeneration strategy. Our group and others have demonstrated in a range of animal and human lung models the ability to decellularize and initially recellularize these scaffolds with a variety of cell types [2–22].
Given potential limitations in clinical sources for donor scaffolds to be utilized in decellularization schemes, it is conceivable that failed donor and cadaveric human lungs for potential use in ex vivo tissue engineering will come from older individuals or from those with previously existing structural lung diseases, such as emphysema and pulmonary fibrosis. Our previous work demonstrated feasibility of decellularizing lungs from mice with experimentally-induced emphysema but found they did not support recellularization as well as acellular lungs from normal, healthy mice [13]. In the current studies, decellularized scaffolds produced from human lungs retained their characteristic normal or emphysematous architecture and showed retention of key ECM proteins by histochemical and immunohistochemical staining. Further, utilizing mass spectrometry as a rapid high throughput and practical semi-quantitative technique for measuring residual proteins in decellularized human lung scaffolds [8, 9, 13, 15, 22], no significant differences were detected between normal and emphysematous lungs. Although strong statistical correlations were not established in the data due to the limited sample size and individual variability associated with the clinical specimens, future studies with large sample numbers will better address these issues. Nonetheless, these initial studies still serve as a discovery platform to identify interesting potential protein candidates that are involved in the recellularization process. For example, HSPG2 protein, different isoforms of collagens, and myosins were identified in lungs from both non-smokers and smokers. Hemoglobin alpha, beta, delta are also consistently present in smoker lungs, but not in non-smoker lungs. Future studies can be conducted to assess the levels of these protein in non-smokers’ vs. smokers’ lungs by quantitative approaches for example, western blotting with isoform-specific antibodies.
It is important to note that the mass spectrometry approach we have used here primarily assesses those proteins which are readily soluble and allows detection of residual cell associated proteins which may be low in abundance. Alternative approaches are necessary to preferentially analyze more insoluble extracellular matrix proteins [27].
While other groups have also investigated the feasibility of decellularizing large animal and human organs [18–21], we have uniquely examined the differences in acellular emphysematous human tissue which has been recellularized in a biologically relevant manner. Initial recellularization attempts of acellular human lungs by others was done by monolayer seeding in thin acellular sections [18, 20] or by nonspecifically injecting cells into thicker acellular tissue segments [19]. However, this can likely result in random attachment and subsequent growth of cells in irrelevant compartments of the lung. Inoculating cells into either blood vessels or airways of a three-dimensional segment of excised decellularized human lung tissue permits the study of recellularization events in a more biologically relevant manner.
The initial day 1 survival and distribution pattern of cell types inoculated through the airways was similar in both normal and emphysematous decellularized lungs. As expected, cells could be found lining airways and parenchymal spaces. Comparably, CBFs demonstrated similar initial (D1) inoculation patterns in both normal and emphysematous human lungs with the majority of cells engrafting in large/small blood vessels and distal capillary beds. However, cells seeded into acellular normal tissue appeared initially healthier and survived longer compared to seeding into acellular emphysematous scaffolds. Collectively, the early seeding data indicates that both scaffolds are likely not cytotoxic and have the necessary cues to support initial binding and proliferation. However, acellular emphysematous lungs were unable to support long term survival as compared to acellular normal human lungs.
These observations match those of our previous work with mice in which both mouse bone marrow-derived mesenchymal stromal cells (mMSCs) and an immortalized type 2 alveolar epithelial cell line (C10) remained viable for less time in acellular lung scaffolds derived from an elastase induced model of emphysema as compared to those derived from normal lungs [13]. The biological underpinnings for these observations in both human and mouse models remains unclear. However, this suggests that much like in the clinical scenario of emphysema, whereby distal lung tissue fails to regenerate, the extracellular matrix from an emphysematous patient may not be capable of supporting ex vivo regeneration without modification.
Interestingly, in direct contrast to cell seedings in the 3-dimensional acellular scaffolds, when acellular scaffolds from either group was solubilized and coated on tissue culture wells, none of the cell types demonstrated enhanced cell death or inhibition of proliferation. This indicates that the ECM proteins present in acellular emphysematous scaffolds are capable of supporting cell proliferation, and that the 3-dimensional micro or macroarchitecture assembly of ECM proteins in the intact scaffolds may be defective in emphysematous lungs. This is a significant finding and one which will be imperative to explore in future work to examine matrix effects of normal versus emphysematous lung ECM on cell behavior.
Conclusions
Our data suggests that emphysematous lungs support initial engraftment and proliferation of a variety of human cell lines, but for reasons presently unknown, do not support prolonged viability. However, COPD, including emphysema, is a heterogenous collection of disorders and more extensive exploration of these findings need to be performed in a wider range of diseased human lungs. Further, other acute pulmonary pathologies at the time of lung harvest from autopsy may also conceivably impact de- and recellularization of the acellular scaffolds. This work highlights that a number of unanswered questions remain concerning the criteria of lung procurement for decellularization and that optimal conditions for recellularization and eventual clinical implantation remain unclear. Therefore, further evaluation of the properties of normal and diseased lungs is needed to assess the potential usefulness of these lungs as scaffolds for recellularization and eventual clinical application. These results also provide valuable information for further study of cell–matrix interactions in normal and diseased lungs.
Supplementary Material
Table S1. Total proteins identified by mass spectrometry
Values represent p-values from two group comparisons of raw unique peptide hits using the non-parametric test. Protein group assignments were established from UniProt entries of positively identified proteins (those containing two or more unique peptide hits. P-values less than 0.05 are considered significant.
Representative sections demonstrate minimal background fluorescence with use of either rabbit or mouse secondary antibodies. Original magnification 200x
Representative H and E photomicrographs depicting characteristic recellularization patterns three days post-inoculation of HLFs. Despite the initial seeding data which indicated lesser immediate adherence rates of HLFs, those cells persisting on day three appeared to be viable and adherent to the acellular normal scaffolds. Original magnification 100x.
Acknowledgments
The authors would like to thank the autopsy staff at Fletcher Allen Hospital, particularly Dr. Nick Hardin, Dr. Barbara Waters from the UVM Department of Pathology for technical assistance in methodologic development for identifying and securing small airways and blood vessels for cell inoculations, Dr. Rachael Oldinski from UVM College of Engineering for assistance with the calcium alginate coating, Dr. Mervin Yoder from Indiana University – Purdue University Indianapolis for supplying the endothelial progenitor cells, Jessica DeJong for assistance with figure file compressions, and Drs. Andrew Hoffman and Joseph Platz for critical comments on the manuscript. Studies were supported by NIH ARRA RC4HL106625 (DJW), NHLBI R21HL094611 (DJW), the UVM Lung Biology Training grant T32 HL076122 from the NHLBI, and UVM Environmental Pathology Training grant T32 ES007122 from the NIEHS. Facilities and equipment were supported by the UVM Lung Biology COBRE (NIH NCRR P20 RR-155557) and the Vermont Cancer Center DNA Analysis facility (NIH P30 CA22435). The Vermont Genetics Network Proteomics Facility is supported through NIH grant 8P20GM103449 from the INBRE Program of the National Institute of General Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orens JB, Garrity ER., Jr General overview of lung transplantation and review of organ allocation. Proc Am Thorac Soc. 2009;6:13–9. doi: 10.1513/pats.200807-072GO. [DOI] [PubMed] [Google Scholar]
- 2.Panoskaltsis-Mortari A, Weiss DJ. Breathing new life into lung transplantation therapy. Mol Ther. 2010;18:1581–3. doi: 10.1038/mt.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price AP, England KA, Matson AM, Blazar BR, Panoskaltsis-Mortari A. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581–91. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortiella J, Niles J, Cantu A, Brettler A, Pham A, Vargas G, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010;16:2565–80. doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 5.Petersen TH, Calle EA, Zhao L, Lee EJ, Gui L, Raredon MB, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–33. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 7.Song JJ, Kim SS, Liu Z, Madsen JC, Mathisen DJ, Vacanti JP, et al. Enhanced in vivo function of bioartificial lungs in rats. Ann Thorac Surg. 2011;92:998–1006. doi: 10.1016/j.athoracsur.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 8.Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA, et al. Initial binding and recellularization of decellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tissue Eng Part A. 2012;18:1–16. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, Allen GB, et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng Part C Methods. 2012;18:420–32. doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen T, Roszell B, Zang F, Girard E, Matson A, Thrall R, et al. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng Part C Methods. 2012;18:632–46. doi: 10.1089/ten.tec.2011.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, et al. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A. 2012;18:2437–52. doi: 10.1089/ten.tea.2011.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10:398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokocevic D, Bonenfant NR, Wagner DE, Borg ZD, Lathrop MJ, Lam YW, et al. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials. 2013;34:3256–69. doi: 10.1016/j.biomaterials.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petersen TH, Calle EA, Colehour MB, Niklason LE. Matrix composition and mechanics of decellularized lung scaffolds. Cells Tissues Organs. 2012;195:222–31. doi: 10.1159/000324896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonenfant NR, Sokocevic D, Wagner DE, Borg ZD, Lathrop MJ, Lam YW, et al. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials. 2013;34:3231–45. doi: 10.1016/j.biomaterials.2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379:943–52. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 17.Wagner DE, Bonvillain RW, Jensen T, Girard ED, Bunnell BA, Finck CM, et al. Can stem cells be used to generate new lungs? Ex vivo lung bioengineering with decellularized whole lung scaffolds. Respirology. 2013;18:895–911. doi: 10.1111/resp.12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–76. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichols JE, Niles J, Riddle M, Vargas G, Schilagard T, Ma L, et al. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng Part A. 2013;19:2045–62. doi: 10.1089/ten.tea.2012.0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neill JD, Anfang R, Anandappa A, Costa J, Javidfar J, Wobma HM, et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann Thorac Surg. 2013;96:1046–56. doi: 10.1016/j.athoracsur.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilpin SE, Guyette JP, Gonzalez G, Ren X, Asara JM, Mathisen DJ, et al. Perfusion Decellularization of Human and Porcine Lungs: Bringing the Matrix to Clinical Scale. The Journal of Heart and Lung Transplantation. doi: 10.1016/j.healun.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 22.Wagner DE, Bonenfant NR, Sokocevic D, Desarno MJ, Borg ZD, Parsons C, et al. Three-dimensional scaffolds of acellular human and porcine lungs for high throughput studies of lung disease and regeneration. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.11.078. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–58. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 24.Yankaskas JR, Haizlip JE, Conrad M, Koval D, Lazarowski E, Paradiso AM, et al. Papilloma virus immortalized tracheal epithelial cells retain a well-differentiated phenotype. Am J Physiol. 1993;264:C1219–30. doi: 10.1152/ajpcell.1993.264.5.C1219. [DOI] [PubMed] [Google Scholar]
- 25.Sekiya I, Larson BL, Smith JR, Pochampally R, Cui JG, Prockop DJ. Expansion of human adult stem cells from bone marrow stroma: conditions that maximize the yields of early progenitors and evaluate their quality. Stem Cells. 2002;20:530–41. doi: 10.1634/stemcells.20-6-530. [DOI] [PubMed] [Google Scholar]
- 26.Zar JH. Biostatistical analysis. Englewood Cliffs, N.J: Prentice-Hall; 1974. [Google Scholar]
- 27.de Castro Bras LE, Ramirez TA, DeLeon-Pennell KY, Chiao YA, Ma Y, Dai Q, et al. Texas 3-step decellularization protocol: looking at the cardiac extracellular matrix. J Proteomics. 2013;86:43–52. doi: 10.1016/j.jprot.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Total proteins identified by mass spectrometry
Values represent p-values from two group comparisons of raw unique peptide hits using the non-parametric test. Protein group assignments were established from UniProt entries of positively identified proteins (those containing two or more unique peptide hits. P-values less than 0.05 are considered significant.
Representative sections demonstrate minimal background fluorescence with use of either rabbit or mouse secondary antibodies. Original magnification 200x
Representative H and E photomicrographs depicting characteristic recellularization patterns three days post-inoculation of HLFs. Despite the initial seeding data which indicated lesser immediate adherence rates of HLFs, those cells persisting on day three appeared to be viable and adherent to the acellular normal scaffolds. Original magnification 100x.