Abstract
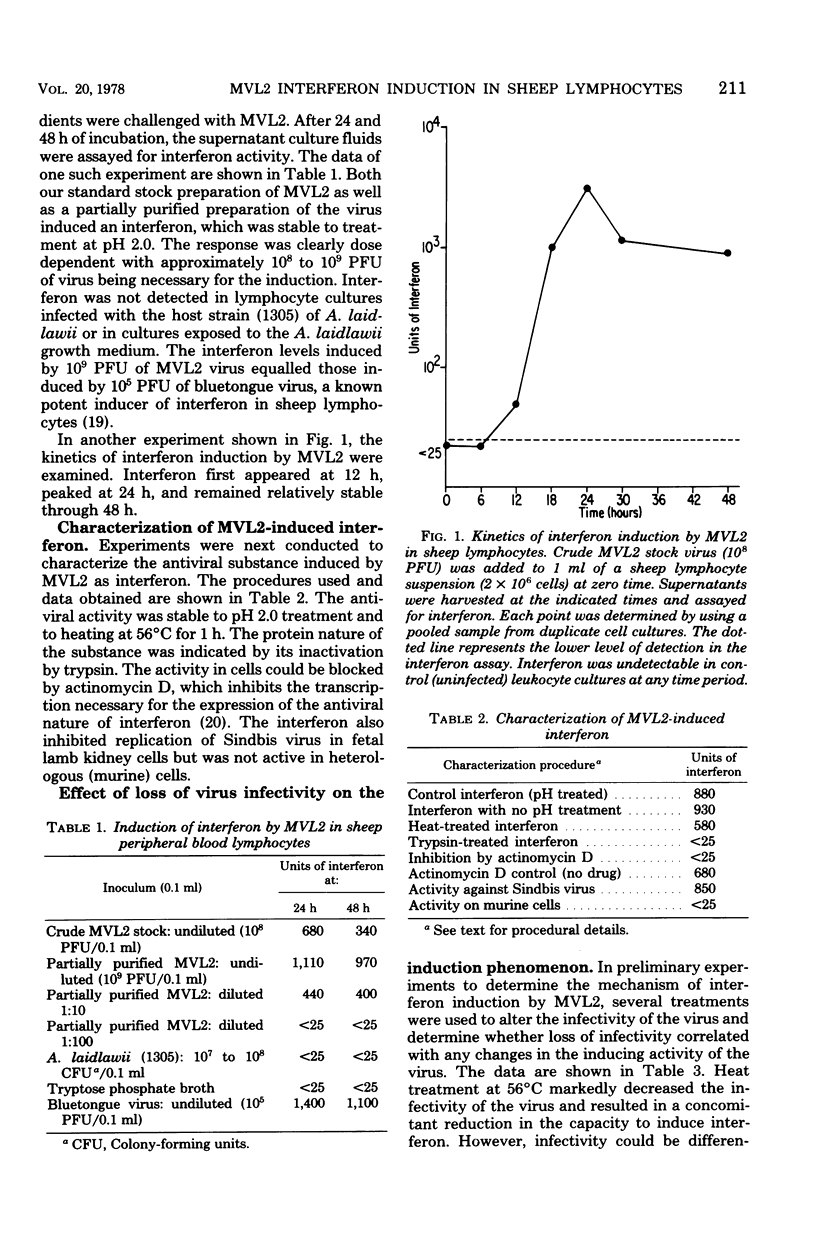
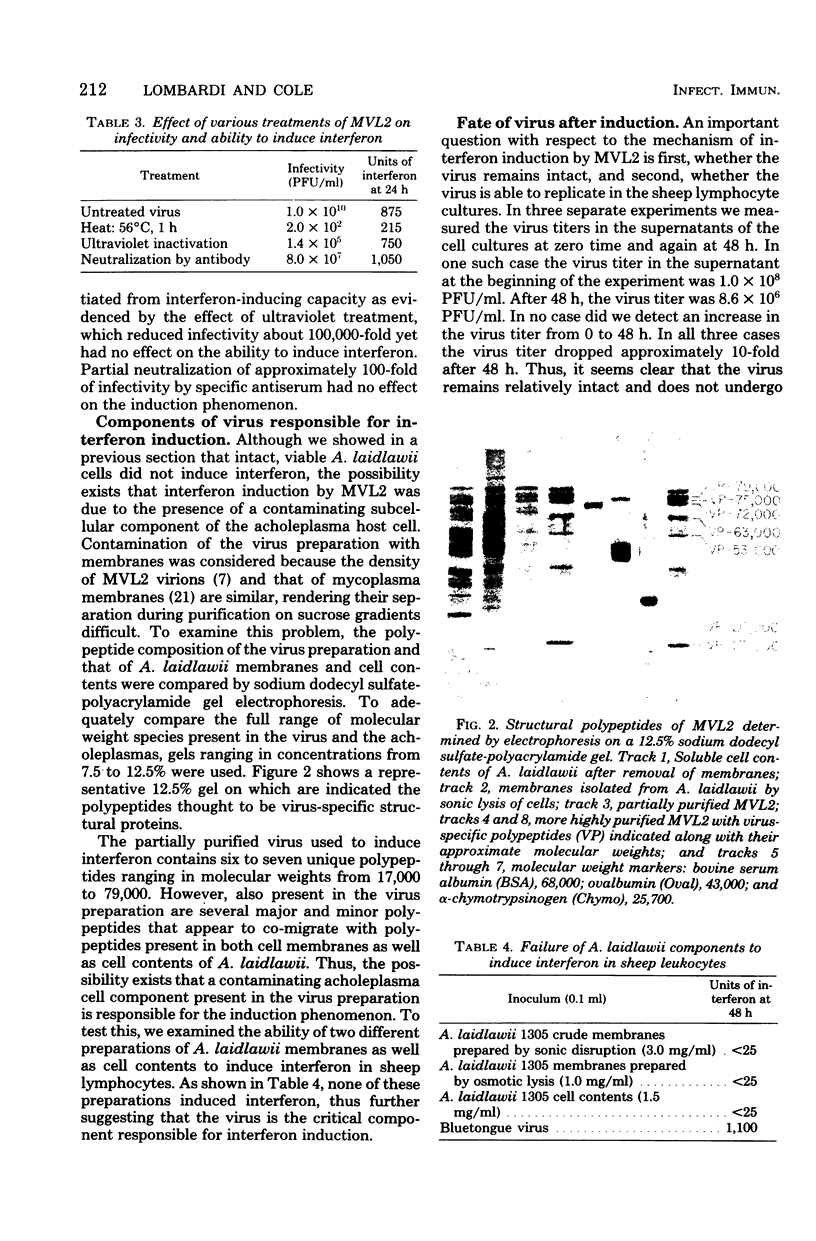
The data presented in this report show that the enveloped Mycoplasmatales virus MVL2 is capable of interferon induction in sheep peripheral blood lymphocytes. The phenomenon was dose dependent, requiring approximately 10(8) plaque-forming units of virus per 2 X 10(6) lymphocytes. The interferon was stable to pH 2.0 treatment, was produced in moderately high levels (greater than 1,000 units), and met many of the criteria for classification as a type I interferon. Heat-inactivated MLV2 lost its ability to induce interferon, whereas ultraviolet-inactivated virus retained its capacity to induce levels comparable to untreated virus. Whereas the MVL2 used in these studies was contaminated with several host cell proteins as determined by sodium dodecyl sulfate-containing polyacrylamide gel electrophoresis, the contaminants probably did not play a role in the induction because isolated cell membranes or soluble cell contents from Achoplasma laidlawii are inactive as inducers. Also presented in this report is a preliminary description of the structural polypeptides of MVL2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks G. T., Buck K. W., Chain E. B., Darbyshire J. E., Himmelweit F., Ratti G., Sharpe T. J., Planterose D. N. Antiviral activity of double stranded RNA from a virus isolated from Aspergillus foetidus. Nature. 1970 Aug 1;227(5257):505–507. doi: 10.1038/227505a0. [DOI] [PubMed] [Google Scholar]
- Cole B. C., Overall J. C., Jr, Lombardi P. S., Glasgow L. A. Induction of interferon in ovine and human lymphocyte cultures by mycoplasmas. Infect Immun. 1976 Jul;14(1):88–94. doi: 10.1128/iai.14.1.88-94.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. K., Lampson G. P., Tytell A. A., Nemes M. M., Hilleman M. R. Inducers of interferon and host resistance, IV. Double-stranded replicative form RNA (MS2-Ff-RNA) from E. coli infected with MS2 coliphage. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2102–2108. doi: 10.1073/pnas.58.5.2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwes D. J., Pike B. V., Wyld S. G., Pocock D. H., Gourlay R. N. Characterization of Mycoplasmatales virus-laidlawii 3. J Gen Virol. 1975 Oct;29(1):11–24. doi: 10.1099/0022-1317-29-1-11. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N., Garwes D. J., Bruce J., Wyld S. G. Further studies on the morphology and composition of Mycoplasmatales virus-laidlawii 2. J Gen Virol. 1973 Feb;18(2):127–133. doi: 10.1099/0022-1317-18-2-127. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N. Isolation of a virus infecting a strain of Mycoplasma laidlawii. Nature. 1970 Mar 21;225(5238):1165–1165. doi: 10.1038/2251165a0. [DOI] [PubMed] [Google Scholar]
- Gourlay R. N. Mycoplasmatales virus-laidlawii 2, a new virus isolated from Acholeplasma laidlawii. J Gen Virol. 1971 Jul;12(1):65–67. doi: 10.1099/0022-1317-12-1-65. [DOI] [PubMed] [Google Scholar]
- Ho M., Armstrong J. A. Interferon. Annu Rev Microbiol. 1975;29:131–161. doi: 10.1146/annurev.mi.29.100175.001023. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt W. J., Douthart R. J., Murphy E. B. Interferon production by T4 coliphage. Nature. 1970 Oct 3;228(5266):27–30. doi: 10.1038/228027a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lampson G. P., Tytell A. A., Field A. K., Nemes M. M., Hilleman M. R. Inducers of interferon and host resistance. I. Double-stranded RNA from extracts of Penicillium funiculosum. Proc Natl Acad Sci U S A. 1967 Aug;58(2):782–789. doi: 10.1073/pnas.58.2.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Isolation of Mycoplasmatales viruses and characterization of MVL1, MVL52, and MVG51. Science. 1971 Aug 20;173(3998):725–727. doi: 10.1126/science.173.3998.725. [DOI] [PubMed] [Google Scholar]
- Lovinger G. G., Ling H. P., Gilden R. V., Hatanaka M. Effect of UV Light on RNA-Directed DNA Polymerase Activity of Murine Oncornaviruses. J Virol. 1975 May;15(5):1273–1275. doi: 10.1128/jvi.15.5.1273-1275.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., Plagemann P. G. Effect of ultraviolet light on mengovirus: formation of uracil dimers, instability and degradation of capsid, and covalent linkage of protein to viral RNA. J Virol. 1974 Mar;13(3):729–739. doi: 10.1128/jvi.13.3.729-739.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M., Huang W. M. The identification of prereplicative bacteriophage T4 proteins. J Biol Chem. 1973 Aug 10;248(15):5499–5501. [PubMed] [Google Scholar]
- RAZIN S., ARGAMAN M., AVIGAN J. CHEMICAL COMPOSITION OF MYCOPLASMA CELLS AND MEMBRANES. J Gen Microbiol. 1963 Dec;33:477–487. doi: 10.1099/00221287-33-3-477. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Cole B. C., Overall J. C., Jr, Glasgow L. A. Induction of interferon in mice by mycoplasmas. Infect Immun. 1974 Dec;10(6):1296–1301. doi: 10.1128/iai.10.6.1296-1301.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Overall J. C., Jr, Cole B. C., Glasgow L. A. Mycoplasma-associated induction of interferon in ovine leukocytes. Infect Immun. 1973 Nov;8(5):796–803. doi: 10.1128/iai.8.5.796-803.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovell D., Cantell K. Kinetics of interferon production in human leukocyte suspensions. J Gen Virol. 1971 Dec;13(3):485–489. doi: 10.1099/0022-1317-13-3-485. [DOI] [PubMed] [Google Scholar]