Abstract
Summary
This study investigated the influence of ovarian hormone deficiency on core circadian regulatory protein (CCRP) in the context of bone loss. Our data suggest that ovarian hormone deficiency disrupts diurnal rhythmicity and CCRP expression in bone. Further studies should determine if chronobiology provides a novel therapeutic target for osteoporosis intervention.
Introduction
CCRP synchronize metabolic activities and display an oscillatory expression profile in murine bone. In vitro studies using bone marrow mesenchymal stromal/stem cells have demonstrated that the CCRP is present and can be regulated within osteoblast progenitors. In vivo studies have shown that the CCRP regulates bone mass via leptin/neuroendocrine pathways. The current study used an ovariectomized murine model to test the hypothesis that ovarian hormone deficiency is associated with either an attenuation and/or temporal phase shift of the CCRP oscillatory expression in bone and that these changes are correlated with the onset of osteoporosis.
Methods
Sham-operated controls and ovariectomized female C57BL/6 mice were euthanized at 4-h intervals 2 weeks post-operatively.
Results
Ovariectomy attenuated the oscillatory expression of CCRP mRNAs in the femur and vertebra relative to the controls and reduced the wheel-running activity profile.
Conclusion
Ovarian hormone deficiency modulates the expression profile of the CCRP with potential impact on bone marrow mesenchymal stem cell lineage commitment.
Keywords: Diurnal, Estrogen, Mesenchymal stem cell, Osteoporosis
Introduction
The body’s circadian clock requires the basic helix loop helix–Periodic/ARNT/Sim domain family [1]. Members of this superfamily, Clock and Brain Muscle Arnt-Like 1 (Bmal1), encode positive transcription factors binding to cis-acting E-box elements within the promoters of the downstream targets cryptochrome (CRY) and period (PER), which act as feedback negative regulators [1]. CRY and PER form heterodimers and their expression levels display a 24-h oscillation [1]. Additional downstream targets include the repressors E4BP4 and nuclear hormone receptors Rev-erb α and retinoic acid receptor related orphan receptor (ROR) and the activator albumin D-box binding protein (DBP) [1]. Identified collectively as the CCRP [2], these genes are expressed in peripheral tissues [3] where their diurnal rhythm can be desynchronized relative to the brain's central clock in response to photic stimuli, feeding schedule, and systemic factors (e.g., nuclear hormone receptor ligands) [1].
Studies show that CCRP genes contribute to bone biology. Mutations in ROR cause abnormal circadian rhythms and skeletal defects [4], and circadian oscillation of PER 2 [5] and multiple CCRP genes [6] have been reported in murine femora. Mice deficient in PER or CRY display increased bone mass [6]. Transcriptomic analyses of murine calvaria determined that ~20% of expressed mRNAs oscillated in a diurnal manner [7]. Mice transgenic for an osteocalcin promoter driven luciferase reporter displayed a diurnal luciferase expression profile in skeletal tissues [8]. Oscillatory expression of representative CCRP genes can be synchronized following dexamethasone exposure in isolated bone marrow-derived mesenchymal stem cells (BMSC) [2]. E4BP4 has been identified in osteoblasts as a parathyroid hormone inducible transcriptional repressor [9] while Rev-erb α, a heme-binding nuclear hormone receptor, plays a regulatory role in directing adipogenesis [10].
An imbalance between BMSC-driven osteogenesis and adipogenesis in favor of adipogenesis contributes to bone loss in osteoporosis [11]. The action of nuclear hormone receptors (androgen, estrogen, glucocorticoid, PPARγ) and their ligands have been linked to bone loss and the pathophysiology of osteoporosis [11]. Furthermore, nuclear hormone receptors, ligands, and co-activators modulate circadian rhythms in murine models [1, 4, 12]. The current study comparing sham-operated and ovariectomized mice indicates that ovarian hormone deficiency desynchronizes CCRP genes in the context of bone loss.
Methods
Animal studies
Under the Institutional Animal Care and Use Committee's approved protocols, sham-operated and ovariectomized (OVX) female 8-week-old C57BL/6 mice purchased from the Charles River Laboratory (Wilmington, MA) (June, 2008) were maintained for 12 days under a constant 12-h light:12-h dark cycle with ad lib Purina 5015 chow and water access. Groups of control or OVX animals (n=3) were euthanized and harvested at 4-h intervals [13]. Each uterus was dissected and weighed and the L3–4 vertebra harvested for RNA isolation. The tibia and the L5 vertebra from n=6 mice were dissected, fixed, and stored in 70% ethanol for µCT analyses.
Physical activity
To determine if OVX altered physical activity patterns consistent with disturbed circadian mechanisms [14], wheel running was performed and analyzed in sham-operated and OVX mice (n=3) under 12 h LD or constant dark conditions from 2 to 6 weeks post ovariectomy [15].
Semi-quantitative real-time RT-PCR
Total RNA from skeletal tissues (femur, vertebra n=3 animals/time point) was analyzed by RT-PCR using an approach that has been validated in multiple prior studies in murine adipose and bone depots and isolated stem cells [7, 13, 16–18]. Results for each CCRP mRNA were normalized relative to the mean expression of four separate control genes (18S RNA, β-actin, β2-microglobulin, and cyclophilin B) at each corresponding time point. The PCR primers can be found in Supplement Table 1.
Trabecular and cortical bone microarchitecture using µCT
X-ray microcomputed tomography was used to evaluate bone microarchitecture of the proximal tibial metaphysis, tibial mid-diaphysis and fifth lumbar vertebra [19]. All scans were performed at 2,048 × 2,048 pixels or at a higher resolution using an integration time of 165 ms per projection on a µCT40 scanner (SCANCO Medical, Switzerland).
Biomechanical testing of vertebra with finite element analysis (FEA)
Trabecular biomechanical parameters were evaluated from µCT images using an FEA software (SCANCO Medical, Switzerland). A micromechanical finite element model was constructed by converting bone voxels from the VOI into 8-node brick elements [20]. Compression testing was simulated on the proximal tibia metaphyseal region and the vertebral body
Statistics
Analyses used the Student's t-test where p<0.05 defined statistical significance. Uterine weights were reported as mean±standard deviation and other parameters as mean±standard error. Cosinor analyses used the Time Series Analysis-Single Cosinor v. 6.0 software (Expert Soft Technologie, Richelieu, France) to determine the period length and acrophase or zenith of expression for each CCRP gene over the 24-h period [21].
Results
Uterine weight analyses
To confirm that ovariectomy resulted in ovarian hormone deficiency after 14 days, the uteri of sham operated and OVX cohorts (n=18) were weighed. The mean sham operated uterine weight (0.75±0.043 g) was significantly (p=0.00007) greater than that of the OVX cohort (0.020±0.013 g).
Physical activity measures
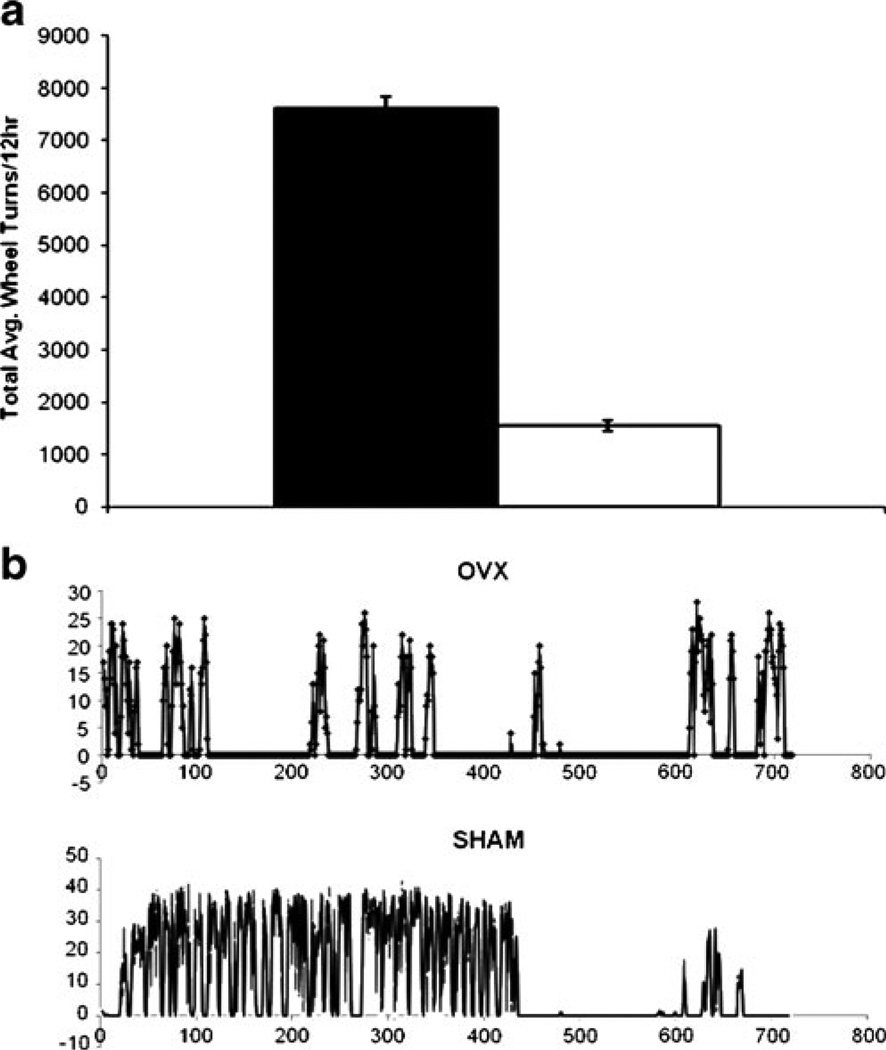
Physical activity provides a well-established physiological measure of circadian rhythms in rodent models. Furthermore, ovariectomy in rodents has been found to reduce physical activity as measured by wheel running [14]. Consistent with these observations, under conditions of 12-h light:12-h darkness, mice in the sham operated cohort displayed a robust periodic wheel running activity over the 24-h period that exceeded that of the OVX cohort by 4.95-fold (n=3 animals, mean of n=6 consecutive days) (Fig. 1a). In both cohorts, the majority of the wheel-running activity occurred immediately prior to and during the lights-off period (Fig. 1b); minimal wheel running activity occurred during the lights-on period.
Fig. 1.
Lights off wheel running in OVX mice, is severely attenuated compared to sham-operated mice. a Averaged wheel turns for the nocturnal bout (ZT, 12–24) of activity were compiled over six consecutive nights (n=3/group, OVX, and sham-operated controls). Significant differences (asterisk) between OVX and sham mice were observed (p<0.00000005, Two 2-tailed Student's t-test). b Representative nocturnal wheel-running activity profile for OVX and sham-operated controls. The OVX mice display a severe attenuation of nocturnal wheel running throughout the 12-h lights-off period relative to sham-operated controls. The X-axis displays the time from 0 min, corresponding to ZT 12 to 720 min, corresponding to ZT 24
µCT analyses and biomechanical testing
By 14 days of post surgery, the OVX cohort experienced a 17.8% and 14.4% decrease (p<0.05) in trabecular bone volume at the proximal tibial metaphysis and vertebral body, respectively. The trabecular thickness (Tb.Th) in the tibial metaphysis was significantly reduced (Sham=33.3±0.4 µm vs OVX=30.6±0.4 0 µm; p<0.01), but the alterations in Tb.Th in the vertebra and trabecular number at both skeletal sites did not reach statistical significance (p < 0.10). No significant changes in trabecular separation (Tb. Sp) occurred at either site. Loss of trabecular bone in the vertebral and tibial metaphyseal regions in the OVX animals was characterized by a decrease in the density (tibia, Sham= 853.8±4.8 mg/cm3 vs OVX=838.9±4.2 mg/cm3; vertebra, Sham=831.5±4.6 mg/cm3 vs OVX=820.8±2.1 mg/cm3; p< 0.05) and a higher mean structure model index (tibia, Sham= 2.04±0.09 vs OVX=2.35+0.03; vertebra, Sham=0.99+0.09 vs OVX=1.25+0.05; p<0.05), indicating that the trabecular bone microarchitecture has been compromised to a more rod-like structure. No alterations in cortical bone parameters at the tibial midshaft were observed (data not show). The OVX-induced alterations in the trabecular microstructure at the proximal tibial metaphysis, but not in the vertebral body, produced a 52% decrease in total compressive force and stiffness.
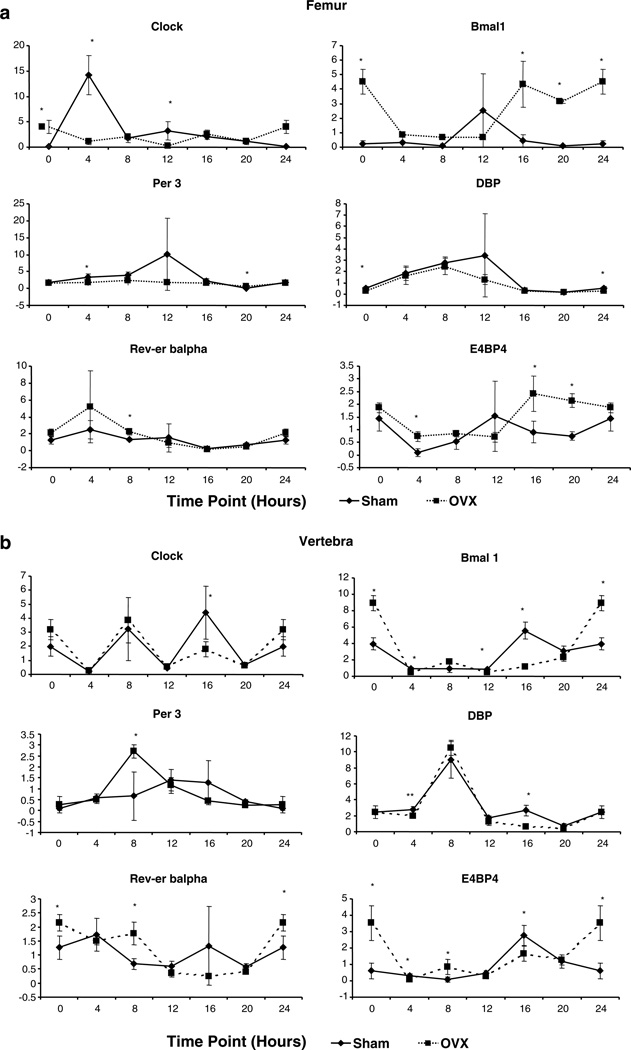
CCRP gene expression profiles in the femora and lumbar vertebra
The expression profile of the genes encoding the CCRP (Fig. 2) was monitored over time in total RNA isolated from the marrow-flushed femora and the marrow-containing lumbar vertebra of the sham-operated and OVX mice. The genes encoding the positive arm of the CCRP (Clock, Bmal1) in the femur of sham-operated mice displayed acrophase at ~16:00 and 18:30, respectively, and were ~4–7 h out of phase relative to genes encoding the negative arm of the CCRP (Cry2, Per 3) (~11:00) (Fig. 2a). Downstream targets of the CCRP, including DBP, Rev-erb α, and E4PB4 displayed oscillatory expression profiles. In all cases except Per 3, the CCRP gene expression profiles in the OVX mice were attenuated relative to the controls. Furthermore, the acrophase for several genes (Bmal1, Clock, E4BP4) was phase delayed in the OVX cohort while the other genes (Cry2, DBP, Rev-erb α) were phase advanced. The absolute phase shift following OVX across all CCRP genes in the femur was 5.4±3.0 h. The CCRP profile in the lumbar vertebra of the sham-operated mice was distinct from that of the femur; the timing of the acrophase for individual genes differed and, in the case of the control Clock, Cosinor analysis did not reveal a statistically significant distinct circadian oscillatory pattern (Fig. 2b). However, like the femur, the expression levels of the CCRP genes in the OVX vertebra were attenuated relative to the controls and displayed a phase delay for Per 3 of ~3 h and a phase advance of ~1–4 h for Bmal1 and DBP. It is possible that hematopoietic cells present in the vertebra as compared to the marrow-flushed femur may contribute to differences observed in the CCRP gene expression profiles in these two bone depots.
Fig. 2.
Diurnal analysis of CCRP expression in the a femur and b lumbar vertebra of sham-operated (square) and OVX (diamond) mice was determined by RT-PCR using total RNA (n=3 mice/4-h time point). Positive (Clock, Bmal1), negative (Per 3), and immediate downstream targets (DBP, E4BP4, Rev-erb α) were normalized relative to the housekeeping controls 18S RNA, β-actin, β2-microglobulin, and cyclophilin B. Values displayed are the mean±SD
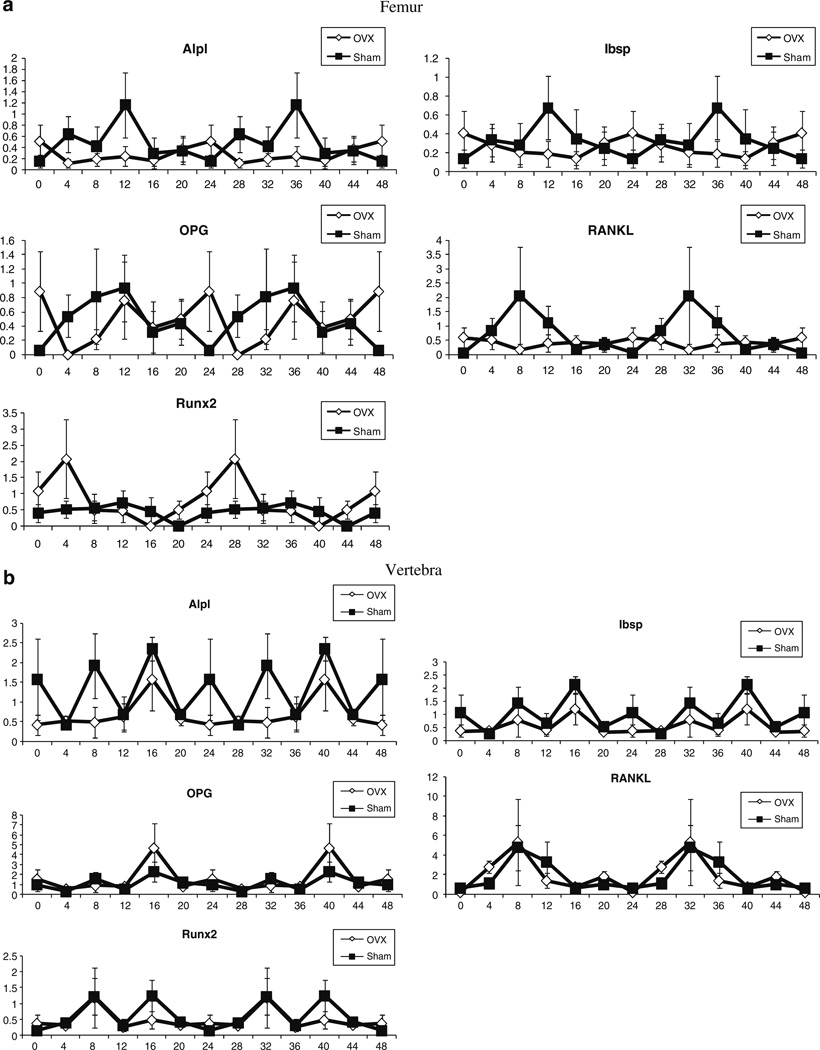
Osteoblast biomarker gene expression profiles in the femora and lumbar vertebra
The circadian expression profile of genes encoding the osteoblast biomarkers alkaline phosphatase (Alpl), bone siaoloprotein (Ibsp), osteoprotegerin (OPG), receptor activator of NF-κB ligand (RANKL), and Runx2 were monitored in marrow-flushed femora (Fig. 3a) and marrow-containing lumbar vertebral bodies (Fig. 3b) of sham and OVX mice. In the femur of sham operated mice, all osteoblast biomarkers displayed a dynamic circadian oscillation. With the exception of Runx2, the expression profile of the osteoblast biomarkers was attenuated in the OVX femora relative to the sham operated controls. In contrast, the majority of osteoblast biomarkers in the lumbar vertebra did not display as robust a circadian amplitude oscillation in either the sham and/or OVX cohorts; the exceptions were OPG and RANKL (Fig. 3b).
Fig. 3.
Diurnal analysis of osteoblast-associated mRNA expression. The expression profile of mRNA encoding the osteoblast-associated genes in the a femur and b lumbar vertebra of sham-operated (square) and OVX (diamond) mice was determined by RT-PCR using total RNA pooled from n=3 mice per time point (ZT h). The mRNA levels of Alpl, Ibsp, OPG, RANKL, and Runx2 were normalized at the individual time points relative to the housekeeping controls 18S RNA, β-actin, β2-microglobulin, and cyclophilin B. Values displayed are the mean±SD
Discussion
The current data shows that mice with ovarian hormone deficiency disrupted diurnal rhythmicity and CCRP expression in bone. This suggests that estrogen and nuclear hormone receptors interact with the CCRP and circadian mechanisms to alter BMSC differentiation. There is reason to postulate that CCRP genes contribute to BMSC lineage commitment [2] and the putative inverse relationship between the adipocyte and osteoblast lineages [11] since adipogenesis is associated with increased expression of Bmal1 and Rev-erb α mRNAs [22]. Consistent with this observation, embryonic fibroblasts isolated from Bmal1−/− mice fail to form adipocytes unless rescued by Bmal1-expressing adenoviral transduction [23]. In 3T3-L1 pre-adipocytes, down regulation of Bmal1 inhibited adipogenesis [23]. While this growing body of literature suggests that circadian mechanisms regulate BMSC differentiation, further studies will be necessary to determine if chronobiology can serve as a novel clinical mechanism and target for osteoporosis therapeutic interventions.
Supplementary Material
Acknowledgements
The authors thank Laura Dallam, Susan Newman, Barry Robert DVM, PhD, and the Genomics and Comparative Biology Cores at PBRC and financial support from the Pennington Biomedical Research Foundation (J.M.G., X.W.) and the Clinical Nutrition Research Unit Center Grant #1P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions” sponsored by NIDDK (A.A. B., J.M.G., G.Y.).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00198-010-1325-z) contains supplementary material, which is available to authorized users.
Presented at the ASBMR New Frontiers in Skeletal Research: Bone, Fat, and Brain Connections on April 27–28, 2009 in Bethesda, MD.
Conflicts of interest None.
Contributor Information
B. J. Smith, Email: bjsmith@okstate.edu, Department of Nutritional Sciences, Oklahoma State University, 423 HES, Stillwater, OK 74078, USA.
G. M. Sutton, Neuropeptides Laboratory, Baton Rouge, LA, USA
X. Wu, Stem Cell Biology Laboratory, Baton Rouge, LA, USA
G. Yu, Stem Cell Biology Laboratory, Baton Rouge, LA, USA Clinical Nutrition Research Unit, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
B. C. Goh, Stem Cell Biology Laboratory, Baton Rouge, LA, USA
T. Hebert, Stem Cell Biology Laboratory, Baton Rouge, LA, USA
G. Pelled, Skeletal Biotechnology Laboratory, Hebrew University–Hadassah Medical Campus, Jerusalem, Israel Department of Surgery, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
Z. Gazit, Skeletal Biotechnology Laboratory, Hebrew University–Hadassah Medical Campus, Jerusalem, Israel Department of Surgery, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
D. Gazit, Skeletal Biotechnology Laboratory, Hebrew University–Hadassah Medical Campus, Jerusalem, Israel Department of Surgery, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
A. A. Butler, Neuropeptides Laboratory, Baton Rouge, LA, USA Clinical Nutrition Research Unit, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
J. M. Gimble, Stem Cell Biology Laboratory, Baton Rouge, LA, USA Clinical Nutrition Research Unit, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
References
- 1.Lowrey PL, Takahashi JS. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genomics Hum Genet. 2004;5:407–441. doi: 10.1146/annurev.genom.5.061903.175925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gimble JM, Floyd ZE, Bunnell BA. The 4th dimension and adult stem cells: can timing be everything? J Cell Biochem. 2009;107:569–578. doi: 10.1002/jcb.22153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jetten AM, Kurebayashi S, Ueda E. The ROR nuclear orphan receptor subfamily: critical regulators of multiple biological processes. Prog Nucleic Acid Res Mol Biol. 2001;69:205–247. doi: 10.1016/s0079-6603(01)69048-2. [DOI] [PubMed] [Google Scholar]
- 5.Horard B, Rayet B, Triqueneaux G, Laudet V, Delaunay F, Vanacker JM. Expression of the orphan nuclear receptor ERRalpha is under circadian regulation in estrogen-responsive tissues. J Mol Endocrinol. 2004;33:87–97. doi: 10.1677/jme.0.0330087. [DOI] [PubMed] [Google Scholar]
- 6.Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 7.Zvonic S, Ptitsyn AA, Kilroy G, Wu X, Conrad SA, Scott LK, Guilak F, Pelled G, Gazit D, Gimble JM. Circadian oscillation of gene expression in murine calvarial bone. J Bone Miner Res. 2007;22:357–365. doi: 10.1359/jbmr.061114. [DOI] [PubMed] [Google Scholar]
- 8.PA GY, Zilberman Y, Pelled G, Gimble JM, Gazit D. Circadian regulation of the osteocalcin promoter: imaging based studies in a transgenic mouse model. J Dent Res. 2009;88:45–50. doi: 10.1177/0022034508328012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozkurt IC, Tetradis S. Parathyroid hormone-induced E4BP4/NFIL3 down-regulates transcription in osteoblasts. J Biol Chem. 2003;278:26803–26809. doi: 10.1074/jbc.M212652200. [DOI] [PubMed] [Google Scholar]
- 10.Burris TP. Nuclear hormone receptors for heme: REV-ERBalpha and REV-ERBbeta are ligand-regulated components of the mammalian clock. Mol Endocrinol. 2008;22:1509–1520. doi: 10.1210/me.2007-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME. Playing with bone and fat. J Cell Biochem. 2006;98:251–266. doi: 10.1002/jcb.20777. [DOI] [PubMed] [Google Scholar]
- 12.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell. 2008;134:728–742. doi: 10.1016/j.cell.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zvonic S, Ptitsyn AA, Conrad SA, Scott LK, Floyd ZE, Kilroy G, Wu X, Goh BC, Mynatt RL, Gimble JM. Characterization of peripheral circadian clocks in adipose tissues. Diabetes. 2006;55:962–970. doi: 10.2337/diabetes.55.04.06.db05-0873. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz de Elvira MC, Persaud R, Coen CW. Use of running wheels regulates the effects of the ovaries on circadian rhythms. Physiol Behav. 1992;52:277–284. doi: 10.1016/0031-9384(92)90271-3. [DOI] [PubMed] [Google Scholar]
- 15.Sutton GM, Perez-Tilve D, Nogueiras R, Fang J, Kim JK, Cone RD, Gimble JM, Tschop MH, Butler AA. The melanocortin-3 receptor is required for entrainment to meal intake. J Neurosci. 2008;28:12946–12955. doi: 10.1523/JNEUROSCI.3615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.XWX GBC, Evans AE, AE ML, Johnson ML, Molly R, GJ HMR. Food entrainment of circadian gene expression altered in PPARα−/− brown fat and heart. Biochem Biophys Res Commun. 2007;360:828–833. doi: 10.1016/j.bbrc.2007.06.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Yu G, Parks H, Hebert T, Goh BC, Dietrich MA, Pelled G, Izadpanah R, Gazit D, Bunnell BA, Gimble JM. Circadian mechanisms in murine and human bone marrow mesenchymal stem cells following dexamethasone exposure. Bone. 2008;42:861–870. doi: 10.1016/j.bone.2007.12.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, Zvonic S, Floyd ZE, Kilroy G, Goh BC, Hernandez TL, Eckel RH, Mynatt RL, Gimble JM. Induction of circadian gene expression in human subcutaneous adipose-derived stem cells. Obesity (Silver Spring) 2007;15:2560–2570. doi: 10.1038/oby.2007.308. [DOI] [PubMed] [Google Scholar]
- 19.Smith BJ, Lerner MR, Bu SY, Lucas EA, Hanas JS, Lightfoot SA, Postier RG, Bronze MS, Brackett DJ. Systemic bone loss and induction of coronary vessel disease in a rat model of chronic inflammation. Bone. 2006;38:378–386. doi: 10.1016/j.bone.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Newitt DC, Majumdar S, van Rietbergen B, von Ingersleben G, Harris ST, Genant HK, Chesnut C, Garnero P, MacDonald B. In vivo assessment of architecture and micro-finite element analysis derived indices of mechanical properties of trabecular bone in the radius. Osteoporos Int. 2002;13:6–17. doi: 10.1007/s198-002-8332-0. [DOI] [PubMed] [Google Scholar]
- 21.Bingham C, Arbogast B, Guillaume GC, Lee JK, Halberg F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia. 1982;9:397–439. [PubMed] [Google Scholar]
- 22.Gimble JM, Floyd ZE. Fat circadian biology. J Appl Physiol. 2009;107(5):1629–1637. doi: 10.1152/japplphysiol.00090.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimba S, Ishii N, Ohta Y, Ohno T, Watabe Y, Hayashi M, Wada T, Aoyagi T, Tezuka M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci U S A. 2005;102:12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.