Abstract
The Intubation-Surfactant-Extubation (INSURE) procedure is used worldwide to treat pre-term newborn infants suffering from respiratory distress syndrome, which is caused by an insufficient amount of the chemical surfactant in the lungs. With INSURE, the infant is intubated, surfactant is administered via the tube to the trachea, and at completion the infant is extubated. This improves the infant’s ability to breathe and thus decreases the risk of long term neurological or motor disabilities. To perform the intubation safely, the newborn infant first must be sedated. Despite extensive experience with INSURE, there is no consensus on what sedative dose is best. This paper describes a Bayesian sequentially adaptive design for a multi-institution clinical trial to optimize the sedative dose given to pre-term infants undergoing the INSURE procedure. The design is based on three clinical outcomes, two efficacy and one adverse, using elicited numerical utilities of the eight possible elementary outcomes. A flexible Bayesian parametric trivariate dose-outcome model is assumed, with the prior derived from elicited mean outcome probabilities. Doses are chosen adaptively for successive cohorts of infants using posterior mean utilities, subject to safety and efficacy constraints. A computer simulation study of the design is presented.
Keywords: Adaptive design, Bayesian design, Clinical Trial, Decision Theory, Dose-finding, Neonatal, Phase I–II trial, Surfactant, Utility
1. Introduction
Respiratory distress syndrome (RDS) in pre-term newborn infants is characterized by an inability to breathe properly. RDS is associated with the facts that the infant’s lungs have not developed fully and do not have a sufficient amount of surfactant, a compound normally produced in the lungs that facilitates breathing. A relatively new but widely used procedure for preterm infants suffering from RDS is Intubation-Surfactant-Extubation (INSURE), which is carried out when the infant is a few hours old. Once RDS has been diagnosed, the INSURE procedure is carried out as soon as possible to reduce the need for mechanical ventilation and risk of bronchopulmonary dysplasia. With INSURE, the infant is intubated, surfactant is administered via the tube to the trachea, and at completion the infant is extubated. The surfactant spreads from the trachea to the surface of the alveola, where it lowers alveolar surface tension and reduces alveolar collapse, thus improving lung aeration and decreasing respiratory effort. The aim is to improve the infant’s ability to breathe and thus increase the probability of survival without long term neurological or motor disabilities (Verder, et al., 1994; Bohlin, et al., 2007; Stevens, et al., 2007). In most cases, the INSURE procedure takes no more than one hour, and ideally it is completed within 30 minutes. Because intubation is invasive, to allow it to be done safely and comfortably the infant first must be sedated. The drugs propofol (Ghanta et al., 2007) and remifentanyl (Welzing et al., 2009) are widely used for this purpose. Although the benefits of the INSURE procedure are well-established, it also carries risks associated with intubation done while the infant is awake, and risks associated with the sedative. These include possible adverse behavioral and emotional effects if the infant is under-sedated as well as adverse haemodynamic effects associated with over-sedation. The goal in choosing a sedative dose is to sedate the infant sufficiently so that the procedure may be carried out, but avoid over-sedating. While it is clear that dose should be quantified in terms of amount per kilogram (kg) of the infant’s body weight, little is known about what the optimal dose of any given sedative may be for the INSURE procedure. Propofol doses that are too high, or that are given recurrently or by continuous infusion, have been associated with serious adverse effects in the neonatal or pediatric populations (Murdoch and Cohen, 1999; Vanderhaegen, et al., 2010; Sammartino, et al. 2010). Unfortunately, there is no broad consensus regarding the dose of any sedative in the community of neonatologists. The doses of that actually are used vary widely, with each neonatologist using their preferred dose chosen based on personal clinical experience and consensus within their neonatal unit.
Pediatric clinical trials are challenging primarily due to ethical considerations, including informed consent, the fact that many pediatricians are hesitant to experiment with children, and the fact that adverse events may have lifelong consequences. These issues are especially difficult with newborn infants just a few hours old. While there is an extensive literature on adaptive dose-finding methods, these have been developed primarily for chemotherapy in oncology, which is a very different medical setting than sedation of neonates as described above. To date, no adaptive dose-finding design has been developed specifically for infants.
The primary aim of the clinical trial described here is to optimize the dose of propofol given at the start of the INSURE procedure. Six possible doses are considered: 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg/kg body weight. Inherent difficulties in determining an optimal propofol dose are that there are both desirable and undesirable clinical outcomes related to dose, the probability of each outcome may vary as a complex, possibly non-monotone function of dose, and the outcomes do not occur independently of each other. In any dose-finding clinical trial in humans, it is not ethical to randomize patients fairly among doses because, a priori, some doses are considered unsafe or ineffective, and as data are obtained some doses may turn out to be either unsafe or to have unacceptably low efficacy. These ethical considerations motivate the use of sequential, outcome-adaptive, “learn-as-you-go” dose-finding methods (cf. O’Quigley, et al., 1990; Thall and Russell, 1998; Chevret, 2006; Cheung, 2011). Such methods are especially important when treating newborn infants diagnosed with RDS, where sedative dose prior to intubation may have adverse haemodynamic effects and failure of the INSURE procedure may result in prolonged mechanical ventilation, a recognized risk factor for long term adverse pulmonary outcomes (Stevens, et al., 2007). Consequently, to optimize propofol dose in a reliable and ethical manner in the setting of the INSURE procedure, a clinical trial design must (1) account for unknown, potentially complex relationships between dose and key clinical outcomes, (2) account for inherent risk-benefit trade-offs between efficacy and adverse outcomes, (3) adaptively learn and make decisions using the accumulating dose-outcome data during the trial, and (4) reliably choose a final, “optimal” dose that can be recommended for future use worldwide with the INSURE procedure.
The clinical trial design described here satisfies all of these requirements. It uses a Bayesian sequentially outcome-adaptive method that relies on subjective utilities, elicited from neonatologists who perform the INSURE procedure, that account for the benefits of desirable outcomes and the risks of adverse outcomes. To characterize propofol dose effects in a realistic and practical way, we define three co-primary outcomes, including two desirable efficacy outcomes and one undesirable adverse outcome. The first efficacy outcome is that a “good sedation state,” GSS, is achieved quickly. GSS is a composite event defined in terms of five established ordinal sedation assessment criteria variables scored within five minutes of the first sedative administration (Hummel, et al., 2008). These five variables are A1 = Crying Irritability, A2 = Behavior State, A3 = Facial Expression, A4 = Extremities Tone, and A5 = Vital Signs. Each variable takes on an integer value in the set {−2, −1, 0, +1, +2}, with Aj = −2 corresponding to highest sedation and Aj = +2 to highest infant discomfort. The Vital Signs criterion score A5 is defined in terms of heart rate (HR), respiration rate (RR), mean blood pressure (BP), and saturated oxygen in the circulating blood (SaO2). Supplementary Table 1 gives detailed definitions of these five assessment variables.
The overall sedation assessment score is defined as , and a good sedation score is defined as GSS = {−7 ≤ Z ≤ −3}. Because a GSS is required to intubate the infant, if it is not achieved with the initial propofol dose then an additional fixed dose of 1.0 mg/kg propofol is given. If this still does not achieve a GSS, then use of another sedative is allowed at the discretion of the attending clinician. A nontrivial dimension reduction is performed in defining GSS, since Z is defined in terms of the variables A1,⋯, A4 and A5, which in turn is a function of three haemodynamic measurements. However, A1,⋯, A5, Z, and GSS were defined by neonatologists who have extensive experience with the INSURE procedure.
Because it is desirable to complete the INSURE procedure as quickly as possible, the design also accounts for the efficacy event, EXT, that the infant is extubated within at most 30 minutes of intubation. This is motivated by the desire to sedate the infant sufficiently so that the INSURE procedure may be carried out, but not over-sedate. In addition to the efficacy events GSS and EXT, it is essential to monitor adverse events and include them in the dose-finding procedure. To do this, a third, composite adverse event was defined. The adverse haemodynamic event, HEM, is defined to have occurred if the baby’s HR falls below 80 beats per minute, SaO2 falls below 60%, or mean BP decreases by more than 5 mm Hg from a chosen inferior limit corresponding to the infant’s gestational age. The time interval for monitoring the infant’s HR, SaO2, and BP values to score HEM includes both the period while the infant is intubated and the subsequent three hours following extubation. Thus, HEM is defined very conservatively.
Our proposed methodology is very different from adaptive dose-finding methods based on a single outcome. For example, a method based on GSS alone might choose a dose to maximize Pr(GSS | dose), maximize information using about this dose-response function using D-optimal or A-optimal designs, or possibly find the “minimum effective dose” for which it is likely that Pr(GSS | dose) ≥ for some fixed target . There is an extensive literature on such methods. Some useful references are Fedorov and Leonov (2001), Atkinson, Donev, and Tobias (2006), Dette, et al. (2008), and Bornkamp, et al. (2011). In the present setting, a method that is ethically acceptable must account for more than one outcome, and must quantify the trade-offs between the risk of HEM and the benefits of GSS and EXT. This requires specifying and estimating a trivariate dose-outcome probability distribution for these three events. Even if this function were known perfectly, however, some numerical representation of the desirabilities of the eight possible elementary outcomes still would be needed to decide which dose is best. We quantify this using elicited utilities, described in Section 3, below.
The propofol trial design uses a sequentially outcome-adaptive Bayesian dose-finding method based on a numerical utility of each of the eight possible combinations of the three outcomes GSS, EXT, and HEM. The numerical utilities, given in Table 1, were elicited from the neonatologists planning the trial, who are experienced with the INSURE procedure and have observed and dealt with these events in their clinical practice. Before the elicitation, the maximum numerical utility 100 was assigned to the best possible event (GSS = yes, EXT = yes, HEM = no), and the minimum numerical utility 0 was assigned to the worst possible event (GSS = no, EXT = no, HEM = yes). The six remaining intermediate values were elicited subject to the obvious constraints that the utility must increase as either GSS or EXT goes from “no” to “yes” and must decrease as HEM goes from “no” to “yes.” The range 0 to 100 was chosen for convenience since it is easy to work with, although in general any numerical domain with which the area experts are comfortable could be used. By quantifying the desirability of each of the eight possible outcomes, the utility function formalizes the inherent trade-off between the INSURE procedure’s risks and benefits, insofar as they are characterized by these three events. An essential property of the numerical utilities is that they quantify the subjective opinions of the area experts. This is an advantage of the methodology since, inevitably, any multidimensional criterion must be reduced to a one-dimensional object if decisions are to be made. However a dimension reduction is done, it is inherently subjective.
Table 1.
Consensus elicited utilities and alternative utilities of the eight possible elementary outcomes. GSS = {good sedation score}, EXT = {extubation within 30 minutes}, HEM = {an adverse value of heartbeat, blood oxygen level, or blood pressure during the INSURE procedure or within 3 hours after extubation}.
| a. Elicited Consensus Utilities. | ||||
| GSS = Yes | GSS = No | |||
| EXT = Yes | EXT = No | EXT = Yes | EXT = No | |
| HEM = Yes | 60 | 20 | 40 | 0 |
| HEM = No | 100 | 80 | 90 | 70 |
| b. Alternative Utilities 1, with GSS given greater importance compared to the consensus utility. | ||||
| GSS = Yes | GSS = No | |||
| EXT = Yes | EXT = No | EXT = Yes | EXT = No | |
| HEM = Yes | 80 | 60 | 20 | 0 |
| HEM = No | 100 | 90 | 45 | 35 |
| c. Alternative Utilities 2, with EXT given greater importance compared to the consensus utility. | ||||
| GSS = Yes | GSS = No | |||
| EXT = Yes | EXT = No | EXT = Yes | EXT = No | |
| HEM = Yes | 80 | 10 | 70 | 0 |
| HEM = No | 100 | 40 | 95 | 35 |
| d. Alternative Utilities 3, with HEM given greater importance compared to the consensus utility. | ||||
| GSS = Yes | GSS = No | |||
| EXT = Yes | EXT = No | EXT = Yes | EXT = No | |
| HEM = Yes | 30 | 10 | 20 | 0 |
| HEM = No | 100 | 90 | 95 | 85 |
For trial conduct, the first cohort is treated at 1.0 mg/kg. The design chooses doses adaptively for all subsequent cohorts, subject to dose safety and efficacy constraints. Each decision is based on the dose-outcome data from all previously treated infants, using the posterior mean utilities of the six doses. To avoid getting stuck at a sub-optimal dose, a well-known problem with “greedy” sequential algorithms that always maximize an objective function (cf. Sutton and Barto, 1988), once a minimal sample is obtained at the current optimal dose, one version of the design randomizes adaptively among acceptable doses with posterior mean utility close to the maximum.
A variety of Bayesian decision theoretic methods have been proposed that are based on the utilities of making correct or incorrect decisions at the end of the trial. These include designs for phase II trials (cf. Stallard, 1998; Stallard, Thall, and Whitehead 1999; Stallard and Thall, 2001; Leung and Wang, 2001; Chen and Smith, 2009) and for randomized phase III trials (cf. Christen, et al., 2004; Lewis, et al., 2007; Wathen and Thall, 2008). These methods optimize benefit to future patients. This is fundamentally different from the present approach, which assigns doses based on elicited joint utilities of the clinical outcomes, and at the end of the trial relies on the same criterion, posterior mean utility of each dose, to make a final recommendation. Bayesian clinical trial designs with similar sequentially adaptive Bayesian decision structures based on utilities have been proposed by Houede, et al. (2010), Thall, et al., (2011), and Thall and Nguyen (2012). The third design is the basis for a currently ongoing trial to optimize the dose of radiation therapy for pediatric brain tumors, based on bivariate ordinal efficacy and toxicity outcomes.
Section 2 describes the Bayesian multivariate dose-outcome model. The utility function and decision criteria used for trial conduct are presented in Section 3, and outcome-adaptive randomization criteria used in a modified version of the design are given in Section 4. An extensive simulation study of the design’s behavior under a range of different possible scenarios is summarized in Section 5. We close with a brief discussion in Section 6.
2. Probability Model
2.1 Dose-Response Functions
Denote the outcome indicators YG = I(GSS) = I{−7 ≤ Z ≤ −3}, YE = I(EXT), YH = I(HEM). In the dose-response model, we will use the standardized doses obtained by dividing the raw doses by their mean, x1 = 0.5/1.75 = 0.286,⋯, x6 = 3.0/1.75 = 1.714, with unsubscripted x denoting any given dose. The observed outcome vector is O = (Z, YE, YH). Because historical data of the form (x, O) are not available, the following dose-outcome model was developed based on the collective experiences and prior beliefs of the neonatologists planning the propofol trial, and extensive computer simulations studying properties of various versions of the model and design.
Adaptive decisions in the trial are based on the behavior of Y = (YG, YE, YH) as a function of x. The distributions of the later outcomes, YE and YH, may depend quite strongly on the sedation score Z achieved at the start of the INSURE procedure, it is unlikely that YE and YH are conditionally independent given Z and x, and the definition of Z includes some of the haemodynamic events used to define HEM. To reflect these considerations, our joint model for [O | x] is based on the probability factorization
| (1) |
where θZ and θE,H are subvectors of the model parameter vector θ. Expression (1) says that x may affect Z, while both x and Z may affect (YE, YH). To account for association between YE and YH, we first specify the conditional marginals of [YE | x, Z] and [YH | x, Z], and use a copula (Nelsen, 1999) to obtain a bivariate distribution. Indexing k = E, H, we define these marginals using logistic regression models (McCullagh and Nelder, 1989),
| (2) |
with linear terms taking the form
| (3) |
where f(Z) = {(Z + 5)/15}2 and we denote θk = (θk,0, θk,1, θk,2, θk,3, θk,4). For k = E, H, θk,1 is the dose effect, θk,2 is the sedation score effect, θk,3 is the effect of not achieving a GSS, and x is exponentiated by θk,4 to obtain flexible dose-response curves. We standardize Z in ηE and ηH so that its numerical value does not have unduly large effects for values in the Z domain far away from −5, with (Z + 5)/15 squared to reflect the functional form of the elicited prior in Table 2. For example, the extreme score Z = +10 is represented by f(Z) = 1 rather than 225.
Table 2.
Prior means, interval probabilities for Z and x = dose, and utilities.
| Propofol Dose (mg/kg) | ||||||
|---|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | |
| a. Elicited prior interval probabilities for Z | ||||||
| −10 ≤ Z ≤ −8 | .05 | .10 | .20 | .30 | .40 | .60 |
| −7 ≤ Z ≤ −3 | .55 | .65 | .75 | .66 | .58 | .39 |
| −2 ≤ Z ≤ 10 | .40 | .25 | .05 | .04 | .02 | .01 |
| b. Elicited prior means of πE(z, x) | ||||||
| Z = −10 | .99 | .98 | .90 | .70 | .60 | .25 |
| Z = −5 | .99 | .98 | .97 | .95 | .90 | .75 |
| Z = 0 | .95 | .90 | .80 | .50 | .20 | .10 |
| Z = +10 | .70 | .30 | .10 | .05 | .03 | .01 |
| c. Elicited prior means of πH (z, x) | ||||||
| Z = −10 | .01 | .10 | .20 | .30 | .50 | .70 |
| Z = −5 | .01 | .02 | .05 | .10 | .15 | .40 |
| Z = 0 | .01 | .20 | .40 | .70 | .80 | .90 |
| Z = +10 | .30 | .40 | .70 | .95 | .98 | .99 |
| d. Prior mean utilities and probabilities, obtained by averaging over Z. | ||||||
| Ū (x | θ) | 94.0 | 91.6 | 90.9 | 83.5 | 74.8 | 50.0 |
| π̅G(x | θ) | .55 | .65 | .75 | .66 | .58 | .39 |
| π̅H (x | θ) | .02 | .08 | .12 | .20 | .32 | .57 |
| π̅E(x | θ) | .97 | .95 | .94 | .84 | .75 | .46 |
| π̅S(x | θ) | .54 | .63 | .71 | .58 | .47 | .24 |
Specifying domains of the elements of θE and θH requires careful consideration. The intercepts θE,0 and θH,0 are real-valued, with the exponents θE,4, θH,4 > 0. Based on clinical experience with propofol and other sedatives used in the INSURE procedure, as reflected by the elicited prior means in Table 2, we assume that θE,1, θE,2, < 0 while θH,1, θH,2 > 0. This says that, given sedation score Z achieved initially, πE(x, Z, θ) decreases and πH(x, Z, θ) increases with dose. Similarly, failure to achieve a GSS can only increase the probability πH(x, Z, θ) of an adverse haemodynamic event and decrease the probability πE(x, Z, θ) of extubation within 30 minutes, so θH,3 > 0 while θE,3 < 0.
Denote the joint distribution πE,H(a, b | x, Z, θk) = Pr(YE = a, YH = b | x, Z, θk), for a, b ∈ {0, 1}. Given the marginals πk(x, Z, θ), k = E, H, temporarily suppressing (x, Z, θ) for brevity, the Gumbel-Morgenstern copula model is
| (4) |
with association parameter −1 < ρ < +1. The joint conditional distribution of [YE, YH | x, Z] is parameterized by θE,H = (θE, θH, ρ), which has dimension 5+5+1 = 11, and θZ, which will be described below. Combining terms, and denoting πZ(z | x, θZ) = Pr(Z = z | x, θZ), the joint distribution of [Z, YE, YH | x] is
| (5) |
for z = −10, −9,⋯, +9, +10 and a, b ∈ {0, 1}.
An important property of the model is that the unconditional marginal distributions of the two later events, YE and YH, may be complex, non-monotone functions of x. This is because their marginals first are defined in (2) conditional on the initial sedation score, Z, and their unconditional marginals are obtained by averaging over the distribution of Z,
The unconditional joint distribution π̅E,H(x, θk, θZ) is computed similarly, from (4) and (5). The probability π̅H(x, θk, θZ) of HEM plays a key role in the design because it is used as a basis for deciding whether x is acceptably safe. Similarly, overall success is defined as S = (GSS and EXT) = (−7 ≤ Z ≤ −3 and YE = 1), which has probability πS(x, θ) that depends on πZ(z | x, θZ). Thus, a key aspect of how the outcomes are observed that affects the statistical model and method is that, for an infant given propofol dose x, π̅H(x, θk, θZ) and πS(x, θ) are averages over the initial sedation score distribution, and thus these probabilities depend on θZ.
2.2 Extended Beta Regression Model for Sedation Score
To specify a flexible distribution of [Z | x], we employ the technical device of first defining a beta regression model for a latent variable W having support [0, 1] with mean that is a decreasing function of x, and then defining the distribution of Z in terms of the distribution of W. We formulate the beta regression model for [W | x] using the common re-parameterization of the Be(a, b) model in terms of its mean μ = a/(a + b) and ψ = a + b, where μ = μx varies with x and the pdf is
| (6) |
(cf. Williams, 1982; Ferrari and Cribari-Neto, 2004), and Γ(·) denotes the gamma function. Denote the indexes of the doses in increasing order by j(x) = 1,⋯, J. We assume a saturated model for the mean of [W | x],
where α1,⋯, αJ > 0. Our preliminary simulations showed that assuming constant ψ in the beta regression model for [W | x] results in a model for [Z | x], shown below, that is not sufficiently flexible across a range of possible dose-outcome scenarios to facilitate reliable utility-based dose-finding. To obtain a more flexible model, we explored the behavior of several parametric functions for ψ. We found that the function
| (7) |
with γ1, γ2 > 0 and γ3 real-valued gives a model that does a good job of fitting a wide range of simulated data. The initial rationale for this particular functional form was to model the standard deviation as the function σx = {μx(1 − μx)}ν/(2 + ζxα), with ν > 0. To ensure the usual beta distribution parameter constraints σx < 0.50 and ψx > 0, it was necessary to modify this so that σx = [{μx(1 − μx)}/(1 + ψx)]1/2 with ψx given by (7). Modeling the ESS parameter as a function of x and μx in this way, in addition to the more common practice of defining a regression model for the mean, is similar in spirit to the generalized beta regression model of Simas, et al. (2010).
Denote the incomplete beta function for 0 < w < 1 and c, d > 0. Using the continuous distribution of [W | x] given in (6), we define the discrete distribution for [Z | x] as
| (8) |
for z = −10, −9,⋯, +9, +10, where θZ = (α, γ) = (α1,⋯, αJ, γ1, γ2, γ3). Since J = 6 propofol doses will be studied, this model for the distribution of Z in terms of the generalized beta latent variable W expresses the probability of a GSS in terms of the incomplete beta function evaluated at arguments characterized by x, the 6 dose-response parameters α = (α1,⋯, α6) of μx, and the three parameters γ = (γ1, γ2, γ3) of ψx. While this model for [Z | x] may seem somewhat elaborate, it must be kept in mind that Z is a sum with 21 possible values and its distribution is a function of J possible doses, so for the propofol trial a 6 × 20 = 120 dimensional distribution is represented by a 9-parameter model.
It follows from (8) that the probability of GSS = (−7 ≤ Z ≤ −3) is
| (9) |
While the distribution of W is monotone in dose by construction, it should be clear from expressions (6) – (9) that πG(x, θZ) is a complex, possibly non-monotone function of dose.
2.3 Prior, Likelihood, and Posterior Computation
Collecting terms, the model parameter vector is θ = (ρ, α, γ, θE, θH), which has 20 elements. To establish a prior, we assumed ρ ~ Unif[−1, +1], and for the remaining 19 parameters, θ−ρ, we used the following pseudo-sample-based approach, similar to that of Thall and Nguyen (2012). The pseudo samples were obtained by treating the elicited means of the probabilities πE(z, x) and πH(z, x) and interval probabilities Pr(l ≤ Z ≤ u | x) in Table 2 as the true state of nature. For each dose x, we used these elicited probabilities to generate a pseudo-sample of 100 iid patient outcomes,
To generate each pseudo-sample, it first was necessary to specify πZ(z | x) for all combinations of x and z = −10,⋯, +10. For each x, we did this by first fitting the three interval probabilities in the corresponding column of Table 2a to a beta(ax, bx), then partitioning [0, 1] into 21 equal subintervals and setting each πZ(z | x) to be the fitted beta probability of the corresponding subinterval. To obtain πE(x, z) for all 21 values of z, we linearly interpolated the rows of Table 2b, and we obtained πH(x, z) similarly from 2c. Using these probabilities, for each i and x, we first simulated Z̃i(x) from πZ(z | x) and then simulated from πk(x, Z̃i(x)) for k = E and H. Given the combined pseudo-sample 𝒟̃= ∪x𝒟̃(x), and assuming a highly non-informative pseudo prior on θ−ρ, we computed a pseudo-posterior p(θ−ρ | 𝒟̃). This entire process was repeated 3000 times, and the average of the 3000 pseudo-posterior means was used as the prior mean of θ−ρ. The pseudo-sample size 100 was chosen to be large enough to provide reasonably reliable pseudo-posteriors, but small enough so that the computations could be carried out feasibly. Pseudo-sampling provides a reliable alternative to nonlinear least squares, which often fails to converge in this type of setting.
For priors, we assumed that {α1,⋯, α6, −θE,1,−θE,2,−θE,3, θH,1, θH,2, θH,3} were normal truncated below at 0, {γ1, γ2, θE,4, θH,4} were lognormal, and {γ3, θE,0, θH,0} were normal. Given the prior means established by the pseudo-sampling method, we calibrated the prior variances to be uninformative in the sense that effective sample size (ESS, Morita et al., 2008) of the prior was 0.10. Numerical prior means and variances are given in Supplementary Table 2.
Let N denote the maximum trial sample size. Index the patients enrolled in the trial by i = 1,⋯, N, and denote the observed outcomes by Oi = (Zi, Yi,E, Yi,H), and the assigned dose by x[i] for the ith patient. Let n = 1,⋯, N denote an interim sample size where an adaptive decision is made during the trial, and 𝒪n = (O1,⋯, On) the observed data from the first n patients. The likelihood for the first n patients in the trial is
The posterior based on this interim sample is
All posterior quantities used for decision making by the trial design were computed using Markov chain Monte Carlo with Gibbs sampling (Robert and Cassella, 1999).
3. Decision Criteria
3.1 Utilities
Denote the utility function by U(y), where y = (yG, yE, yH) ∈ {0, 1}3 is an elementary outcome. The numerical utilities for the propofol trial outcomes were obtained by first fixing the scores of the best and worst possible elementary outcomes to be U(1, 1, 0) = 100 and U(0, 0, 1) = 0, and eliciting the remaining six scores as values between 100 and 0 from neonatologists familiar with the INSURE procedure. An admissible utility U(yG, yE, yH) must increase in yG and yE and decrease in yH. While these admissibility requirements may seem obvious, they must be kept in mind during the elicitation process. Although we used the range [0, 100] for U, in general for a given application any convenient interval may be used, depending on what the area experts find intuitively appealing.
To construct dose-finding criteria from the utility function U(y), we first define the mean utility of dose x given θ,
| (10) |
where the joint distribution πG,E,H is as given earlier. This expression says that, if one knew the parameters θ, then the mean utility (10) is what one would expect to achieve by giving an infant dose x. Since θ is not known, it must be estimated. Rather than computing a frequentist estimator θ̂ and basing decisions on Ū (x | θ̂), we will exploit our Bayesian model to compute statistical decision criteria, as follows. Let datan denote the observed dose-outcome data from n babies at any interim point in the trial, 1 ≤ n < N. Let p(θ | datan) denote the current posterior of θ. The posterior mean utility of dose x given datan is
| (11) |
In words, based on what has been learned from the observed the data from n babies, the posterior mean utility u(x | datan) is what one would expect to achieve if the next baby were given dose x. An important point is that, with small sample sizes, some of the eight elementary events may not occur, and in this case u(x | datan) will be based partly on the prior. Note that (11) is obtained by averaging over the distribution of [Y|x, θ] in (10) to obtain Ū (x | θ), and then averaging this mean utility over the posterior of θ. We denote by the dose having maximum u(x | datan) among the doses under study. For brevity, we denote . Subject to the restriction that an untried dose may not be skipped when escalating, the design Uopt chooses each successive cohort’s dose to maximize u(x | datan) among all x ∈ {x1,⋯, x6}.
It may seem appropriate to place a probability distribution on the utility function U to reflect uncertainty about what alternative utilities others may have. If a distribution q(U) is assumed for U, using the elicited consensus utility as the mean Uq under q, then one would need to integrate over q(U) as well as πG,E,H(y) and p(θ | datan) to obtain u(x | datan). This computation gives the original posterior mean utility (11), however, essentially because the trial data provide no new information about U. We will address this issue by sensitivity analyses to U, in Section 5.
3.2 Dose Acceptability Criteria
A critical issue is that a dose that is “optimal” in terms of the utility alone may be unacceptable in terms of either safety or overall success rate. To ensure that any administered dose has both an acceptably high success rate and an acceptably low adverse event rate, based on the current data, we define the following two posterior acceptability criteria. Given the fixed upper limit , we say that a dose x is unsafe if
| (12) |
for fixed upper limit pU,H. Recall that the overall success event is S = (YG = 1 and YE = 1), that a GSS was achieved with the initial propofol administration and the INSURE procedure was completed with extubation within 30 minutes. Denoting πS(x, θ) = Pr(S = 1 | x, θ), the probability of this event is given by
parameterized by (θE, θZ). We say that a dose x has unacceptably low overall success probability if
| (13) |
for fixed upper limit pU,S. We will refer to the subset of doses that do not satisfy either (12) or (13) as acceptable doses. We denote this subset by 𝒜n, and we denote the modification of design Uopt restricted to 𝒜n by Uopt + Acc.
4. Adaptive Randomization
Intuitively, it may seem that the best dose is simply the one maximizing the posterior mean utility, possibly enforcing the additional acceptability criteria given above. However, it is well known in sequential decision making that a “greedy” algorithm that always chooses each successive action by optimizing some decision criterion risks getting stuck at a suboptimal action. A greedy algorithm may get stuck at a suboptimal action due to the fact that, because it repeatedly takes the suboptimal action, it fails to take and thus obtain enough data on an optimal action to determine, statistically, that it is truly optimal. This problem is sometimes known as the “optimization versus exploration” dilemma. (cf. Robbins, 1952; Gittins, 1979; Sutton and Barto, 1998). This fact has been recognized only recently in the context of dose-finding clinical trials (Azriel, et al., 2011; Thall and Nguyen, 2012; Oron and Hoff, 2013). In the propofol trial, always choosing an “optimal” dose x by maximizing u(x | datan) is an example of a greedy algorithm, even if x is restricted to 𝒜n. A simple aspect of this problem is that the statistics u(x1 | datan),⋯, u(xK | datan) are actually quite variable for most values of n during the trial, and simply maximizing their means ignores this variability. This problem has both ethical and practical consequences, since maximizing the posterior mean utility for each cohort may lead to giving suboptimal doses to a substantial number of the infants in the trial, and it also may increase the risk of recommending a suboptimal dose at the end. To deal with this problem, we use adaptive randomization (AR) to improve this greedy algorithm and thus the reliability of the trial design. Our AR criterion is similar to that used by Thall and Nguyen (2012). One goal of the AR is to obtain a design that, on average, treats more patients at doses with higher actual utilities and is more likely to choose a dose with maximum or at least high utility at the end of the trial. At the same time, it must not allow an unacceptable risk for the two infants in each cohort. Thus, while the AR is implemented using probabilities proportional to the posterior mean utilities, it is restricted to the set 𝒜n of acceptable doses. Given current datan, the next cohort is randomized to dose xj ∈ 𝒜n with probability
| (14) |
The following algorithm is a hybrid of utility maximization and AR. It chooses doses according to Uopt + Acc, unless the current optimal dose has at least δ more patients than any other acceptable dose. In this case, it applies the AR criterion (14) to choose a dose, as follows. Denote the sample size at dose xj after n patients have been treated by mn(xj), so that mn(x1) + ⋯ mn(xK) = n. Among the doses in 𝒜n if mn(xopt) ≥ mn(xj) + δ for all xj ≠ xopt, then assign xj with probability pj,n. Otherwise, assign xopt.
For ethical reasons, AR must be applied carefully. Once enough data have been obtained to apply AR reliably, it is ethically inappropriate to randomize patients to a dose that is unlikely to be best. Formally, we say that x is unlikely to be best if
| (15) |
for fixed lower limit pL. Thus, AR is applied to the set of doses that not only are acceptable in terms of the safety and efficacy criteria (12) and (13), but that also do not satisfy (15), i.e. that are not unlikely to be best. This restriction is most useful when larger sample sizes are available, later in the trial, and has the effect of reducing the numbers of patients treated at inferior doses. We denote this hybrid algorithm by Uopt + Acc + ARδ.
For each design Uopt, Uopt + Acc, and Uopt + Acc + ARδ, the first cohort is treated with 1.0 mg/kg, untried doses may not be skipped when escalating, but there is no constraint on de-escalation. Acc restricts doses to 𝒜n. For Uopt + Acc + ARδ, doses unlikely to be best also are excluded, and the AR criterion is used only if, within this subset of doses, xopt has at least δ more patients than any other dose. For both Uopt +Acc, and Uopt + Acc + ARδ, if it is determined that 𝒜n = ϕ, the trial is stopped and no dose is selected. For all three designs, if the trial is not stopped early, at the end of the trial, the dose xselect having maximum posterior mean utility, udataN, is selected.
While the trial will be shut down if 𝒜n is empty, i.e. no dose is acceptable, we consider this very unlikely. If this happens, then for neonatologists performing the INSURE procedure using propofol, in practice a safe dose with HEM rate < 0.10 but a success rate lower than 0.60 would be used. This might motivate a subsequent trial to study the idea of titrating the dose in more than one administration for each infant. However, optimizing such a multi-stage procedure is a much more complex problem, and would require a very different design.
5. Simulation Study
In the simulations, the trial has maximum sample size N = 60, cohort size c = 2, and acceptability cut-offs pU,H = pU,S = 0.95, with pL = 0.05 when ARδ is used. In preliminary simulations, these design parameters were varied, along with the prior variances, to study their effects and obtain a design with desirable properties. The hybrid Uopt + Acc + ARδ was studied for δ = 2, 4, 6, 8, and 10. Since the results were insensitive to δ in this range, only the case δ = 2 is reported.
We also included the following ad hoc non-model-based 4-stage design suggested by a Referee as a comparator. Stage 1: Randomize 24 patients to each of the 6 doses (4 per dose). Select the 4 doses with the highest mean utility Ū (x) for evaluation in stage 2. Stage 2: Randomize 16 patients to each of the 4 selected doses (4 per dose), and select the 3 doses (from all 6) with highest Ū (x) for evaluation in stage 3. Stage 3: Randomize 12 patients to each of the 3 newly selected doses (4 per dose), and select the 2 doses (from all 6) with the highest Ū (x) for evaluation in stage 4. Stage 4: Randomize 8 patients to each of the 2 remaining doses (4 per dose), and select the best dose, having highest Ū (x) across all 6 doses. This design uses 60 patients, evaluates at least 4 patients per dose, and the selected dose has information on up to 16 patients. While it interimly selects (drops) doses with higher (lower) empirical mean utilities, it does not have rules that drop doses in terms of their empirical HEM or Success rates.
We used the following criteria to assess and compare the designs. The first is the proportion of the difference between the utilities of the best and worst possible doses achieved by xselect, scaled to the domain [0, 100],
The second criterion quantifies how well a method assigns doses to patients in the trial,
where utrue(x[i]) is the true utility of the dose given to the ith patient. Larger value correspond to better design performance, with Rselect quantifying benefit to future patients while Rtreat, which may be regarded as an ethical criterion, quantifying benefit to the patients treated in the trial.
Table 3 compares the four designs, based on mean values across 3000 simulated trials under each of 9 different dose-outcome scenarios, given in Supplementary Tables 3.1 – 3.9. Scenario 1 is based on the elicited prior probabilities. The beta regression model was used to obtain all 21 true πZ(x) values from three interval probabilities, and linear interpolation was used to obtain true πE(x) and πH(x). Otherwise, none of the scenarios are model-based. The scenarios assume that a larger dose will shift the Z distribution toward −10, which is reasonable given the nature of the sedative drug. Given this, the interval probabilities for Z vary widely across the scenarios. The scenarios’ true πE(x) and πH(x) have the same general trends as the prior in that πE(x) decreases and πH(x) increases with x given Z. To reflect the prior belief that YE and YH are slightly negatively correlated (Table 2), we set ρ = − 0.1 when generating the true joint distributions of each scenario. Preliminary simulation results were insensitive to the assumed true ρ value. Both Uopt and 4-Stage have no early stopping rules, so these designs always treat 60 patients. Due to the much larger number adverse HEM events of 4-Stage in Scenarios 1, 2 and 8, the fact that it treats 60 patients in Scenarios 8 and 9 where no doses are acceptable, and the much lower Rtreat values across all scenarios, this design is unethical. Compared to Uopt + Acc and Uopt + Acc + AR, 4-Stage has Rselect values that are slightly higher in Scenarios 1 and 2 with the price being many more occurrences of HEM, and in Scenarios 3 – 7 it has lower Rselect values. Comparison of Uopt to Uopt + Acc shows the effects of including dose acceptability criteria in a sequentially adaptive utility-based design. While these two designs have similar values of Rselect and Rtreat for Scenarios 1 – 4, the importance of the acceptability rules is shown clearly by the other scenarios, where Uopt + Acc has greatly superior performance. Moreover, the mean of 19.2 adverse HEM events for Uopt in Scenario 8 illustrates the potential danger of using a design with a utility-based decision criterion without an early stopping rule for safety. The much higher values of Rselect and Rtreat for Uopt + Acc in Scenarios 5 – 7 show that it is both more reliable and more ethical in these cases compared to Uopt.
Table 3.
Comparison of alternative designs. Scenarios 8 and 9 have no acceptable dose, so Rselect values are less relevant and thus have a gray background. A dose x is unacceptable if either π̅H(x, θ) > .10 or πS(x, θ) < .60 with posterior probability > .95.
| Scenario | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Design | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Uopt | Rselect | 96 | 93 | 99 | 90 | 73 | 49 | 30 | 95 | 48 |
| Rtreat | 96 | 92 | 98 | 90 | 64 | 46 | 21 | 95 | 42 | |
| % None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| # Pats | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | |
| # HEM | 4.1 | 2.7 | 2.4 | 2.8 | 2.2 | 2.3 | 2.1 | 19.3 | 2.0 | |
| # Succ | 36.7 | 40.8 | 39.6 | 33.0 | 25.7 | 20.2 | 9.2 | 37.1 | 11.0 | |
| Uopt + Acc | Rselect | 95 | 93 | 99 | 95 | 93 | 89 | 88 | 96 | 99 |
| Rtreat | 96 | 92 | 98 | 92 | 79 | 69 | 64 | 96 | 73 | |
| % None | 4 | 0 | 1 | 2 | 4 | 7 | 10 | 100 | 93 | |
| # Pats | 58.9 | 59.8 | 59.8 | 59.4 | 59.0 | 58.1 | 56.9 | 15.4 | 40.6 | |
| # HEM | 4.2 | 2.6 | 2.4 | 2.9 | 2.3 | 2.6 | 2.9 | 4.9 | 1.9 | |
| # Succ | 36.4 | 40.6 | 39.3 | 35.1 | 32.2 | 28.7 | 25.5 | 9.4 | 11.9 | |
| Uopt + Acc + AR2 | Rselect | 95 | 94 | 94 | 95 | 92 | 89 | 94 | 98 | 97 |
| Rtreat | 92 | 84 | 87 | 87 | 76 | 71 | 69 | 94 | 72 | |
| % None | 4 | 1 | 1 | 4 | 5 | 6 | 7 | 100 | 95 | |
| # Pats | 59.0 | 59.7 | 59.7 | 59.2 | 58.9 | 58.5 | 57.9 | 15.4 | 39.7 | |
| # HEM | 5.7 | 4.5 | 2.8 | 3.8 | 2.6 | 2.9 | 3.0 | 5.0 | 1.9 | |
| # Succ | 36.5 | 39.0 | 36.4 | 35.1 | 32.4 | 30.3 | 28.2 | 9.5 | 11.6 | |
| 4-Stage | Rselect | 97 | 97 | 92 | 93 | 89 | 82 | 84 | 90 | 86 |
| Rtreat | 83 | 65 | 73 | 76 | 66 | 63 | 59 | 74 | 69 | |
| % None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| # Pats | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | |
| # HEM | 8.8 | 8.9 | 3.2 | 5.0 | 2.7 | 2.9 | 2.7 | 22.8 | 2.7 | |
| # Succ | 34.5 | 35.4 | 33.3 | 31.9 | 29.8 | 28.4 | 23.1 | 36.4 | 16.8 | |
After excluding Uopt and 4-Stage as ethically unacceptable, comparison between Uopt + Acc and Uopt + Acc + AR2 shows the effects of including AR. Recall that AR2 randomizes patients among acceptable doses having u(x | data) close to u(xopt | data), to better explore the dose domain. These designs have very similar Rselect values for Scenarios 1 – 6, with Uopt + Acc + AR2 showing a slight advantage in Scenario 7. As expected, Uopt + Acc has slightly larger Rtreat values and slightly smaller mean numbers of HEM events in most scenarios. Consequently, for the propofol trial, Uopt + Acc is the better of the two ethical designs, but by a small margin.
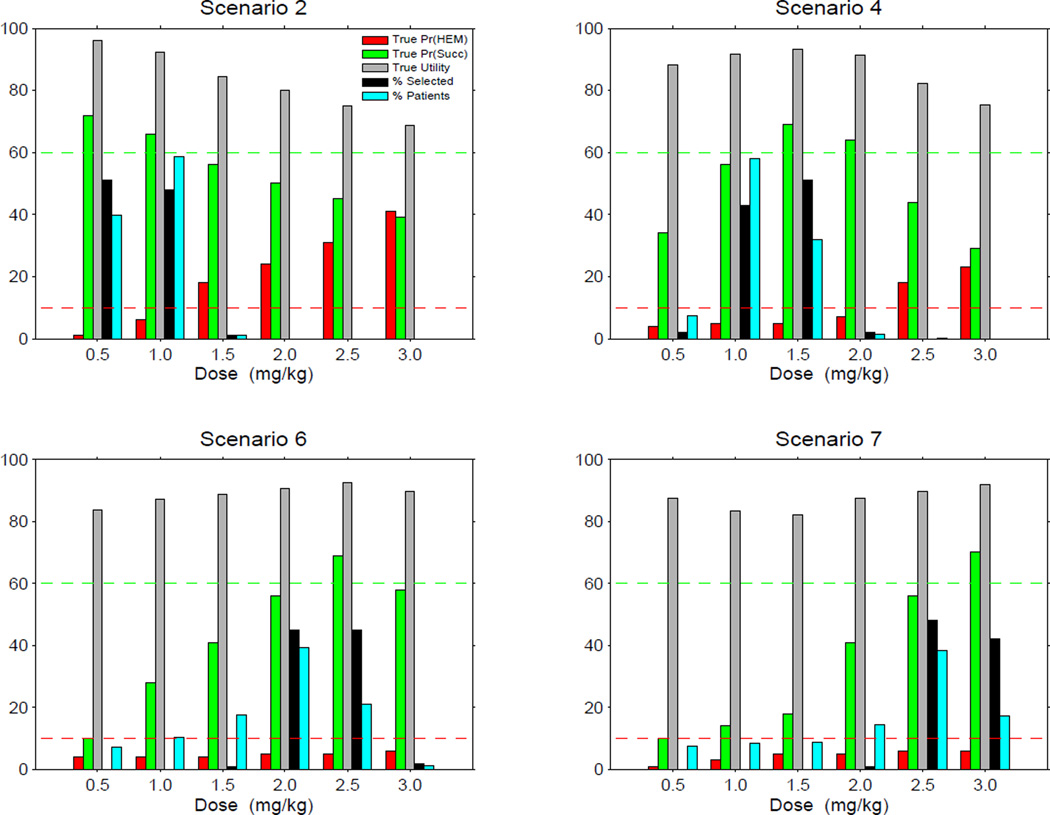
Table 4 summarizes the simulations in more detail for Uopt + Acc. In each of Scenarios 1 – 7, the selection rates, subsample sizes, and success event rates for the 6 doses all follow the utrue(x) values, and doses with comparatively low utrue(x) are selected seldom or not at all. The design is very likely to stop the trial and select no dose in both Scenario 8, where all doses are unsafe with , and Scenario 9, where all doses have a low success probability with . In particular, Uopt + Acc does a good job of controlling the HEM event rate at very low values across all scenarios. Figure 1 illustrates properties of Uopt + Acc in four selected scenarios.
Table 4.
Simulation results using the Uopt + Acc design. A dose x is unacceptable if either π̅H(x, θ) > .10 or πS(x, θ) < .60 with posterior probability > .95. Utilities of unacceptable doses have a gray background. The highest utility among acceptable doses is given in boldface.
| dose (mg/kg) | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | % none, Sum | |
|---|---|---|---|---|---|---|---|---|
| Scenario 1 | utrue | 94.0 | 91.6 | 90.9 | 83.5 | 74.7 | 49.9 | |
| % Sel | 18 | 69 | 9 | 0 | 0 | 0 | 4 | |
| # Pats | 12.1 | 42.8 | 3.9 | 0.1 | 0.0 | 0.0 | 58.9 | |
| # HEM | 0.2 | 3.5 | 0.5 | 0.0 | 0.0 | 0.0 | 4.2 | |
| # Succ | 6.5 | 27.1 | 2.8 | 0.0 | 0.0 | 0.0 | 36.4 | |
| Scenario 2 | utrue | 95.9 | 92.3 | 84.3 | 79.9 | 75.0 | 68.7 | |
| % Sel | 51 | 48 | 1 | 0 | 0 | 0 | 0 | |
| # Pats | 23.9 | 35.2 | 0.7 | 0.0 | 0.0 | 0.0 | 59.8 | |
| # HEM | 0.3 | 2.2 | 0.1 | 0.0 | 0.0 | 0 | 2.6 | |
| # Succ | 17.2 | 23.0 | 0.4 | 0.0 | 0.0 | 0.0 | 40.6 | |
| Scenario 3 | utrue | 93.0 | 94.4 | 92.2 | 88.7 | 86.0 | 80.6 | |
| % Sel | 8 | 89 | 2 | 0 | 0 | 0 | 1 | |
| # Pats | 8.2 | 50.0 | 1.4 | 0.1 | 0.0 | 0.0 | 59.8 | |
| # HEM | 0.3 | 2.0 | 0.1 | 0.0 | 0.0 | 0.0 | 2.4 | |
| # Succ | 4.4 | 34.0 | 0.9 | 0.0 | 0.0 | 0.0 | 39.3 | |
| Scenario 4 | utrue | 88.2 | 91.7 | 93.2 | 91.3 | 82.1 | 75.3 | |
| % Sel | 2 | 43 | 51 | 2 | 0 | 0 | 2 | |
| # Pats | 4.4 | 34.8 | 19.2 | 0.8 | 0.1 | 0.0 | 59.4 | |
| # HEM | 0.2 | 1.6 | 1.0 | 0.1 | 0.0 | 0.0 | 2.9 | |
| # Succ | 1.5 | 19.7 | 13.3 | 0.5 | 0.0 | 0.0 | 35.1 | |
| Scenario 5 | utrue | 80.6 | 85.7 | 90.9 | 92.9 | 90.4 | 84.4 | |
| % Sel | 0 | 0 | 35 | 58 | 2 | 0 | 4 | |
| # Pats | 3.7 | 6.4 | 28.4 | 19.7 | 0.8 | 0.1 | 59.0 | |
| # HEM | 0.1 | 0.2 | 1.1 | 0.9 | 0.0 | 0.0 | 2.3 | |
| # Succ | 0.3 | 1.8 | 15.6 | 13.9 | 0.5 | 0.0 | 32.2 | |
| Scenario 6 | utrue | 83.6 | 87.0 | 88.8 | 90.7 | 92.6 | 89.6 | |
| % Sel | 0 | 0 | 1 | 45 | 45 | 2 | 7 | |
| # Pats | 4.4 | 6.3 | 10.6 | 23.5 | 12.6 | 0.7 | 58.1 | |
| # HEM | 0.1 | 0.2 | 0.4 | 1.1 | 0.7 | 0.0 | 2.6 | |
| # Succ | 0.4 | 1.8 | 4.3 | 13.1 | 8.7 | 0.4 | 28.7 | |
| Scenario 7 | utrue | 87.5 | 83.4 | 82.1 | 87.5 | 89.8 | 91.8 | |
| % Sel | 0 | 0 | 0 | 1 | 48 | 42 | 10 | |
| # Pats | 4.6 | 5.0 | 5.2 | 8.7 | 23.1 | 10.3 | 56.9 | |
| # HEM | 0.1 | 0.2 | 0.2 | 0.4 | 1.3 | 0.6 | 2.9 | |
| # Succ | 0.5 | 0.7 | 0.9 | 3.5 | 12.8 | 7.2 | 25.5 | |
| Scenario 8 | utrue | 82.1 | 80.5 | 78.5 | 75.2 | 69.7 | 59.9 | |
| % Sel | 0 | 0 | 0 | 0 | 0 | 0 | 100 | |
| # Pats | 7.2 | 7.8 | 0.4 | 0.0 | 0.0 | 0.0 | 15.4 | |
| # HEM | 2.1 | 2.6 | 0.1 | 0.0 | 0.0 | 0.0 | 4.9 | |
| # Succ | 4.2 | 4.9 | 0.2 | 0.0 | 0.0 | 0.0 | 9.4 | |
| Scenario 9 | utrue | 79.9 | 82.2 | 83.9 | 85.1 | 86.0 | 85.9 | |
| % Sel | 0 | 0 | 0 | 0 | 1 | 6 | 93 | |
| # Pats | 4.6 | 5.1 | 5.9 | 6.9 | 9.4 | 8.6 | 40.6 | |
| # HEM | 0.1 | 0.2 | 0.2 | 0.3 | 0.5 | 0.6 | 1.9 | |
| # Succ | 0.4 | 0.8 | 1.4 | 2.2 | 3.6 | 3.5 | 11.9 | |
Figure 1.
Simulation results for the design Uopt + Acc using the greedy algorithm with safety and efficacy acceptability rules, under four selected scenarios. For convenience, probabilities as percentages, utilities, and selection percentages are given together in the same plot. Horizontal dashed lines show the upper limit 10% for πH(dose) and lower limit 60% for πS(dose).
The numerical limits πH(x) ≤ 0.10 and πS(x) ≥ 0.60 in the propofol trial are very demanding, and they constrain the acceptable dose set severely. This is ethically appropriate for a trial where the patients are newborn infants and, although the optimal sedative dose is not known, the INSURE procedure has been very successful. Recall that adding AR to the design is motivated by the desire to reduce the chance of getting stuck at a suboptimal dose. In other structurally similar settings, different numerical values for the dose admissibility limits pU,H and pU,S may produce substantively different behavior of Uopt + Acc and Uopt + Acc + ARδ. As a hypothetical but realistic example, consider an oncology trial of an anti-cancer agent where G is a desirable early biological effect, E is tumor response, and H is toxicity. Suppose that, based on what has been seen with standard chemotherapy, pU,H = 0.25 and pU,S = 0.40 are appropriate numerical values for the dose acceptability criteria (12) and (13). Changing only these two design parameters to reflect this hypothetical oncology setting, we re-simulated Uopt + Acc and Uopt + Acc + AR2 to assess the effect of including AR in the design, under Scenarios 1 – 7. Table 5 summarizes the results. In terms of both Rselect and Rtreat, the design Uopt + Acc performs slightly better in Scenarios 1 – 3, where the optimal dose is close to the starting dose, but Uopt + Acc + AR2 is greatly superior in Scenarios 5 –7, where the optimal dose is far away from the starting dose. The general message is that including AR may be regarded as an insurance policy against extremely poor behavior in some cases, with the price being a small drop in Rselect and Rtreat in other cases.
Table 5.
Simulation study comparing Uopt + Acc and Uopt + Acc + AR2 for a hypothetical trial where the acceptability limits πH(x) ≤ .25 and πS(x) ≥ 0.40 are appropriate.
| Scenario | ||||||||
|---|---|---|---|---|---|---|---|---|
| Design | 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Uopt + Acc | Rselect | 96 | 93 | 99 | 91 | 82 | 61 | 65 |
| Rtreat | 96 | 92 | 98 | 90 | 73 | 53 | 50 | |
| % None | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| # Pats | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 60.0 | 59.3 | |
| # HEM | 4.1 | 2.7 | 2.4 | 2.8 | 2.2 | 2.5 | 2.8 | |
| # Succ | 36.7 | 40.7 | 39.4 | 33.2 | 29.0 | 22.9 | 21.7 | |
| Uopt + Acc + AR2 | Rselect | 95 | 93 | 95 | 96 | 91 | 84 | 90 |
| Rtreat | 92 | 83 | 88 | 88 | 76 | 67 | 66 | |
| % None | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| # Pats | 60.0 | 60.0 | 60.0 | 60.0 | 59.9 | 59.9 | 59.5 | |
| # HEM | 5.7 | 4.6 | 2.7 | 3.7 | 2.6 | 2.7 | 3.1 | |
| # Succ | 36.7 | 39.1 | 36.7 | 35.4 | 32.6 | 29.0 | 27.6 | |
We also evaluated our design’s performance under simpler versions of the model obtained by dropping f(Z), YG, or both from the linear term (3). We found that dropping f(Z) results in a design that escalates far too slowly or often fails to escalate when higher doses have higher utility. Dropping YG, so that neither πE(x, θ) nor πH(x, θ) depends on YG, causes the design to stop early far too often in cases where YE or YH actually are associated with YG. As a final comparator, we used the bivariate CRM (Braun, 2002) with Success as ‘efficacy’ and HEM as ‘toxicity’, since this method is model-based but simpler than our method (Supplementary Table 8). Because the bivariate CRM requires that the probability of efficacy must increase with dose, and our elicited prior has non-monotone πS(x), to implement it we adjusted the prior mean success probabilities to be nearly at over the last 4 doses rather than decreasing. For the one stopping rule allowed by the available bivariate CRM software, we chose the toxicity rule with upper limit 0.10. The simulation results show that the bivariate CRM performs much worse than our method in six scenarios (2 through 7), and about the same in the other three.
To evaluate robustness to the model assumptions, we perturbed the scenarios’ true probabilities in each of three ways: (1) mixing the true beta score distribution with a piecewise uniform score distribution in various proportions (Supplementary Table 5), (2) changing the assumed optimal Z scores for Pr(EXT) and Pr(HEM) (Supplementary Table 6), and (3) increasing the true risks by various amounts when GSS is not achieved (Supplementary Table 7). We found that, when the model was misspecified by these perturbations, in most cases the early stopping probability tended to increase, but the dose selection performance (both Rselect and Rtreat) remained relatively high.
A key issue is that the elicited neonatologists’ consensus utilities are subjective, and others may have different utilities. To address this, we carried out two sensitivity analyses. For the first, which addresses this concern by anticipating how the trial results may be interpreted by others after its completion, we evaluated the results of the trial conducted as before using the elicited consensus utility, but analyzed using each of the three alternative utilities given in Table 1. These alternative utilities numerically reflect the respective viewpoints that, compared to the consensus utility, GSS is more important, EXT is more important, or HEM is more important. Note that, for each alternative, several numerical values of U(y) differ substantially from the corresponding values of the consensus utility. For the second sensitivity analysis, we simulated the trial conducted using each alternative utility in place of the consensus utility. The results, summarized in Table 6, show that the design appears to be quite robust to changes in numerical utility values, either for trial conduct or data analysis. Thus, the trial results based on the consensus utility should be acceptable for a wide audience of other neonatologists who may have differing opinions.
Table 6.
| a. Comparison of results obtained by conducting the trial using the consensus utility with the design Uopt + Acc, but analyzing the resulting data using each of the alternative utilities. Rselect values have a gray background in Scenarios 8 and 9 because these have no acceptable doses. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scenario | ||||||||||
| Utility Used for Analysis | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Consensus | Rselect | 95 | 93 | 99 | 95 | 93 | 89 | 88 | 96 | 99 |
| Rtreat | 96 | 92 | 98 | 92 | 79 | 69 | 64 | 96 | 73 | |
| Alternative 1: | Rselect | 87 | 92 | 95 | 86 | 90 | 88 | 85 | 76 | 98 |
| GSS more important | Rtreat | 87 | 90 | 92 | 77 | 73 | 66 | 55 | 75 | 64 |
| Alternative 2: | Rselect | 96 | 93 | 99 | 97 | 95 | 90 | 90 | 97 | 98 |
| EXT more important | Rtreat | 97 | 92 | 99 | 94 | 84 | 73 | 72 | 97 | 75 |
| Alternative 3: | Rselect | 92 | 93 | 99 | 98 | 94 | 90 | 84 | 93 | 73 |
| HEM more important | Rtreat | 93 | 92 | 99 | 97 | 81 | 73 | 68 | 93 | 73 |
| b. Comparison of results if different alternative utilities are used to conduct the trial in place of the consensus utility, for the design Uopt + Acc. Rselect values have a gray background in Scenarios 8 and 9 because these have no acceptable doses. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Scenario | ||||||||||
| Utility | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Consensus | Rselect | 95 | 93 | 99 | 95 | 93 | 89 | 88 | 96 | 99 |
| Rtreat | 96 | 92 | 98 | 92 | 79 | 69 | 64 | 96 | 73 | |
| % None | 4 | 0 | 1 | 2 | 4 | 7 | 10 | 100 | 93 | |
| # Pats | 58.9 | 59.8 | 59.8 | 59.4 | 59.0 | 58.1 | 56.9 | 15.4 | 40.6 | |
| # HEM | 4.2 | 2.6 | 2.4 | 2.9 | 2.3 | 2.6 | 2.9 | 4.9 | 1.9 | |
| # Succ | 36.4 | 40.6 | 39.3 | 35.1 | 32.2 | 28.7 | 25.5 | 9.4 | 11.9 | |
| Alternative 1: | Rselect | 88 | 89 | 97 | 89 | 92 | 90 | 86 | 72 | 97 |
| GSS more important | Rtreat | 87 | 89 | 92 | 78 | 75 | 68 | 55 | 75 | 64 |
| % None | 4 | 1 | 1 | 2 | 4 | 6 | 9 | 99 | 94 | |
| # Pats | 59.1 | 59.8 | 59.7 | 59.4 | 59.0 | 58.3 | 57.2 | 15.4 | 40.3 | |
| # HEM | 4.4 | 2.8 | 2.4 | 2.9 | 2.4 | 2.7 | 2.9 | 4.8 | 1.9 | |
| # Succ | 36.8 | 40.4 | 39.3 | 35.5 | 32.6 | 29.4 | 26.0 | 9.4 | 11.8 | |
| Alternative 2: | Rselect | 96 | 94 | 99 | 96 | 95 | 89 | 90 | 98 | 96 |
| EXT more important | Rtreat | 97 | 92 | 99 | 94 | 85 | 73 | 72 | 97 | 75 |
| % None | 4 | 0 | 1 | 2 | 3 | 6 | 9 | 100 | 93 | |
| # Pats | 58.9 | 59.9 | 59.7 | 59.3 | 59.0 | 58.4 | 57.5 | 15.4 | 40.8 | |
| # HEM | 4.2 | 2.5 | 2.4 | 2.9 | 2.4 | 2.7 | 2.9 | 4.9 | 1.9 | |
| # Succ | 36.5 | 40.9 | 39.2 | 34.9 | 32.5 | 29.2 | 25.9 | 9.4 | 12.0 | |
| Alternative 3: | Rselect | 93 | 94 | 99 | 98 | 93 | 90 | 84 | 94 | 74 |
| HEM more important | Rtreat | 93 | 92 | 98 | 97 | 80 | 73 | 67 | 93 | 73 |
| % None | 4 | 1 | 1 | 2 | 3 | 6 | 9 | 100 | 94 | |
| # Pats | 59.0 | 59.9 | 59.7 | 59.2 | 59.1 | 58.3 | 57.3 | 15.4 | 40.7 | |
| # HEM | 4.1 | 2.2 | 2.4 | 2.8 | 2.3 | 2.6 | 2.9 | 4.8 | 1.9 | |
| # Succ | 36.4 | 41.3 | 39.3 | 34.8 | 32.0 | 28.6 | 25.6 | 9.4 | 11.9 | |
6. Discussion
We have presented a Bayesian model and method for choosing sedative doses in a clinical trial involving newborn babies being treated for RDS with the INSURE procedure. The design is based on elicited utilities of three binary clinical outcome variables. The proposed method sequentially optimizes doses using posterior expected utilities, with additional restrictions to exclude doses that are likely to be either unsafe or inefficacious.
Using the utility function to reduce the three-dimensional outcome (YG, YE, YH) to a single quantity may be regarded as a technical device that is ethically desirable. Comparison of Uopt to Uopt + Acc clearly shows that use of the greedy utility-based algorithm per se gives a design that is ethically unacceptable, but that this can be fixed by adding dose admissibility criteria. As shown by the hypothetical example where the limits on πU,H and πU,S were replaced with different numerical values that might be more appropriate in an oncology trial (Table 5), in some settings using AR may be preferable.
Important caveats are that a particular utility function is setting-specific, and it may not be reasonable to attempt to include outcomes having dramatically different clinical importance in the utility function. For example, in cancer trials it may not be possible to construct a utility including both death and tumor response. This is a practical and ethical limitation of this type of utility-based methodology.
Application of a complex outcome-adaptive clinical trial design presents several important practical challenges. The first step, which has been our focus here, is to establish the design, write the necessary computer program, and obtain approval from the physicians who will treat patients enrolled in the trial. Key elements in implementation include (1) establishing a database and procedure for data entry in the clinic, (2) obtaining approval of the trial protocol by the Institutional Review Boards of all participating medical centers, and (3) implementing the design using the database and computer program as patients are enrolled, treated, and evaluated. Updating the database in real time, which is critically important for outcome-adaptive designs, is challenging since it requires research nurses or data managers to enter patient outcomes in a timely manner. The required data usually are simple, however. For example, the vector (x, Z, YE, YH) is all that is required by the propofol trial design. Computing each assigned dose is straightforward, since it requires only one run of the computer program using the updated database.
Upon completion of the trial, in addition to recommending an optimal dose, inferences from the final data will include summaries of the posterior distributions of the key outcome probabilities, including πG(x, θZ), π̅E(x, θE, θZ), π̅H(x, θH, θZ), and the success event probability, πS(x, θE, θZ). This will be done by cross-tabulating posterior means and 95% credible intervals (ci’s) with dose x. This table also will include the posterior means u(x | dataN) and 95% ci’s of the utilities Ū (x | θ), which provide a set of natural summary statistics for evaluating and comparing the doses. Corresponding plots of the posteriors will provide a graphical illustration of what has been learned about each of these parametric quantities. As suggested in our sensitivity analyses, the summaries of u(x | dataN) could be repeated for each of several reasonable alternative utilities, such as those in Table 1. Finally, it also will be important to include non-model-based summaries of the empirical distribution of the sedation score Z and the count of each of event G, E, H, and S for each dose.
The propofol trial design synthesizes ideas from several areas, including phase I–II dose-finding, sequential optimization, decision analysis, Bayesian statistics, and intervention in preterm newborns. For future studies in neonatal care and similar medical settings, several potential extensions and improvements are worth mentioning. More general regimes might include multiple agents, two or more different administration schedules, or more than one cycle of therapy. Use of multi-category ordinal rather than binary outcomes would provide a more refined assessment of treatment or dose effects, and thus a more informed basis for decision-making. Accounting for effects of known prognostic covariates to optimize so-called “individualized” therapies also is highly desirable, although such a design is likely to be complex and logistically difficult, since it would require rapid evaluation of the necessary covariates and adaptive computation of the dose in real time.
Designing clinical trials in children is challenging, both technically and ethically. Successful use of this type of statistical methodology in the propofol trial may serve as proof-of-concept, and possibly provide a bridge to future pediatric trials using similar approaches.
Supplementary Material
Acknowledgments
The authors thank the editor, an associate editor, and two referees for their detailed and constructive comments. This research was supported by NIH NCI grant 2RO1 CA083932.
References
- Atkinson AC, Donev A, Tobias R. Optimal Experimental Designs, with SAS. Oxford Statistical Series. Vol. 34. London: Oxford University Press; 2006. [Google Scholar]
- Azriel D, Mandel M, Rinott Y. The treatment versus experimentation dilemma in dose-finding studies. Journal of Statistical Planning and Inference. 2011;141:2759–2768. [Google Scholar]
- Bekele BN, Shen Y. A Bayesian approach to jointly modeling toxicity and biomarker expression in a phase I/II dose-finding trial. Biometrics. 2004;60:343–354. doi: 10.1111/j.1541-0420.2005.00314.x. [DOI] [PubMed] [Google Scholar]
- Berger, James O. Statistical Decision Theory and Bayesian Analysis. 2nd Edition. New York: Springer-Verlag; 1985. [Google Scholar]
- Bohlin K, Gudmundsdottir T, Katz-Salamon M, Jonsson B, Blennow M. Implementation of surfactant treatment during continuous positive airway pressure. Journal of Perinatology. 2007;27:422–427. doi: 10.1038/sj.jp.7211754. [DOI] [PubMed] [Google Scholar]
- Brook RH, Chassin MR, Fink A, Solomon DH, Kosecoff J, Park RE. A method for the detailed assessment of the appropriateness of medical technologies. International Journal of Technology Assessment and Health Care. 1986;2:53–63. doi: 10.1017/s0266462300002774. [DOI] [PubMed] [Google Scholar]
- Bornkamp B, Bretz F, Dette H, Pinheiro J. Response-adaptive dose-finding under model uncertainty. Annals of Applied Statistics. 2011;5:1611–1631. [Google Scholar]
- Braun TM. The bivariate continual reassessment method: extending the CRM to phase I trials of two competing outcomes. Controlled Clinical Trials. 2002;23:240–256. doi: 10.1016/s0197-2456(01)00205-7. [DOI] [PubMed] [Google Scholar]
- Chen Y, Smith BJ. Adaptive group sequential design for phase II clinical trials: A Bayesian decision theoretic approach. Statistics in Medicine. 2009;28:3327–3362. doi: 10.1002/sim.3711. [DOI] [PubMed] [Google Scholar]
- Chevret S, editor. Statistical Methods for Dose-Finding Experiments. West Sussex, UK: John Wiley and Sons; 2006. [Google Scholar]
- Cheung Y-K. Dose Finding by the Continual Reassessment Method. New York: Chapman and Hall/CRC Press; 2011. [Google Scholar]
- Christen J, Muller P, Wathen K, Wolf J. Bayesian randomized clinical trials: a decision-theoretic sequential design. Canadian Journal of Statistics. 2004;32:387–402. [Google Scholar]
- Dette H, Bretz F, Pepelyshev A, Pinhiero J. Optimal designs for dose-finding studies. J. American Statistical Association. 2008;103:1225–1237. [Google Scholar]
- Fedorov V, Leonov SL. Optimal Design of dose response expriments: A model-oriented approach. Drug Information Journal. 2001;35:1373–1383. [Google Scholar]
- Fedorov V. Optimal experimental design. Wiley Interdisciplinary Reviews: Computational Statistics. 2010;2(5):581589. [Google Scholar]
- Ferrari SLP, Cribari-Neto F. Beta regression for modelling rates and proportions. Journal of Applied Statistics. 2004;31(7):799815. [Google Scholar]
- Gittins JC. Bandit processes and dynamic allocation indices. Journal of the Royal Statistical Society, Series B. 1979;41:148–177. [Google Scholar]
- Houede N, Thall PF, Nguyen H, Paoletti X, Kramar A. Utility-based optimization of combination therapy using ordinal toxicity and efficacy in phase I/II trials. Biometrics. 2010;66:532–540. doi: 10.1111/j.1541-0420.2009.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatology. 2008;28:55–60. doi: 10.1038/sj.jp.7211861. [DOI] [PubMed] [Google Scholar]
- McCullagh P. Regression models for ordinal data (with discussion) J. Royal Statistical Society, Series B. 1980;42:109142. [Google Scholar]
- McCullagh P, Nelder JA. Generalized Linear Models. 2nd Edition. New York: Chapman and Hall; 1989. Evaluating the impact of prior assumptions in Bayesian biostatistics. Statistics in Biosciences2 1–17. [Google Scholar]
- Morita S, Thall PF, Mueller P. Determining the effective sample size of a parametric prior. Biometrics. 2008;64:595–602. doi: 10.1111/j.1541-0420.2007.00888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch SD, Cohen AT. Propofol-infusion syndrome in children. Lancet. 1999;353(9169):2074–2075. doi: 10.1016/s0140-6736(05)77897-1. [DOI] [PubMed] [Google Scholar]
- Nelsen RB. An Introduction to Copulas. Lecture Notes in Statistics. Vol. 139. New York: Springer-Verlag; 1999. [Google Scholar]
- OQuigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase 1 clinical trials in Cancer. Biometrics. 1990;46:3348. [PubMed] [Google Scholar]
- O'Quigley J, Hughes MD, Fenton T. Dose-finding designs for HIV studies. Biometrics. 2001;57:1018–1029. doi: 10.1111/j.0006-341x.2001.01018.x. [DOI] [PubMed] [Google Scholar]
- Oron AP, Hoff PD. Small-sample behavior of novel phase I cancer trial designs. Clinical Trials. 2013;10:63–80. doi: 10.1177/1740774512469311. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bornkamp B, Bretz F. Design and analysis of dose finding studies combining multiple comparisons and modeling procedures. Journal of Biopharmaceutical Statistics. 2006;16:639656. doi: 10.1080/10543400600860428. [DOI] [PubMed] [Google Scholar]
- Robbins H. Some aspects of the sequential design of experiments. Bulletin of the American Mathematical Society. 1952;58:527535. [Google Scholar]
- Robert CP, Cassella G. Monte Carlo Statistical Methods. New York: Springer; 1999. [Google Scholar]
- Sammartino M, Garra R, Sbaraglia F, Papacci P. Propofol overdose in a preterm baby: may propofol infusion syndrome arise in two hours? Paediatr Anaesth. 2010;20(10):973–974. doi: 10.1111/j.1460-9592.2010.03395.x. [DOI] [PubMed] [Google Scholar]
- Simas AB, Barreto-Souza W, Rocha AV. Improved estimators for a general class of beta regression models. J. Computational Statistics and Data Analysis. 2010;54:348–366. [Google Scholar]
- Stallard N, Thall PF, Whitehead J. Decision theoretic designs for phase II clinical trials with multiple outcomes. Biometrics. 1999;55:971–977. doi: 10.1111/j.0006-341x.1999.00971.x. [DOI] [PubMed] [Google Scholar]
- Stallard N, Thall PF. Decision-theoretic designs for pre-phase II screening trials in oncology. Biometrics. 2001;57:1089–1095. doi: 10.1111/j.0006-341x.2001.01089.x. [DOI] [PubMed] [Google Scholar]
- Stevens TP, Harrington EW, Blennow M, Soll RF. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Syst Rev. 2007;4 doi: 10.1002/14651858.CD003063.pub3. CD003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RS, Barto AG. Reinforcement Learning: An Introduction. Cambridge, MA: MIT Press; 1998. [Google Scholar]
- Thall PF, Nguyen HQ. Adaptive randomization to improve utility-based dose- finding with bivariate ordinal outcomes. J Biopharmaceutical Statistics. 2012;22:785–801. doi: 10.1080/10543406.2012.676586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thall PF, Russell KT. A strategy for dose finding and safety monitoring based on efficacy and adverse outcomes in phase I/II clinical trials. Biometrics. 1998;54:251–264. [PubMed] [Google Scholar]
- Thall PF, Szabo A, Nguyen HQ, Amlie-Lefond CM, Zaidat OO. Optimizing the concentration and bolus of a drug delivered by continuous infusion. Biometrics. 2011;67:1638–1646. doi: 10.1111/j.1541-0420.2011.01580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaegen J, Naulaers G, Van Huffel S, Vanhole C, Allegaert K. Cerebral and systemic hemodynamic effects of intravenous bolus administration of propofol in neonates. Neonatology. 2010;98:57–63. doi: 10.1159/000271224. [DOI] [PubMed] [Google Scholar]
- Verder H, Robertson B, Greisen G, et al. Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. New England J. Medicine. 1994;331:10511055. doi: 10.1056/NEJM199410203311603. [DOI] [PubMed] [Google Scholar]
- Wathen JK, Thall PF. Bayesian adaptive model selection for optimizing group sequential clinical trials. Statistics in Medicine. 2008;27:5586–5604. doi: 10.1002/sim.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA. Extra binomial variation in logistic linear models. Applied Statistics. 1982;31(2):144148. [Google Scholar]
- Zohar S, Chevret S. Recent developments in adaptive designs for phase I/II dose-finding studies. Journal of Biopharmaceutical Statistics. 2007;17:1071–1083. doi: 10.1080/10543400701645116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.