Abstract
Introduction
Platycodi radix is a radish used in food, such as Korean kimchi, and has been shown to cause weight loss in rodents. Platycodin D is considered its active ingredient and has been shown to inhibit lipases. The authors hypothesized that platycodi radix and the platycodin D it contains inhibit angiogenesis; another mechanism for weight loss.
Methods
This study tested platycodi radix extract, platycodin D, and an extract of platycodi radix standardized to platycodin D for their ability to inhibit angiogenesis in a human adipose tissue assay. This study treated five healthy volunteers, orally, with platycodi radix extract standardized to 414 mg of platycodin D. Three volunteers were treated under fasting conditions, one volunteer with a 400 kcal meal, and one volunteer treated with a placebo. Blood was drawn over 5 hours to compare serum inhibition of the human adipose tissue angiogenesis.
Results
Platycodin radix extract, platycodin D, and platycodi radix extract standardized to platycodin D all inhibited angiogenesis. The three volunteers who consumed platycodi radix extract standardized to 414 mg of platycodin D had a 25.76% reduction in angiogenesis from baseline at 60 minutes (P<0.002), and had a statistically significant reduction in angiogenesis from 30 to 240 minutes (P<0.05 to P<0.002). The placebo decreased angiogenesis by 5.6% between 30 and 240 minutes, compared with 17.8% by the extract. The meal delayed absorption by approximately 3.5 hours.
Conclusion
Platycodi radix extract standardized to platycodin D inhibited angiogenesis in human volunteers, and paves the way for a dose-response study and a human clinical obesity trial.
Keywords: angiogenesis, dietary herbal supplement, medicinal food, platycodi radix, platycodin D
INTRODUCTION
Being overweight and obesity are increasing in prevalence around the world.1 Being overweight, defined as body mass index (BMI) above 25 kg/m2, now affects 68% of the US adult population, and obesity, defined as a BMI over 30 kg/m2, affects 34%.2 Obesity increased from 15% in 1980 to 30.9% in 2000.3 An increase in the prevalence of obesity is followed in 10 years by an increase in the prevalence of diabetes.4 The prevalence of diabetes is rising in the USA,5 and it affects ethnic minorities and the economically disadvantaged disproportionately.6 Both diabetes and obesity cost the USA approximately $100 billion per year, or more.7,8 Thus, being overweight and obesity represent one of the most concerning public health problems of our time.
The treatments for being overweight and obese have given disappointing results. The mainstay of treatment is a calorie-restricted diet combined with a lifestyle change program.9 This type of treatment induces a 5%–10% weight loss; however, one-third of the weight is regained in one year and 50% of subjects have returned to their baseline weight by 5 years.10 Medications are available for the treatment of obesity, but give about the same degree of weight loss as diet and lifestyle change.11 Treating obesity through surgery gives a sustainable weight loss of 30% of initial body weight, but is appropriate only for those who are severely obese.12 Thus, there is a clear need for better obesity treatments.
Platycodi radix is the name for the roots of Platycodon grandiflorum (Jacq.) A.DC., a plant that is also known by the common name, “balloon flower.” Platycodi radix is used as a food, such as in recipes for Korean kimchi, as an herbal medicine, and as a dietary herbal supplement. It is used as an herbal medicine to treat colds, and there is a belief in Korea that it can prevent obesity.13,14
Han et al. demonstrated that an aqueous extract of platycodi radix inhibited lipase activity in vitro, reduced the elevation of plasma triglycerides that occurs 2–4 hours after a triolean emulsion is given orally, and localized the lipase inhibitory activity to the total saponin fraction.15 The same group also showed that the body weight of mice fed a high-fat diet, and 5% weight per volume (w/v) aqueous extract of platycodi radix, was reduced compared with the high-fat diet-fed controls.15 The group then tested the crude saponin fraction of platycodi radix in mice fed a high-fat diet at 10 or 30 g/kg of crude saponins compared with a high-fat diet-fed control. Mice eating the high-fat diet, plus 30 g/kg of crude saponins from platycodi radix, gained 17% less weight over 9 weeks compared with the high-fat diet-fed control. Platycodin D was shown to be the active saponin, which inhibited pancreatic lipase in vitro and, when given orally to mice at a dose of 244 mg/kg, inhibited the rise in post-prandial blood triglycerides.16 Zhou et al. treated rats with a high-fat diet and 35 or 70 mg/kg of platycodi radix saponins, or a high-fat diet-fed control. Both groups given the platycodi radix saponins gained 13% less weight than the high-fat diet-fed controls. The low-density lipoprotein (LDL) cholesterol and triglycerides were also decreased compared with the high-fat diet-fed controls, and there was a dose-dependent 2.1–3.2-fold increase in stool triglyceride.17
Adipose tissue has been shown to be regulated through the vasculature. Ob/ob mice and other genetic rodent obesity models given angiogenesis inhibitors experienced a reduction in adipose tissue mass.18 TNP-470, a fumagillin derivative that is an inhibitor of methionine aminopeptidase-type 2 and angiogenesis, returned weight of leptin-deficient mice to the level of the C57B6 mouse with normal leptin levels, but not below.18,19 TPN-470 increased metabolic rate, increased fat oxidation, increased endothelial apoptosis, and appeared to act on the mature vessels in the fat tissue.18 A subsequent study has extended these observations to murine diet induced obesity.20
This study developed an in vitro human adipose tissue assay to assess angiogenesis.21 This assay has two major advantages over other angiogenesis assays. First, it can assess the “angiogenic switch” in which a quiescent vessel is converted into a proliferating vessel.22 Secondly, it uses human tissue, which is predictive of what happens in humans, while non-human assays may not be.23 The reduction of weight gain in the Zhou et al. study of Platycodi radix saponins seemed larger than what could be attributed to lipase inhibition alone. The authors postulated that, in addition to lipase inhibition, platycodi radix may inhibit angiogenesis.
METHODS
Study 1
An aqueous extract of platycodi radix saponins at 1% w/v was tested in this study’s human adipose tissue assay. This method has been described in detail previously.24 Briefly however, subcutaneous adipose tissue was removed from patients having cosmetic surgical procedures. The fat was placed directly into sterile assay media (Medium 199 [GibcoBRL, Gaithersburg, MD, USA]) transported directly to the laboratory from the surgery suite in the sterile container, and processed under a laminar flow hood. The tissue was cut into fragments approximately 1 mm thick and 2 mm in diameter. These fragments were placed in 96-well plates containing 4 µL human thrombin solution (0.05 IU in 4 µL per well) and covered with 100 µL clotting media (3 mg/mL fibrinogen; Sigma Chemical Co., St Louis, MO, USA), 0.5% epsilon-amino caproic acid (Sigma Chemical Co.) in angiogenesis media containing 100 U/mL penicillin, 100 U/mL streptomycin sulfate, and 2.5 µg/mL amphotericin beta in Medium 199 (GibcoBRL). The mixture was allowed to clot by incubation in 6% CO2, in 94% air, at 37°C in a humidified incubator. After the media had gelled overnight, the fat-containing clot was supplemented with 100 µL angiogenesis media containing 20% fetal bovine serum (GibcoBRL). The total volume of each well was 200 µL. There were 30 replicates for the 1% w/v platycodi radix saponins and the media control. The angiogenesis media with 20% fetal bovine serum were replaced every 48 hours and appropriate concentrations of fresh platycodi radix saponins, or media control with 20% fetal bovine serum, were added. Wells were evaluated for the angiogenic response as described by Greenway et al.21
An observer, unbiased to the treatment protocols, evaluated the angiogenic response using a semi-quantitative visual rating scale.25 Essentially, the tissue was viewed under an inverted microscope, visually divided into four quadrants and each quadrant was given a numeric score, from 0 to 4, based on the neovessel’s length, density, and percentage of the quadrants’ circumference involved with the angiogenic response. Numeric results from the four quadrants were summed and expressed as an angiogenic index ranging from 0 (no neovessels apparent in any quadrant) to 16 (highly vascularized in all four quadrants). This rating scale has been validated by Hornick et al. using multiple independent observers.25 The extract of platycodi radix saponins at 1% w/v was tested in the assay. Platycodin D was isolated from the mixture of other platycodi radix saponins and was tested in the assay over a concentration range from 10−7 M to 10−3 M. The platycodi radix saponins standardized to its platycodin D content was also tested in the assay.
Study 2
Five normal, healthy volunteers between the ages of 18 and 65 years with a BMI between 18 kg/m2 and 35 kg/m2 were included in this study. Subjects taking regular medication other than oral contraceptives and women that were pregnant, nursing, or who refused to avoid pregnancy during the study were excluded. During screening all subjects had a medical history, physical examination, electrocardiogram, a fasting chemistry panel (glucose, creatinine, potassium, uric acid, albumin, calcium, magnesium, creatine phosphokinase, alanine-leucine transaminase, alkaline phosphatase, iron, cholesterol, triglycerides, high-density lipoprotein [HDL] cholesterol, and LDL cholesterol), and a complete blood count (CBC) (hemoglobin, hematocrit, mean cell volume, platelet count, white blood cell count, granulocyte number, neutrophil number, eosinophil number, and basophil number). Subjects who passed screening reported for the study visit in the morning, after having nothing to eat or drink, except for water, from 9:00 pm the previous night. Subjects had an intravenous line placed from which 50 mL of blood was drawn at times 0, 30, 60, 120, 180, 240, and 300 minutes.
After the baseline blood draw, three subjects took platycodi radix extract standardized to 414 mg of platycodin D orally, one subject took a placebo orally, and one subject took platycodi radix extract standardized to 414 mg of platycodin D with a 400 kcal omelet; made from egg, butter, flour, and dried onions (40% of energy as fat, 40% as carbohydrate, and 29% as protein). Serum was separated from the blood, drawn during the test day, and frozen at –70°C until analyzed in the angiogenesis assay described in study 1; except the 20% fetal bovine serum was replaced with 80% subject serum. Subjects returned on the morning following their test day, having had nothing to eat or drink except water from the previous night. Subjects had blood drawn for a chemistry-15 panel, a CBC, an electrocardiogram, and subjects were questioned about any adverse events.
Ethical Approval and Informed Consent
The clinical study was approved by the Pennington Biomedical Research Center Institutional Review Board and each subject signed a written informed consent.
Statistical Analysis
The mean and standard deviation of the percent reduction in angiogenesis from baseline at each time point was compared with baseline by t-test. The time course of the inhibition of angiogenesis was described, as was the effect of food and a placebo on angiogenesis.
RESULTS
Study 1
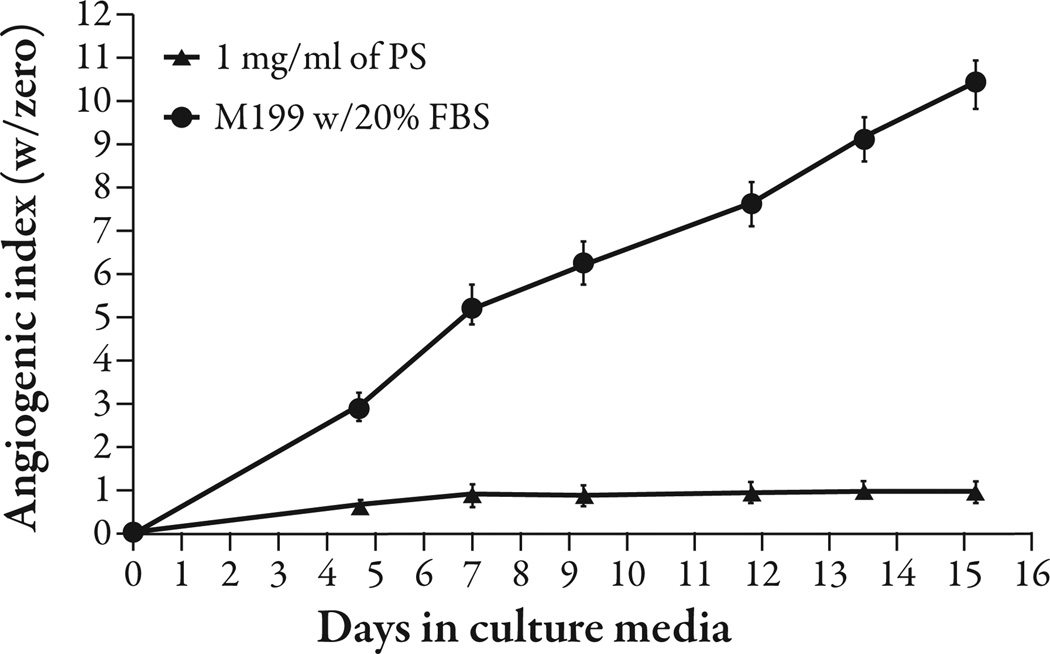
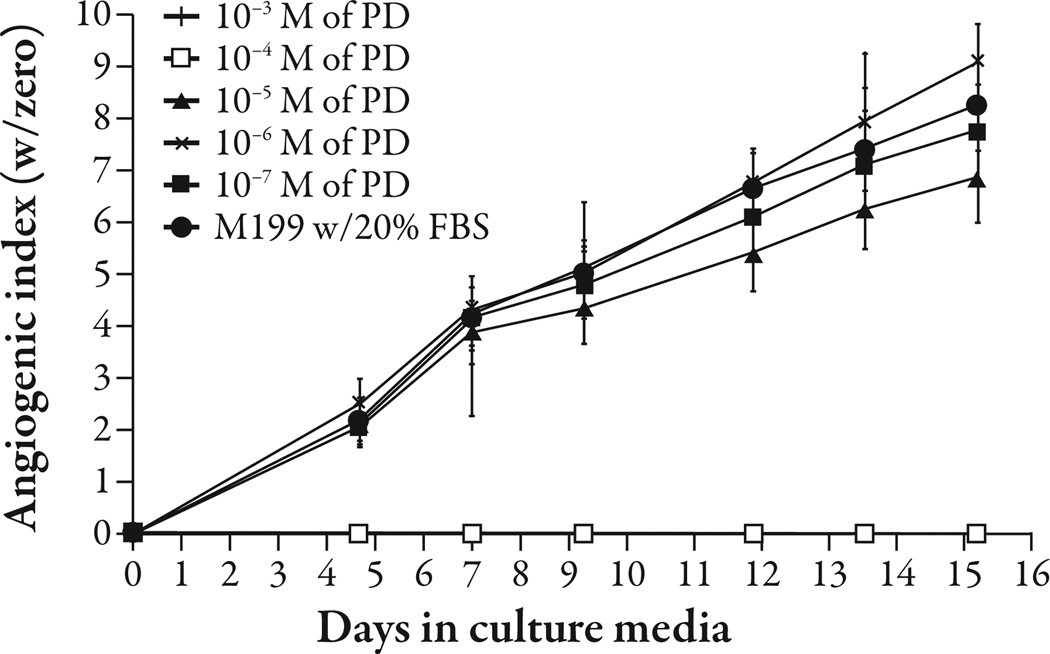
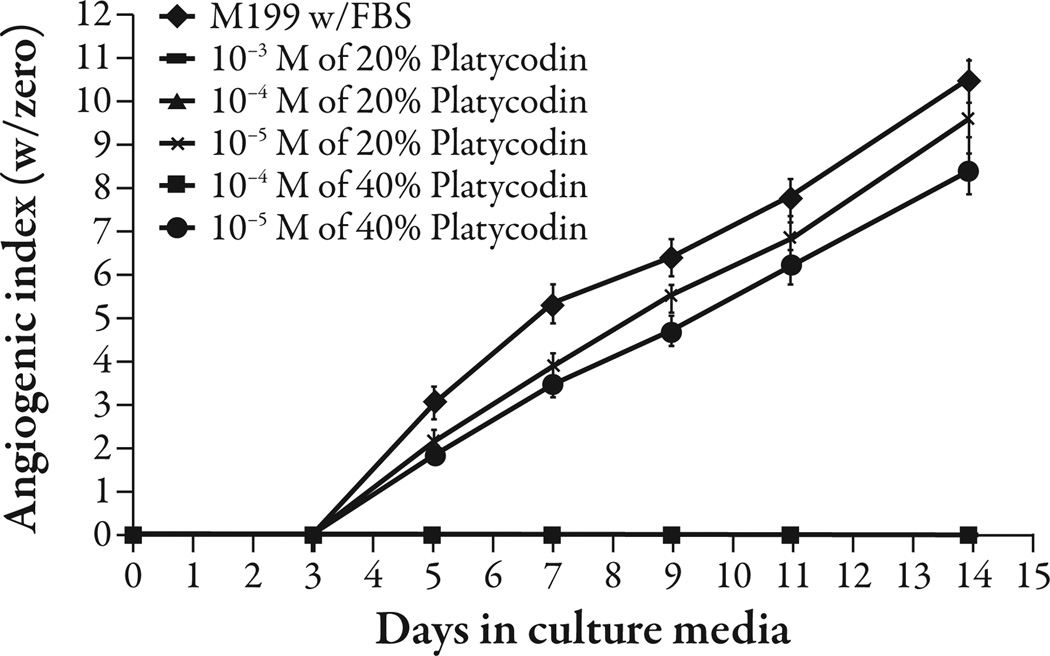
Platycodi radix saponins at 1% w/v concentration inhibited angiogenesis (P<0.001) (Figure 1). Platycodin D gave complete inhibition of angiogenesis at 10−4 M (P<0.001) and appeared to give partial inhibition at 10−5 M; however, the difference was not statistically significant (Figure 2). The platycodi radix extract standardized to platycodin D gave complete inhibition at a 10−4 M concentration of platycodin D (P<0.001) (Figure 3).
Figure 1.
Platycodin saponins (PS) from platycodi radix inhibited angiogenesis at 1% weight per volume (w/v) in a human adipose tissue assay compared with the media control (P<0.001). FBS=fetal bovine serum.
Figure 2.
Platycodin D (PD) was tested in the human adipose tissue angiogenesis assay at concentrations between 10−7 M and 10−3 M. Concentrations of 10−4 M and above completely inhibited angiogenesis.
Figure 3.
Platycodi radix extract standardized to platycodin D was tested at two concentrations, 20% and 40% platycodin D. Te extracts were tested at their platycodin D contents from 10−3 M to 10−5 M. Te extracts with 10−4 M and above completely inhibited angiogenesis compared with the media control (P<0.001). FBS=fetal bovine serum.
Study 2
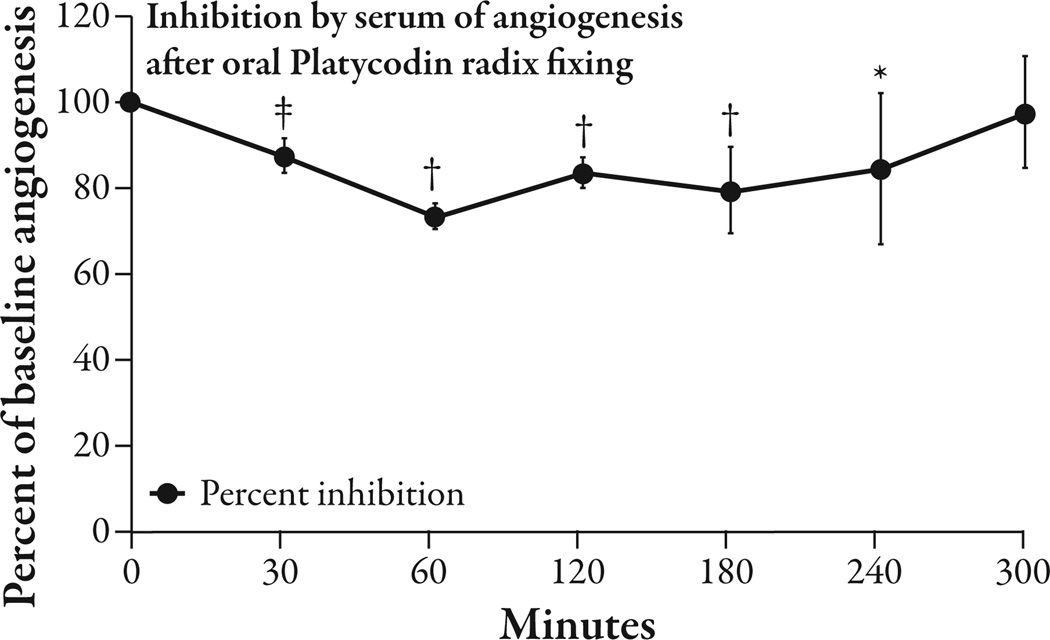
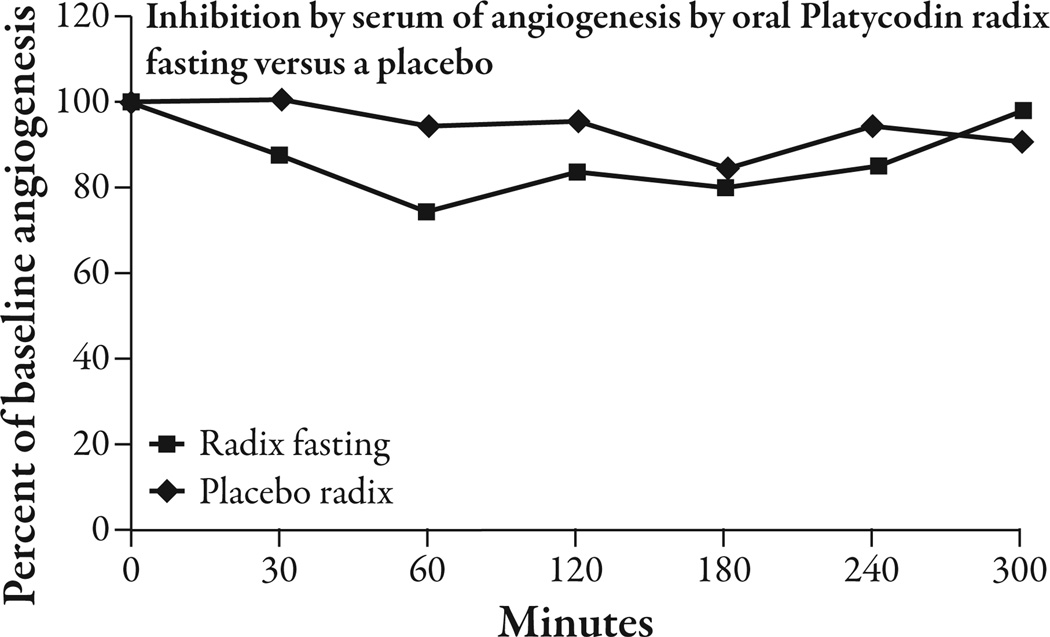
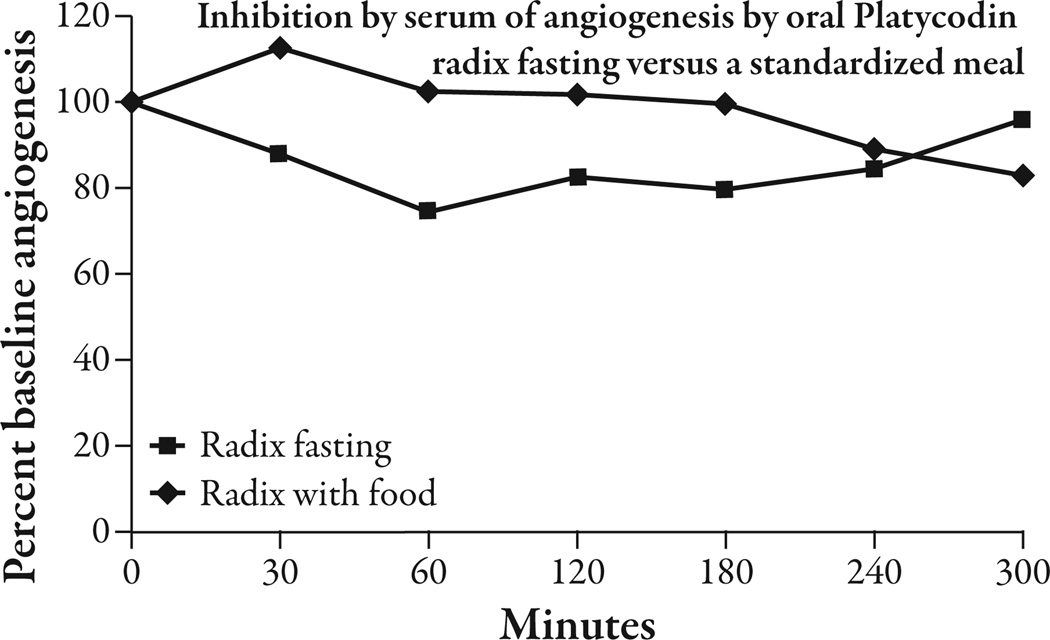
The five healthy volunteers had an average BMI of 23.1 ± 2.2 kg/m2 (mean ± SEM) and an average weight of 63.1 ± 5.9 kg. The three subjects who consumed oral platycodi radix extract standardized to 414 mg of platycodin D had a peak reduction of 25.76% ± 4.93 (P<0.002) in angiogenesis from baseline at 60 minutes. Between 30 minutes and 240 minutes there was a significant reduction of angiogenesis from baseline that varied from 12% to 25.7% (P<0.05 to P<0.002) and averaged 17.8% (Figure 4). The average reduction of angiogenesis in the placebo condition between 30 and 240 minutes was 5.6%, compared with 17.8% in the platycodi radix extract fasting condition (Figure 5). As there was only one placebo-treated subject, statistics could not be done on this comparison. There was only one subject that took the platycodi radix with food. This subject seemed to have a delayed response by approximately 3.5 hours, and at 5 hours, when the test ended, the reduction in angiogenesis was still increasing (Figure 6). One subject had moderate nausea and some heartburn after taking the platycodi radix extract and another complained of a mild toothache. The nausea and heartburn may have been related to the extract, but the toothache was concluded not to be.
Figure 4.
Te serum drawn at time points afer three fasting volunteers orally took platycodi radix extract standardized to 414 mg of platycodin D was tested in the human adipose tissue angiogenesis assay. Te peak reduction in angiogenesis was 25.76% at the 60 minute time-point (P<0.002). Angiogenesis was reduced by an average of 17.8% between 30 and 240 minutes (P<0.05 to P<0.002). n=3; *P<0.05; †P<0.01; ‡P<0.002.
Figure 5.
Te placebo reduced angiogenesis by an average of 5.6% between 30 minutes and 240 minutes, compared with 17.8% by the platycodi radix extract standardized to platycodin D.
Figure 6.
Taking the platycodi radix standardized to platycodin D orally with a 400 kcal meal appeared to delay the absorption of the extract by approximately 3.5 hours.
There were no other adverse events during the study and all five subjects completed the trial. The laboratory work, consisting of the chemistry panel and CBC (the same as those described as being drawn at screening) drawn on the day following the test day, was normal, as was the case at screening, except for the anticipated drop in hemoglobin and hematocrit related to the serial blood draws performed during the test.
DISCUSSION
This pilot study demonstrated that platycodi radix, and the platycodin D it contains, are inhibitors of angiogenesis. There was a statistically significant reduction of angiogenesis from baseline following uptake of a 414 mg oral dose of platycodi radix from 30 minutes to 240 minutes. Thus, it appears that the platycodi radix extract standardized to its platycodin D content would need to be dosed three times a day, as the inhibition of angiogenesis had returned to baseline by 5 hours. The standardized platycodi radix extract gave more inhibition of angiogenesis than a placebo, and food seemed to delay absorption by approximately 3.5 hours. There were no serious adverse events, which is not unexpected, as platycodi radix is consumed as a food.
The bar for safety of obesity drugs has been very high, which is emphasized by the recent rejection by the Food and Drug Administration (FDA) of lorcaserin and two combination drugs, phentermine-topiramate and bupropion-naltrexone, in addition to the removal of sibutramine from the market for safety reasons. In the interest of maximizing the safety of obesity therapeutics, the authors have looked for foods with folklore suggesting their efficacy in treating obesity, and show inhibitory activity in the study’s human adipose tissue angiogenesis assay. The authors were able to show that black raspberry, a member of the Rubus family, inhibited angiogenesis and that gallic acid was partially responsible for this effect.26.27 Number Ten (NT) is an herbal combination of rhubarb, ginger, astragalus, red sage, and turmeric that has been demonstrated to cause dramatic weight loss in rodents, but when taken in humans resulted in diarrhea.28,29 The authors attempted to combine smaller amounts of NT with gallic acid, but this was ineffective in achieving human weight loss. It appeared that the transport mechanism across the gut was saturated at a level lower than what was required to inhibit angiogenesis.30
When finding that platycodin D was effective in inhibiting angiogenesis, the authors were concerned that, despite its demonstrated weight loss efficacy in rodents, it would not be bioavailable in humans. The authors performed this pilot pharmacokinetic study, which confirmed that platycodi radix extract standardized to platycodin D is absorbed, and the human serum inhibits angiogenesis in the study’s ex vivo human angiogenesis assay. The authors intend to construct a dose-response curve and plan to perform a human trial for the treatment of obesity.
The primary uses of angiogenesis in adulthood are for menstrual periods, fetal development, and wound healing. Thus, the potential for toxicity should be low, and the fact that platycodi radix is used as a food is further evidence of its safety. Having lighter menstrual periods may be an advantage during obesity treatment, as pregnancy is not recommended during weight loss, and an inhibitor of angiogenesis can always be stopped if a serious injury, or the need for surgery, should occur. The authors tested gallic acid, another food component absorbed in rodents, and was found to cause weight loss.31 The authors found no evidence of any reproductive toxicity at 430 mg/kg per day, or less, giving further reassurance of the safety of angiogenic inhibitors that are components of food, and anticipate a similar experience with platycodin D.32
One could consider the absence of measuring blood markers of angiogenesis, such as circulating endothelial cells, vascular endothelial growth factor (VEGF), and VEGF splice variants, a weakness of this study. Clearly, this information would have been interesting to have, and with this positive pilot data can be obtained in future studies. Previous studies using this assay, however, have shown that VEGF alone is insufficient to initiate angiogenesis, and antibodies that target only one element of the VEGF pathway fail to consistently inhibit angiogenesis.33 Angiogenesis inhibitors, such as PTK787, a tyrosine kinase inhibitor that targets multiple VEGF and non-VEGF receptors, profoundly inhibit physiologic and pathologic angiogenesis.33 Thus, for a pilot study, the authors felt that testing individual angiogenic factors was premature.
CONCLUSION
This study has demonstrated that platycodi radix and its active ingredient, platycodin D, which generated weight loss in rodents, are active in inhibiting angiogenesis in vitro. The orally active feasibility in humans was demonstrated by the inhibitory effect of serum, from treated volunteers, on angiogenesis in a human adipose tissue assay. The next steps will be to define a dose-response effect of the extract, in a similar pharmacokinetic study, using a larger number of subjects before testing the standardized extract in human clinical trials to induce weight loss.
ACKNOWLEDGMENTS
The authors would like to thank Drs James Wade, Ann Reilly, Michael Teague, their staff, and patients for donating the adipose tissue used in the assay. The mechanistic in-vitro studies were partially supported by a CNRU Center Grant # 1P30 DK072476 entitled “Nutritional Programming: Environmental and Molecular Interactions,” sponsored by NIDDK. The study was also partially supported by an unrestricted grant from General Nutrition Corporation. Dr Greenway is a paid consultant to General Nutrition Corporation. Dr Gimble is a consultant to ATRM & Mentor (subsidiaries of Johnson & Johnson), a collaborator with Cognate Bioservices, Vesta Therapeutics and Zen-Bio and a co-founder of LaCell LLC and Articel in addition to being an inventor on patents relating to the production of human adipose stem cells for regenerative medical applications. Dr Greenway is the guarantor for this article, and takes responsibility for the integrity of the work as a whole.
Contributor Information
Emma M. Twiner, Pennington Biomedical Research Center, Louisiana State University System, 6400 Perkins Road, Baton Rouge, LA 70808, USA
Zhijun Liu, Louisiana State University Agricultural Center, Baton Rouge, LA 70803, USA.
Jeffrey Gimble, Pennington Biomedical Research Center, Louisiana State University System, 6400 Perkins Road, Baton Rouge, LA 70808, USA.
Ying Yu, Pennington Biomedical Research Center, Louisiana State University System, 6400 Perkins Road, Baton Rouge, LA 70808, USA.
Frank Greenway, Email: frank.greenway@pbrc.edu, Pennington Biomedical Research Center, Louisiana State University System, 6400 Perkins Road, Baton Rouge, LA 70808, USA.
REFERENCES
- 1.Low S, Chin MC, Deurenberg-Yap M. Review on epidemic of obesity. Ann Acad Med Singapore. 2009;38:57–59. [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA. Obesity: a time bomb to be defused. Lancet. 1998;352:160–161. doi: 10.1016/S0140-6736(98)22029-0. [DOI] [PubMed] [Google Scholar]
- 5.Skyler JS, Oddo C. Diabetes trends in the USA. Diabetes Metab Res Rev. 2002;18(suppl. 3):S21–S26. doi: 10.1002/dmrr.289. [DOI] [PubMed] [Google Scholar]
- 6.Ferdinand KC, Clark LT. The epidemic of diabetes mellitus and the metabolic syndrome in African Americans. Rev Cardiovasc Med. 2004;5(suppl. 3):S28–S33. [PubMed] [Google Scholar]
- 7.Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6:97–106. doi: 10.1002/j.1550-8528.1998.tb00322.x. [DOI] [PubMed] [Google Scholar]
- 8.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 9.National Institutes of Health, National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults-the evidence report. Obes Res. 1998;6(suppl. 2):51S–209S. [Published erratum appears in Obes Res. 1998:6:464] [PubMed] [Google Scholar]
- 10.Wadden TA, Butryn ML, Byrne KJ. Effcacy of lifestyle modification for long-term weight control. Obes Res. 2004;12(suppl.):151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 11.Bray GA, Greenway FL. Current and potential drugs for treatment of obesity. Endocr Rev. 1999;20:805–875. doi: 10.1210/edrv.20.6.0383. [DOI] [PubMed] [Google Scholar]
- 12.Greenway FL. Surgery for obesity. Endocrinol Metab Clin North Am. 1996;25:1005–1027. doi: 10.1016/s0889-8529(05)70367-4. [DOI] [PubMed] [Google Scholar]
- 13.Pharmacopoeia of the People’s Republic of China (English ed.) Guangzhou: Guangdong Science and Technology Press; [Google Scholar]
- 14.Method for producing platycodi radix root kimchi. [Accessed December 5, 2010]; Available at http://www.sumobrain.com/patents/jp/Method-producing-platycodi-radix-root/JP2002186416.html.
- 15.Han LK, Xu BJ, Kimura Y, Zheng Y, Okuda H. Platycodin radix affects lipid metabolism in mice with high fat diet-induced obesity. J Nutr. 2000;130:2760–2764. doi: 10.1093/jn/130.11.2760. [DOI] [PubMed] [Google Scholar]
- 16.Han LK, Zheng YN, Xu BJ, Okuda H, Kimura Y. Saponins from platycodin radix ameliorate high fat diet-induced obesity in mice. J Nutr. 2002;132:2241–2245. doi: 10.1093/jn/132.8.2241. [DOI] [PubMed] [Google Scholar]
- 17.Zhao HL, Sims JS, Shim SH, Ha YW, Kang SS, Kim Ys. Antiobese and hypolipidemic effects of platycodin saponins in diet-induced obese rats: evidence for lipase inhibition and calorie restriction. Int J Obes. (London) 2005;29:983–990. doi: 10.1038/sj.ijo.0802948. [DOI] [PubMed] [Google Scholar]
- 18.Rupnick MA, Panigrahy D, Zhang CY, et al. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci. 2002;99:10703–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lijnen HR, Frederix L, Van Hoef B. Fumagillin reduces adipose tissue formation in murine models of nutritionally induced obesity. Obesity (Silver Spring) 2010;18:2241–2246. doi: 10.1038/oby.2009.503. [DOI] [PubMed] [Google Scholar]
- 20.Bråkenhielm E, Cao R, Gao B, et al. Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res. 2004;94:1579–1588. doi: 10.1161/01.RES.0000132745.76882.70. [DOI] [PubMed] [Google Scholar]
- 21.Greenway FL, Liu Z, Yu Y, et al. An assay to measure angiogenesis in human fat tissue. Obes Surg. 2007;17:510–515. doi: 10.1007/s11695-007-9089-z. [DOI] [PubMed] [Google Scholar]
- 22.Jung P, Siegrist B, Wade MR, Anthony CT, Woltering EA. Inhibition of human angiogenesis with heparin and hydrocortisone. Angiogenesis. 2001;4:175–186. doi: 10.1023/a:1014089706107. [DOI] [PubMed] [Google Scholar]
- 23.Jung SP, Siegrist B, Hornick CA, et al. Effect of human recombinant Endostatin protein on human angiogenesis. Angiogenesis. 2002;5:111–118. doi: 10.1023/a:1021540328613. [DOI] [PubMed] [Google Scholar]
- 24.Woltering EA, Lewis JM, Maxwell PJ, IV, et al. Development of a novel in vitro human tissue-based angiogenesis assay to evaluate the effect of antiangiogenic drugs. Ann Surg. 2003;237:790–798. doi: 10.1097/01.SLA.0000072111.53797.44. discussion 798–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornick CA, Myers A, Sadowska-Krowicka H, Anthony CT, Woltering EA. Inhibition of angiogenic initiation and disruption of newly established human vascular networks by juice from Morinda citrifolia (noni) Angiogenesis. 2003;6:143–149. doi: 10.1023/B:AGEN.0000011800.04507.4b. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Schwimer J, Liu D, Greenway FL, Anthony CT, Woltering EA. Black raspberry extract and fractions contain angiogenesis inhibitors. J Agric Food Chem. 2005;53:3909–3915. doi: 10.1021/jf048585u. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Schwimer J, Liu D, et al. Gallic acid is partially responsible for the antiangiogenic activities of Rubus leaf extract. Phytother Res. 2006;20:806–813. doi: 10.1002/ptr.1966. [DOI] [PubMed] [Google Scholar]
- 28.York DA, Thomas S, Greenway FL, Liu Z, Rood JC. Effect of an herbal extract Number Ten (NT) on body weight in rats. Chin Med. 2007;2:10. doi: 10.1186/1749-8546-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenway FL, Liu Z, Martin CK, et al. Safety and efficacy of NT, an herbal supplement, in treating human obesity. Int J Obes (London) 2006;30:1737–1741. doi: 10.1038/sj.ijo.0803343. [DOI] [PubMed] [Google Scholar]
- 30.Roberts AT, Martin CK, Liu Z, et al. The safety and efficacy of a dietary herbal supplement and gallic acid for weight loss. J Med Food. 2007;10:184–188. doi: 10.1089/jmf.2006.272. [DOI] [PubMed] [Google Scholar]
- 31.Glick Z. Modes of action of gallic acid in suppressing food intake of rats. J Nutr. 1981;111:1910–1916. doi: 10.1093/jn/111.11.1910. [DOI] [PubMed] [Google Scholar]
- 32.Booth A, Amen RJ, Scott M, Greenway FL. Oral dose-ranging developmental toxicity study of an herbal supplement (NT) and gallic acid in rats. Adv Ther. 2010;27:250–255. doi: 10.1007/s12325-010-0021-x. [DOI] [PubMed] [Google Scholar]
- 33.Lyons JM, Schwimer JE, Anthony CT, et al. The role of VEGF pathways in human physiologic and pathologic angiogenesis. J Surg Res. 2010;159:517–527. doi: 10.1016/j.jss.2008.12.014. [DOI] [PubMed] [Google Scholar]