Abstract
Purpose.
We tested the viability of human limbal mesenchymal cells (LMCs) to support the expansion of human corneal epithelial stem/progenitor cells (LSCs).
Methods.
Human LMCs were isolated from sclerocorneal tissue using collagenase A. Primary limbal epithelial cells (LECs) in the form of single cell suspension or cell clusters were cocultured on a monolayer of either 3T3 cells (control) or LMCs (SC-LMC culture). The LEC clusters also were grown directly on LMCs (CC-LMC culture) and in an optimized 3-dimensional culture method (3D CC-LMC culture). Colony-forming efficiency (CFE) and LEC proliferation were analyzed. The phenotype of the cultured LECs was assessed by their expression level of putative stem cell markers and a differentiation marker by qRT-PCR and immunocytochemistry.
Results.
The LECs in the SC-LMC culture had a very limited growth and the stem/progenitor phenotype was lost compared to the control. Growth and cell morphology improved using the CC-LMC culture. The 3D CC-LMC culture method was the best to support the growth of the LSC population. Expression of ATP-binding cassette family G2 and ΔNp63 at the mRNA level was maintained or increased in CC-LMCs and 3D CC-LMC cultures compared to the control. The percentage of the K14+ and K12+ cells was comparable in these three cultures. There was no significant difference in the percentage of p63α high expressing cells in the control (21%) and 3D CC-LMC culture (17%, P > 0.05).
Conclusions.
Human LMCs can substitute 3T3 cells in the expansion of LSCs using the 3-dimensional culture system.
Keywords: limbal stem cells, limbal stem cell deficiency, limbus, stem cells, cornea epithelium, stem cell niche, fibroblasts, mesenchymal
This study shows that limbal mesenchymal cells can substitute 3T3 cells in the expansion of limbal stem cells using the novel 3-dimensional culture method.
Introduction
Corneal epithelial stem/progenitor cells or limbal stem/progenitor cells (LSCs) have long been thought to locate at the limbus. These progenitor cells differentiate into transamplifying cells, which migrate centripetally toward the central cornea.1,2 Mature corneal epithelial cells maintain the outer corneal surface.3 The LSC niche, similar to niches of other tissues, has been proposed to have a key role in maintaining LSCs in an undifferentiated state.4 The LSC niche likely contains several critical components that can regulate LSC function. These include the extracellular matrix (ECM), limbal vasculature, and various supporting cells within the niche. All components provide specific signals to the LSCs through soluble factors and various cell–cell/matrix interactions for LSC homeostasis. Several types of cells are present in the limbal region and might function as LSC niche cells, which include limbal mesenchymal cells (LMCs), melanocytes, vascular endothelial cells, and smooth muscle cells.5 All these niche components are thought to have a critical role for the growth and regulation of LSCs.
When LSCs are deficient, either due to congenital defect or secondary to injury, conjunctival cells migrate onto the corneal surface as a result of lack of normal corneal epithelium.6,7 To treat this condition called limbal stem cell deficiency (LSCD), autologous transplantation of ex vivo expanded LSCs onto the diseased eye has been the preferred treatment and has long-term success.8–10 A number of protocols have been developed to culture and expand LSCs in vitro. The most common method uses growth-arrested mouse 3T3 fibroblasts as feeder cells.11 This protocol is based on the work by Rheinwald and Green.12 Another method of cultivating LSCs is from the outgrowth of limbal tissue explants, where small pieces of intact limbal explants are placed in Petri dishes and LSCs were expanded in the absence of feeder cells.13–15 Amniotic membrane (AM) has been used as a culture substrate.16–19 A comprehensive study on the successful growth of LSCs using various methods of AM preparation and technique highlights the challenge in determining an optimal protocol for AM.20
One major concern for the use of ex vivo-expanded LSCs in human transplantation is the use of animal components in the culture. Indeed, with recent work showing contamination of cultured human retinal pigment epithelial cells with xenogenic mouse RNA from 3T3 cells,21 it is plausible to eliminate mouse 3T3 fibroblasts to avoid cross-contamination. Several human cells, including mesenchymal stem cells, dermal and foreskin fibroblasts have been reported to maintain the LSC phenotype when used as feeder cells.22–26 Recently, there is a growing interest in using human limbal fibroblasts or stromal cells to replace 3T3 cells in vitro.27,28 The advantage of using human stromal cells is that they are presumed as a component of the LSC native niche and they could be isolated from the same patients with LSCD to allow for autologous LSC culture.
The LSCs have been expanded on limbal niche cells or limbal stromal cells, but the expansion rate is significantly reduced when using the standard culture system on a monolayer of feeder cells as described by Li et al.28 Thus, the culture conditions are critical and must be further investigated to improve the LSC expansion efficiency using the potential limbal niche cells as feeder cells.
We previously showed that a 3-dimensional (3D) culture method using 3T3 cells as feeder cells can increase the LSC expansion efficiency using LSC clusters. The undifferentiated state of cultured LSCs in the 3D method was comparable to the conventional method of culturing LSCs on a monolayer of 3T3 cells. Therefore, maintaining cell–cell contact and the epithelial polarity in the 3D method seem to be important factors to maintain the LSC phenotype. In addition to the presence of the correct niche components, the appropriate culture conditions also must be considered to efficiently grow the LSCs. In this study, we investigated the ability of LMCs, a potential LSC niche component to support LSCs growth in several culture conditions.
Methods
Human Sclerocorneal Tissue
Human sclerocorneal tissues of healthy donors were obtained from the Lions Eye Institute for Transplant and Research (Tampa, FL, USA), the Tissue Bank International (Baltimore, MD, USA), or the San Diego Eye Bank (San Diego, CA, USA). Experimentation on human tissue adhered to the tenets of the Declaration of Helsinki. The experimental protocol was evaluated and exempted by the Institutional Review Board, University of California Los Angeles. The ages of the donors ranged from 20 to 65 years. The death to preservation time was less than 8 hours. To avoid interdonor variations, the sclerocorneal buttons from the same donors were used in each individual experiment.
Primary LSC Culture
Human limbal epithelial cells (LECs) were isolated from the limbus of eye bank corneas after the central cornea button was used for transplantation. The residue iris tissue, endothelium, and conjunctiva were surgically removed. The tissues were incubated in 2.4 U/mL of Dispase II (Roche, Indianapolis, IN, USA) in supplemental hormone epithelial medium (SHEM) consisting of Dulbecco's modified Eagle's medium (DMEM)/F12 (Life Technologies, Carlsbad, CA, USA) with 5% fetal bovine serum (FBS; Life Technologies), penicillin/streptomycin (Life Technologies), gentamicin/amphotericin (Life Technologies), dimethyl sulfoxide (DMSO; Sigma-Aldrich Corp., St. Louis, MO, USA), N-2 supplement (Life Technologies), recombinant human epidermal growth factor (Life Technologies), cholera toxin (Sigma-Aldrich Corp.), and hydrocortisone (Sigma-Aldrich Corp.) at 37°C for 2 hours. The limbal epithelium layer was isolated under a dissecting microscope. The LEC clusters were collected after dissection and a portion of the LEC clusters was treated with 0.25% trypsin-1 mM EDTA (Life Technologies) for 10 to 20 minutes to obtain single LEC suspensions.
Human LMCs were isolated from corneal rims after removal of the limbal epithelium with dispase II and mechanical scraping. The endothelium also was removed. Limbal stroma was cut into small pieces and incubated with 4 mg/mL collagenase A (ColA; Roche Applied Science, Mannheim, Germany) in DMEM (ATCC, Manassas, VA, USA) containing 10% FBS overnight at 37°C. The ColA solution was removed and the resulting cells were cultured in fresh MesenPRO RS medium (Life Technologies). After 5 to 7 days of culture, colonies of LMCs were harvested and passaged. To confirm the mesenchymal origin phenotype, LMCs were characterized at passage 3 for the expression of the mesenchymal markers vimentin, N-cadherin, CD105, and CD34 (Supplementary Table S1).
To prepare growth-arrested feeder layers, subconfluent 3T3-J2 cells (referred to herein as 3T3 cells; Howard Green Lab, Harvard Medical School, Boston, MA, USA) or LMCs were incubated with 4 or 30 μg/mL mitomycin C (Sigma-Aldrich Corp.), respectively, for 2 hours at 37°C. The mitomycin C–treated 3T3 cells then were subcultured in DMEM (ATCC) supplemented with 10% bovine calf serum (BCS; Thermo Fisher Scientific, Waltham, MA, USA) and penicillin/streptomycin at a density of 3 × 104 cells/cm2. The mitomycin C–treated LMCs were seeded at the density of 2 × 104 cells/cm2. Freshly isolated single LECs and LEC clusters were seeded directly on growth-arrested feeder cells (direct method) at a density of 300 cells/cm2 in SHEM with 5% FBS. The media was changed every 2 to 3 days and cells were cultured for 14 days. Single LECs grown on 3T3 feeder cells were used as a control in all of the experiments.
The 3D culturing was described previously.29 Briefly, mitomycin C–treated feeder cells were seeded onto the culture insert membrane at a density between 2 and 3 × 104 cells/cm2 overnight at 37°C. The next day, single LECs or LEC clusters were seeded onto the inner membrane of the insert at a density of 300 cells/cm2 and cultured for 14 days. Images of cell outgrowth and cell colonies were captured using an inverted DM IL LED microscope (Leica Microsystems, Wetzlar, Germany) and Insight 11.2 color mosaic camera (Spot Imaging Solutions, Sterling Heights, MI, USA).
To assess the colony-forming efficiency (CFE) of LSCs, the culture 6-well plates were rinsed once with Dulbecco's PBS (DPBS; Life Technologies), fixed with fresh 4% paraformaldehyde (PFA; Thermo Fisher Scientific), and stained with 0.5% rhodamine B (Sigma-Aldrich Corp.) for 15 minutes. The CFE was calculated by dividing the number of colonies by the number of limbal epithelial cells seeded.
RNA Extraction and Quantitative (q) RT-PCR
Total RNA from cells was extracted with a Qiagen RNeasy Mini Kit (Qiagen, Valencia, CA, USA). The quantity and quality of total RNA were assessed by a NanoDrop 1000 spectrophotometer (NanoDrop, Wilmington, DE, USA). Total RNA was reverse-transcribed using Superscript II RNase H2 reverse transcriptase (Life Technologies) according to the manufacturer's recommendations. The relative abundance of transcripts was detected through qRT-PCR by using a KAPA SYBR FAST qPCR Master Mix (KAPA Biosystems, Woburn, MA, USA). The protocol used a Mastercycler ep realplex2 qPCR System (Eppendorf, Hamburg, Germany). Fast cycling conditions were as follows: an initial denaturing step of 20 seconds at 95°C and subsequent 40 cycles of amplification in which each cycle consisted of 3 seconds at 95°C, 20 seconds at 60°C, and 8 seconds at 72°C. To generate a dissociation curve after the amplification cycles, samples were incubated at 95°C for 15 seconds, 60°C for 15 seconds, a melting curve program at 60°C to 95°C (rate of 0.4°C resolution), and followed by 95°C for 15 seconds. The fluorescence intensity of each sample was normalized in relation to that of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). At least two independent qRT-PCR experiments were performed on each cell sample. The primers used for qRT-PCR are listed in Supplementary Table S2.
Immunocytochemistry
Cultured LECs were transferred onto glass slides (Thermo Fisher Scientific) using a Cytospin 4 centrifuge (Thermo Fisher Scientific). Cells were air-dried for 15 minutes at room temperature (RT) and then stored at −80°C. Slides with cells were fixed in fresh 4% PFA for 15 minutes at RT, permeabilized with 0.3% Triton X-100 (Sigma-Aldrich Corp.) in PBS (Life Technologies) three times, and blocked with 10% donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in PBS for 30 minutes. Slides were incubated with primary antibodies in 1% BSA (Sigma-Aldrich Corp.)/PBS overnight at 4°C. The slides then were washed three times with 1% BSA/PBS and labeled with secondary antibodies (Life Technologies) in 1% BSA/PBS for 1 hour at RT. Slides were washed three times with 0.3% Triton X-100 in 1% BSA/PBS. Primary and secondary antibodies used are summarized in Supplementary Table S1. The nuclei were labeled with Hoechst 33342 (Life Technologies) for 15 minutes at RT. The slides then were washed with PBS five times and mounted in Fluoromount mounting medium (Sigma-Aldrich Corp.). Pictures were taken using a Zeiss Image.A2 fluorescent microscope (Carl Zeiss, Inc., Oberkochen, Germany) and an Olympus FV1000 confocal laser scanning microscope (Olympus, Tokyo, Japan). Image capture and analysis was performed using Olympus FV10-ASU software and Definiens Tissue Studio software (Definiens, München, Germany).
Quantification analysis of p63α high expressing cells (p63αbright cells) was performed on LECs as described previously.30
Statistical Analysis
To eliminate the variation between experiments, a minimum of three independent experiments were performed using the same sclerocorneal tissue in each experiment. Pairwise comparison was performed on the ratio values. Bar graphs represent mean ± SEM from three separate experiments. A P value < 0.05 was considered statistically significant.
Results
Comparison of Cultured LEC Morphology
The LMCs were characterized at passage 3 to confirm their mesenchymal phenotype. LMCs showed a spindle-like morphology typical of mesenchymal cells and expressed the appropriate mesenchymal cell markers, vimentin, N-cadherin, CD105, and CD34 (Fig. 1). To determine the optimal density of mitomycin C–treated LMCs, 2 × 104, 3 × 104, and 4 × 104 cells/cm2 were seeded. The density of 2 × 104 cells/cm2 was chosen since it provided the highest CFE and it was used in the rest of the experiments.
Figure 1.
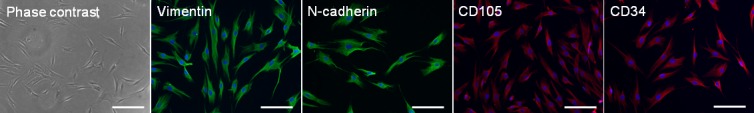
Cell morphology and characterization of the LMCs. Morphology of LMCs in culture (scale bar: 100 μm), and expression of vimentin, N-cadherin, CD105, and CD34 in LMCs (scale bar: 50 μm).
The growth of LECs that contained LSCs in all four different culture methods is summarized in the Table. Consistent growth was observed in single LSCs grown on 3T3 cells (control) as expected, while single LEC growth on LMCs (SC-LMC culture) was poor. Growth was observed in only 20% of the culture attempts. When LSCs were cultured as LEC clusters, the growth success rate increased to 67% in the direct method (CC-LMC culture) and 63% in the 3D method (3D CC-LMC culture).
Table.
Success Rate of Culturing Methods
|
Feeder |
Cell Isolation |
Method |
Success* |
Total† |
Percentage |
Abbreviation |
| 3T3 | Single cells | Direct | 27 | 27 | 100 | Control |
| LMCs | Single cells | Direct | 2 | 10 | 20 | SC-LMC |
| LMCs | Cell clusters | Direct | 6 | 9 | 67 | CC-LMC |
| LMCs | Cell clusters | 3D | 5 | 8 | 63 | 3D CC-LMC |
Number of experiments with successful LEC growth.
Total number of experiments.
The control culture produced holoclone colonies that exhibit tight compact hexagonal cells and a clearly defined colony border (Fig. 2A). In contrast, SC-LMC culture had very poor LEC growth, and the LSC colonies were sparse and tiny. The epithelial cells were larger and less uniform, demonstrating a differentiated morphology (Fig. 2A). The LECs from CC-LMC cultures were smaller and more uniform in shape (Fig. 2A). The 3D SC-LMC culture had very poor LEC growth (Fig. 2A). When comparing all of the culture methods, LECs from the 3D CC-LMC culture method demonstrated an undifferentiated morphology (Fig. 2A). The cultured LECs were small and compact. These findings suggested that the 3D cultures using LMCs as a feeder cell could better support the growth of stem/progenitor-like LECs.
Figure 2.
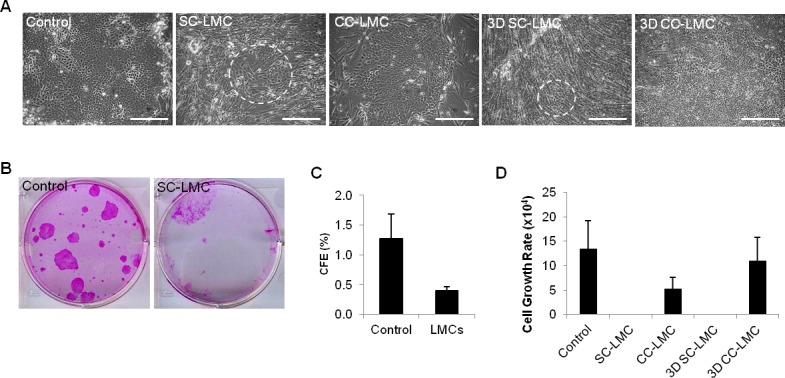
Cell morphology, CFE, and cell growth rate of cultivated LECs from different culture methods. (A) Morphology of LECs from different culture methods. Dashed circles indicate areas containing epithelial-like cells. (B, C) The CFE of control and SS-LMC. (D) Cell growth rate of LECs from different culture methods. Scale bars: 100 μm.
LSC Proliferation in Different Cultures
We first examined the CFE of the control and SC-LMC cultures. The CFE analysis was not possible for the LEC clusters, because colony formation number would be from cell clusters instead of a single LEC. The CFE of the control was 3.2-fold higher compared to that of the SC-LMC culture (Figs. 2B–C). Although there was some growth from SC-LMC, the growth was much inferior to that in the control. We next looked at the proliferation of LECs among the four culture methods. The expansion rate of the LECs in the 3D CC-LMC culture was comparable to that of the control and superior to that of CC-LMC culture (Fig. 2D). There was very limited LEC growth when cultured as SC-LMC and we eliminated this culture method from further analysis in this study.
Expansion of the LSC Population Using Different Culture Methods
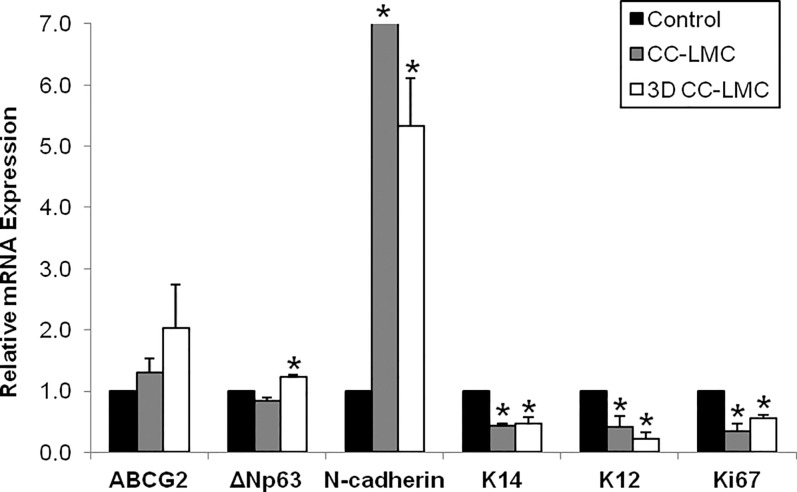
To identify the LSC population in different culture methods, we next examined the phenotype of cultivated LECs using several putative corneal epithelial stem cells markers, including ATP-binding cassette family G2 (ABCG2), ΔNp63, N-cadherin, and K14.31–36 We used K12 as a marker of mature cornea epithelial cells,1 whereas Ki67 was used as a marker for active cell proliferation. When compared to the control, we observed a steady increase in expression of ABCG2 in the CC-LMCs (1.3-fold, P = 0.314) and 3D CC-LMC (2.0-fold, P = 0.251) cultures compared to the control (Fig. 3). Likewise, we saw a similar increase in ΔNp63 expression (20%, P = 0.01) in 3D CC-LMC culture, but a 20% decrease was observed in CC-LMC culture (P = 0.04). The N-cadherin was expressed at high levels in the CC-LMC (27.6-fold, P = 0.02) and 3D CC-LMC (5.4-fold, P = 0.01) cultures. Interestingly, we did observe an almost identical decrease in K14 mRNA expression in the CC-LMC (2.3-fold, P = 0.001) and 3D CC-LMC (2.1-fold; P = 0.011) cultures compared to that in the control. Conversely, K12 mRNA expression was significantly lower in CC-LMC (1.4-fold decrease, P = 0.045) and 3D CC-LMC (3.5-fold decrease, P = 0.005) cultures. Finally, Ki67 expression from all culture methods was consistent with the cell count numbers quantitated. The Ki67 expression was the highest in the control (0.34-fold decrease in CC-LMC, P = 0.015 and 0.55-fold decrease in 3D CC-LMC, P = 0.003).
Figure 3.
Gene expression of corneal epithelial markers in cultured LECs. Expression of putative corneal epithelial stem cell markers ABCG2, ΔNp63, N-cadherin, and K14, the differentiation marker K12 and proliferation marker Ki67 in CC-LMC and 3D CC-LMC cultures compared to the control.
To confirm the gene expression levels, we next examined the protein level of p63α, K14, and K12 in cultured LECs to investigate the LSC population. Percentage of the p63αbright cells has been suggested as a predictor of clinical success.8 We examined the proportion of these cells in different culture methods (Fig. 4A). The percentage of p63αbright cells was similar in the control and 3D CC-LMC culture (21% vs. 17%, P = 0.89; Fig. 4B). We normalized the number of p63αbright cells by using the ratio of p63αbright cells/total cells seeded in each experiment. The normalized p63αbright cells number was 17.2 ± 13.7 in the control, 8.5 ± 0.0 in CC-LMC, and 23.5 ± 9.9 in the 3D CC-LMC culture (P = 0.89; Fig. 4C).
Figure 4.
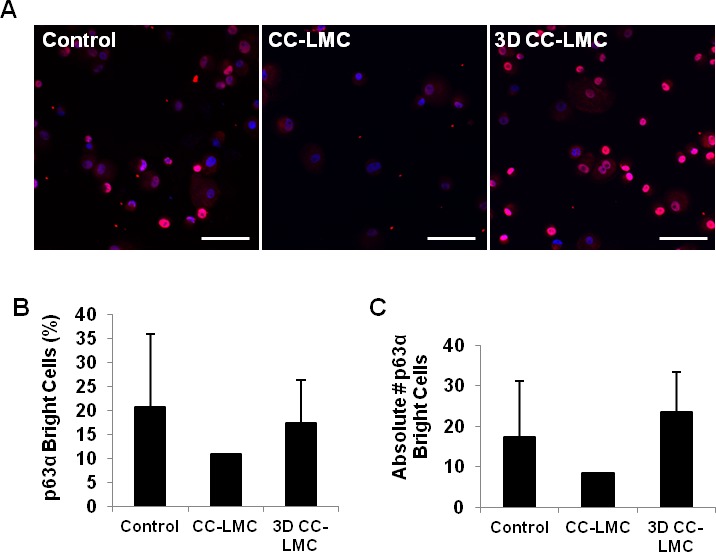
Quantification of p63αbright cells in cultured LECs. (A) Representative images of p63α expression of cultured LECs in the control, CC-LMC, and 3D CC-LMC cultures. (B) Percentage of p63αbright cells in each culture system. (C) Yield of p63αbright cells per each LEC seeded in each culture system. Scale bars: 100 μm.
In addition to the p63α expression, we evaluated K14+ and K12+ LECs in each culture method. There was no difference in the percentage of K14+ cells among the three methods (P > 0.05; Figs. 5A, 5B). The percentage of K12+ was the lowest in the 3D CC-LMC culture (0.4%) followed by the control (0.9%) and CC-LMC culture (1.2%, P > 0.05; Figs. 5C, 5D).
Figure 5.
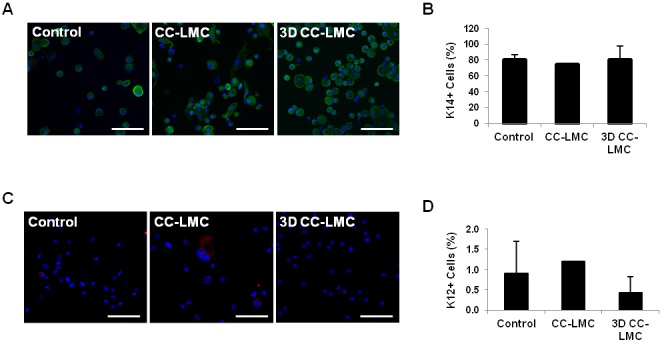
Quantification of K14+ and K12+ cells. (A) Representative images of K14+ LECs in control, CC-LMC, and 3D CC-LMC culture. (B) Percentage of K14+ cells in each culture system. (C) Representative images of K12+ LECs in the control, CC-LMC, and 3D CC-LMC culture. (D) Percentage of K12+ cells in each culture system. Scale bars: 100 μm.
Discussion
The LMCs have been proposed to act as niche cells in the limbus and they may have a similar role to that of BM-MSCs in the hematopoietic stem cell niche.37 Polisetti et al.37 identified several shared groups of genes between the LMCs and BM-MSCs, including cell-cell adhesion, ECM components, and osteoblast-related genes. In addition, they identified several highly expressed mitogenic factors for epithelial cells in LMCs. Additional evidence has shown that LMCs and the ECM components can recapitulate the LSC niche and promote differentiation of human embryonic stem cells (hESCs) into corneal epithelial-like cells in vitro.38
The LMCs have been shown to secrete several cytokines that could have a role in the cross-talk between LMCs and epithelial cells.39 Conditioned medium collected from human limbal fibroblasts was shown to promote the growth of corneal epithelial cells in a mouse model of limbal stem cell deficiency, and conditioned media from human foreskin fibroblasts led to conjunctivalization of the cornea.40 Proteomic analysis of fibroblast lines among the cornea, conjunctiva, and limbus revealed several specific proteins, including the expression and secretion of secreted protein acid and rich in cysteine (SPARC) in LMCs only.41 Furthermore, our previous study on the differential gene expression profiles of the limbus, cornea, and conjunctiva42 shows that many ECM, including several collagens and fibronectin-1, were preferentially expressed in the limbus. These examples highlight the importance of the effects of LMCs on corneal epithelial cell fate and demonstrate the potential effect of LMCs in the cultivation of LECs for the expansion of LSCs in vitro.
The LMC population was characterized to confirm their mesenchymal origin. The limbal stroma is derived from the neural crest, and the stromal cells have been reported to express mesenchymal (CD13, CD29, CD44, CD56, CD73, CD9, and CD105) and hematopoietic (CD11b, CD34, and CD133) markers.43 There also is some evidence suggesting that the proximal stroma in closest contact with the LECs is supposed to harbor a population of stem cells.44 Marker CD34 has been published to be expressed in almost all the keratocytes in the limbal stroma,45 although the function of these CD34+ cells is unclear in the limbus. Cells expressing CD105 and N-cadherin have been observed underlying the limbal crypts as potential important niche cells.46 The LMCs isolated in the present study expressed all four mesenchymal markers, vimentin, N-cadherin, CD105, and CD34. This finding confirms that LMCs maintain their mesenchymal phenotype in culture.47,48
Expansion rate of LECs in the 3D CC-LMC culture was comparable to that in the control. A higher cell proliferation does not represent a higher expansion of the LSC population. Further characterization of the LEC populations from each culturing method confirmed a high percentage of p63αbright cells and the highest mRNA expression of ΔNp63 in the 3D CC-LMC method. These data indicated that the 3D CC-LMC culture could expand a comparable amount of LSCs as the control.
Single LECs could not grow efficiently when cultured directly on a monolayer of LMCs. Instead, clusters of LECs (CC-LMC) better supported the LSC phenotype. It has been reported that when LSCs were cultured as LEC clusters on 3T3 feeder cells, the undifferentiated state was better maintained.49,50 The fact that LECs could not efficiently grow on the monolayer of LMCs could be due to the limited space for growth in a 2D culture so they failed to attach and grow. Li et al.28 recently used a lower density of LMCs that achieved expansion of LECs. However, the CFE appeared to be significantly reduced when using the standard culture method on a monolayer of feeder cells.28 Our LMC population might be slightly different from that of Li et al.28 Our LMCs contain all population of the deep stromal cells, whereas their limbal niche cells were from the superficial stroma. Despite the difference in the cell population, our LMCs have excellent capacity to support the expansion of LSCs in the 3D culture. Therefore, other factors, such as cell-cell contact in the LEC clusters and maintaining the polarity of LSCs in the 3D culture, are important in the expansion of the stem cell population in vitro.
In conclusion, the current study showed that human LMCs have the potential to support the expansion of LSCs at the same efficiency as 3T3 cells in vitro. The method of culture appears to be important in their ability to serve as feeder cells.
Acknowledgments
The authors thank Elfren Baclagon for his technical assistance.
Supported by the National Eye Institute (R01EY021797 and 5P30EY000331; Bethesda, Maryland, USA), the California Institute for Regenerative Medicine (TR2-01768; San Francisco, California, USA), and Research to Prevent Blindness.
Disclosure: M.N. Nakatsu, P; S. González, P; H. Mei, P; S.X. Deng, P
References
- 1. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986; 103: 49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lavker RM, Tseng SCG, Sun TT. Corneal epithelial stem cells at the limbus: looking at some old problems from a new angle. Exp Eye Res. 2004; 78: 433–446 [DOI] [PubMed] [Google Scholar]
- 3. Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971; 229: 560–561 [DOI] [PubMed] [Google Scholar]
- 4. Ordonez P, Di Girolamo N. Limbal epithelial stem cells: role of the niche microenvironment. Stem Cells. 2012; 30: 100–107 [DOI] [PubMed] [Google Scholar]
- 5. Li W, Hayashida Y, Chen YT, et al. Niche regulation of corneal epithelial stem cells at the limbus. Cell Res. 2007; 17: 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen JJ, Tseng SC. Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci. 1990; 31: 1301–1314 [PubMed] [Google Scholar]
- 7. Kinoshita S, Kiorpes TC, Friend J, et al. Limbal epithelium in ocular surface wound healing. Invest Ophthalmol Vis Sci. 1982; 23: 73–80 [PubMed] [Google Scholar]
- 8. Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010; 363: 147–155 [DOI] [PubMed] [Google Scholar]
- 9. Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology. 2001; 108: 1569–1574 [DOI] [PubMed] [Google Scholar]
- 10. Sangwan VS, Matalia HP, Vemuganti GK, et al. Clinical outcome of autologous cultivated limbal epithelium transplantation. Indian J Ophthalmol. 2006; 54: 29–34 [DOI] [PubMed] [Google Scholar]
- 11. Lindberg K, Brown ME, Chaves HV, et al. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci. 1993; 34: 2672–2679 [PubMed] [Google Scholar]
- 12. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975; 6: 331–343 [DOI] [PubMed] [Google Scholar]
- 13. Joseph A, Powell-Richards AOR, Shanmuganathan VA, et al. Epithelial cell characteristics of cultured human limbal explants. Br J Ophthalmol. 2004; 88: 393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim H-S, Jun Song X, de Paiva CS, et al. Phenotypic characterization of human corneal epithelial cells expanded ex vivo from limbal explant and single cell cultures. Exp Eye Res. 2004; 79: 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sobrin L, Liu Z, Monroy DC, et al. Regulation of MMP-9 activity in human tear fluid and corneal epithelial culture supernatant. Invest Ophthalmol Vis Sci. 2000; 41: 1703–1709 [PubMed] [Google Scholar]
- 16. Koizumi N, Fullwood NJ, Bairaktaris G, et al. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000; 41: 2506–2513 [PubMed] [Google Scholar]
- 17. Koizumi N, Inatomi T, Quantock AJ, et al. Amniotic membrane as a substrate for cultivating limbal corneal epithelial cells for autologous transplantation in rabbits. Cornea. 2000; 19: 65–71 [DOI] [PubMed] [Google Scholar]
- 18. Koizumi N, Cooper LJ, Fullwood NJ, et al. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest Ophthalmol Vis Sci. 2002; 43: 2114–2121 [PubMed] [Google Scholar]
- 19. Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000; 343: 86–93 [DOI] [PubMed] [Google Scholar]
- 20. Shortt AJ, Secker GA, Lomas RJ, et al. The effect of amniotic membrane preparation method on its ability to serve as a substrate for the ex-vivo expansion of limbal epithelial cells. Biomaterials. 2009; 30: 1056–1065 [DOI] [PubMed] [Google Scholar]
- 21. Johnen S, Wickert L, Meier M, et al. Presence of xenogenic mouse RNA in RPE and IPE cells cultured on mitotically inhibited 3T3 fibroblasts. Invest Ophthalmol Vis Sci. 2011; 52: 2817–2824 [DOI] [PubMed] [Google Scholar]
- 22. Omoto M, Miyashita H, Shimmura S, et al. The use of human mesenchymal stem cell-derived feeder cells for the cultivation of transplantable epithelial sheets. Invest Ophthalmol Vis Sci. 2009; 50: 2109–2115 [DOI] [PubMed] [Google Scholar]
- 23. Li Y, Inoue T, Takamatsu F, et al. Development of genetically modified eliminable human dermal fibroblast feeder cells for ocular surface regeneration medicine. Invest Ophthalmol Vis Sci. 2013; 54: 7522–7531 [DOI] [PubMed] [Google Scholar]
- 24. Scafetta G, Tricoli E, Siciliano C, et al. Suitability of human Tenon's fibroblasts as feeder cells for culturing human limbal epithelial stem cells. Stem Cell Rev. 2013; 9: 847–857 [DOI] [PubMed] [Google Scholar]
- 25. Sugiyama H, Maeda K, Yamato M, et al. Human adipose tissue-derived mesenchymal stem cells as a novel feeder layer for epithelial cells. J Tissue Eng Regen Med. 2008; 2: 445–449 [DOI] [PubMed] [Google Scholar]
- 26. Lu R, Bian F, Lin J, et al. Identification of human fibroblast cell lines as a feeder layer for human corneal epithelial regeneration. PLoS One. 2012; 7: e38825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen S-Y, Mahabole M, Tseng SCG. Optimization of ex vivo expansion of limbal epithelial progenitors by maintaining native niche cells on denuded amniotic membrane. Transl Vis Sci Technol. 2013; 2: 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, Inoue T, Takamatsu F, et al. Differences between niche cells and limbal stromal cells in maintenance of corneal limbal stem cells. Invest Ophthalmol Vis Sci. 2014; 55: 1453–1462 [DOI] [PubMed] [Google Scholar]
- 29. Mei H, González S, Nakatsu MN, et al. A three-dimensional culture method to expand limbal stem/progenitor cells. Tissue Eng Part C Methods. 2013; 20: 393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Di Iorio E, Barbaro V, Ferrari S, et al. Q-FIHC: quantification of fluorescence immunohistochemistry to analyse p63 isoforms and cell cycle phases in human limbal stem cells. Microsc Res Tech. 2006; 69: 983–991 [DOI] [PubMed] [Google Scholar]
- 31. Chen Z, de Paiva CS, Luo L, et al. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004; 22: 355–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. De Paiva CS, Chen Z, Corrales RM, et al. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005; 23: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hayashi R, Yamato M, Sugiyama H, et al. N-Cadherin is expressed by putative stem/progenitor cells and melanocytes in the human limbal epithelial stem cell niche. Stem Cells. 2007; 25: 289–296 [DOI] [PubMed] [Google Scholar]
- 34. Kurpakus MA, Maniaci MT, Esco M. Expression of keratins K12, K4 and K14 during development of ocular surface epithelium. Curr Eye Res. 1994; 13: 805–814 [DOI] [PubMed] [Google Scholar]
- 35. Kawasaki S, Tanioka H, Yamasaki K, et al. Expression and tissue distribution of p63 isoforms in human ocular surface epithelia. Exp Eye Res. 2006; 82: 293–299 [DOI] [PubMed] [Google Scholar]
- 36. Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001; 98: 3156–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Polisetti N, Agarwal P, Khan I, et al. Gene expression profile of epithelial cells and mesenchymal cells derived from limbal explant culture. Mol Vis. 2010; 16: 1227–1240 [PMC free article] [PubMed] [Google Scholar]
- 38. Ahmad S, Stewart R, Yung S, et al. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007; 25: 1145–1155 [DOI] [PubMed] [Google Scholar]
- 39. Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995; 163: 61–79 [DOI] [PubMed] [Google Scholar]
- 40. Amirjamshidi H, Milani BY, Sagha HM, et al. Limbal fibroblast conditioned media: a non-invasive treatment for limbal stem cell deficiency. Mol Vis. 2011; 17: 658–666 [PMC free article] [PubMed] [Google Scholar]
- 41. Shimmura S, Miyashita H, Higa K, et al. Proteomic analysis of soluble factors secreted by limbal fibroblasts. Mol Vis. 2006; 12: 478–484 [PubMed] [Google Scholar]
- 42. Nakatsu MN, Vartanyan L, Vu DM, et al. Preferential biological processes in the human limbus by differential gene profiling. PLoS One. 2013; 8: e61833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lv FJ, Tuan RS, Cheung MC, et al. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014; 32: 1408–1419 [DOI] [PubMed] [Google Scholar]
- 44. Pinnamaneni N, Funderburgh JL. Concise review: stem cells in the corneal stroma. Stem Cells. 2012; 30: 1059–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Joseph A, Hossain P, Jham S, et al. Expression of CD34 and L-selectin on human corneal keratocytes. Invest Ophthalmol Vis Sci. 2003; 44: 4689–4692 [DOI] [PubMed] [Google Scholar]
- 46. Branch MJ, Hashmani K, Dhillon P, et al. Mesenchymal stem cells in the human corneal limbal stroma. Invest Ophthalmol Vis Sci. 2012; 53: 5109–5116 [DOI] [PubMed] [Google Scholar]
- 47. Dziasko MA, Armer HE, Levis HJ, et al. Localisation of epithelial cells capable of holoclone formation in vitro and direct interaction with stromal cells in the native human limbal cryopt. PLoS One. 2014; 9: e94283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen SY, Hayashida Y, Chen MY, et al. A new isolation method of human limbal progenitor cells by maintaining close association with their niche cells. Tissue Eng Part C Methods. 2011; 17: 537–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kawakita T, Shimmura S, Higa K, et al. Greater growth potential of p63-positive epithelial cell clusters maintained in human limbal epithelial sheets. Invest Ophthalmol Vis Sci. 2009; 50; 4611–4617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. González S, Deng SX. Presence of native limbal stromal cells increases the expansion efficiency of limbal stem/progenitor cells in culture. Exp Eye Res. 2013; 116: 169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]