Abstract
The role of the immune response in the pathogenesis of Rickettsia rickettsii infection in guinea pigs was investigated by immunosuppression, using antilymphocyte serum. Twenty guinea pigs were inoculated with R. rickettsii, Sheila Smith strain, on day 0. Fifteen animals received antilymphocyte serum on days --1, 0, 2, 4, and 6. Five animals received normal rabbit serum on the same schedule. At necropsy, specimens were collected for histological examination, rickettsial immunofluorescence, rickettsial titration, and antirickettsial antibody titration. All normal rabbit serum recipients and 12 of 15 antilymphocyte serum recipients developed typical disease. Comparison of animals in terminal stages of disease revealed the same clinical course and gross lesions, but differing rickettsial burden and cellular response. Immunosuppressed animals had higher titers of splenic rickettsiae and greater numbers of immunofluorescent rickettsiae. Thus, although antibody was undetectable in both groups, there appeared to be an inhibition of antirickettsial immunity. Microscopic vasculitis was similar quantitatively, but differed qualitatively, with immunocompetent animals having the typical monouclear/lymphocytic inflammation and immunosuppressed animals having neutrophilic predominance. This study demonstrates that immunopathological mechanisms are not necessary for the pathogenesis of experimental Rocky Mountain spotted fever. The rickettsiae themselves seem capable of causing cellular and tissue damage.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AIKAWA J. K., HARRELL G. T. The immunophysiology of serum sickness. Alterations in the blood volume and thiocyanate space in relation to the development of humoral antibodies in the rabbit. J Clin Invest. 1951 Apr;30(4):360–368. doi: 10.1172/JCI102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg G. H., Jr, Osterman J. V. Experimental scrub typhus immunogens: gamma-irradiated and formalinized rickettsiae. Infect Immun. 1977 Jan;15(1):124–131. doi: 10.1128/iai.15.1.124-131.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman L., Green I., Frank M. Genetically controlled total deficiency of the fourth component of complement in the guinea pig. Science. 1970 Oct 2;170(3953):74–75. doi: 10.1126/science.170.3953.74. [DOI] [PubMed] [Google Scholar]
- Frank M. M., May J., Gaither T., Ellman L. In vitro studies of complement function in sera of C4-deficient guinea pigs. J Exp Med. 1971 Jul 1;134(1):176–187. doi: 10.1084/jem.134.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRELL G. T. Rocky Mountain spotted fever. Medicine (Baltimore) 1949 Dec;28(4):333–370. doi: 10.1097/00005792-194912000-00001. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
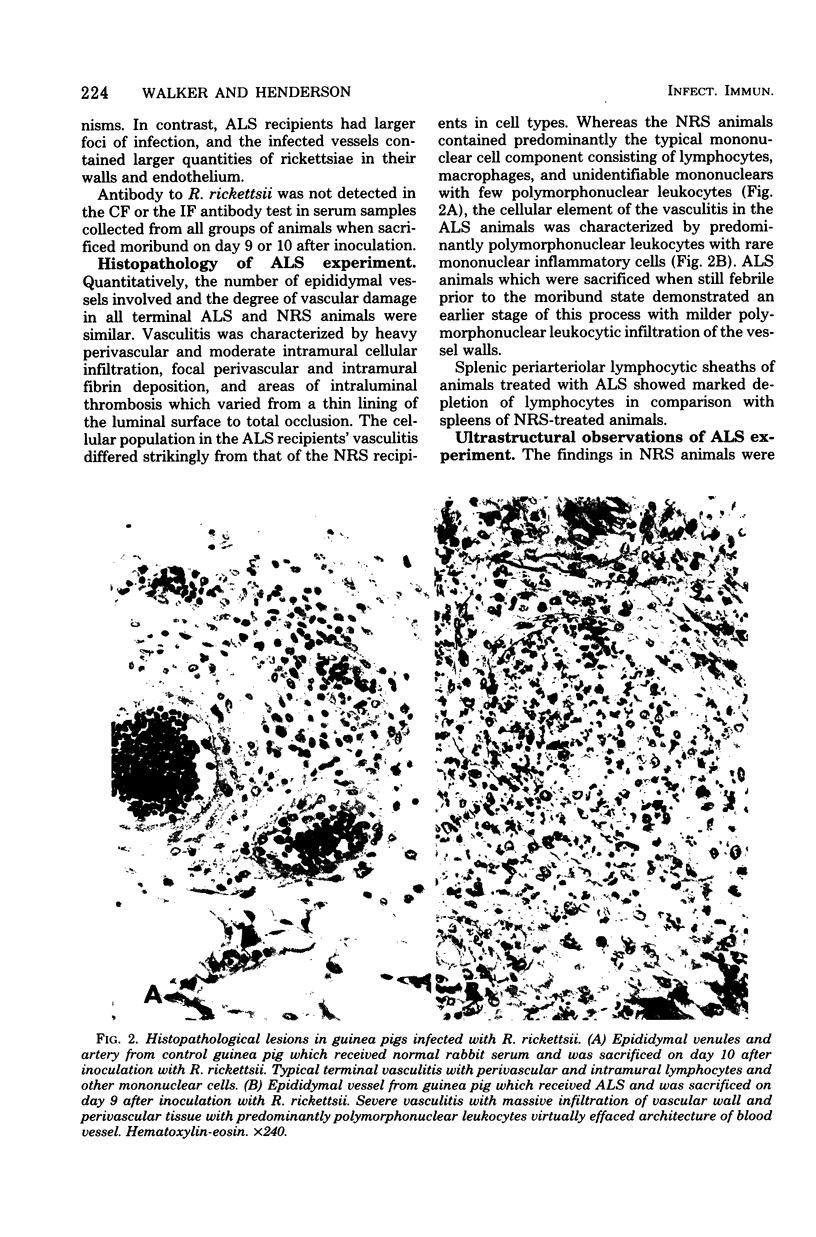
- Moe J. B., Mosher D. F., Kenyon R. H., White J. D., Stookey J. L., Bagley L. R., Fine D. P. Functional and morphologic changes during experimental Rocky Mountain spotted fever in guinea pigs. Lab Invest. 1976 Sep;35(3):235–245. [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Ormsbee R. A., Peacock M. G., Burgdorfer W. Microimmunofluorescence test for the serological study of rocky mountain spotted fever and typhus. J Clin Microbiol. 1976 Jan;3(1):51–61. doi: 10.1128/jcm.3.1.51-61.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A., Catanzaro P. J., Phillips S. M., Osterman J. V. Host defenses in experimental scrub typhus: role of cellular immunity in heterologous protection. Infect Immun. 1976 Jul;14(1):39–46. doi: 10.1128/iai.14.1.39-46.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
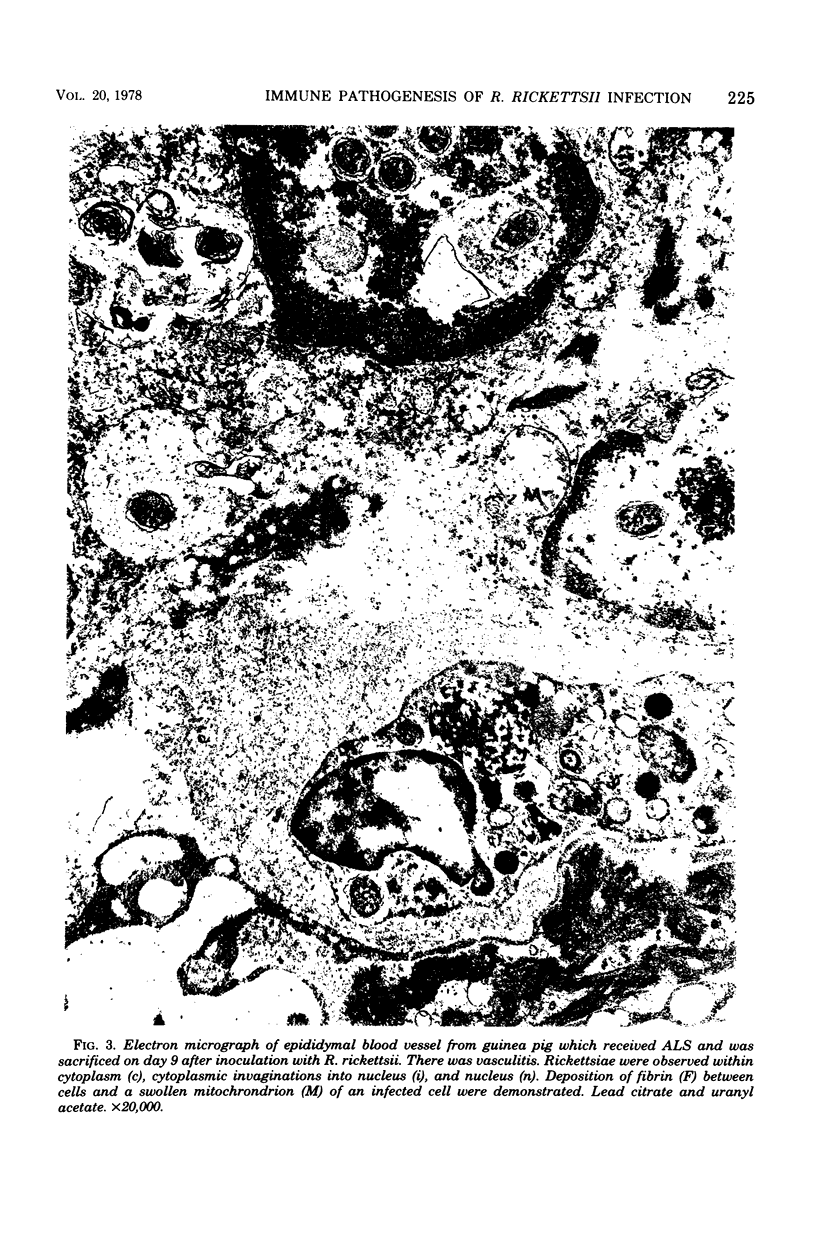
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. H., Harrison A., Henderson F., Murphy F. A. Identification of Rickettsia rickettsii in a guinea pig model by immunofluorescent and electron microscopic techniques. Am J Pathol. 1977 Feb;86(2):343–358. [PMC free article] [PubMed] [Google Scholar]
- Wike D. A., Tallent G., Peacock M. G., Ormsbee R. A. Studies of the rickettsial plaque assay technique. Infect Immun. 1972 May;5(5):715–722. doi: 10.1128/iai.5.5.715-722.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito T., Hoshino-Shimizu S., Pereira M. O., Rigolon N. The pathogenesis of the vascular lesions in experimental rickettsial disease of the guinea pig (Rocky Mountain Spotted fever group). A light, immunofluorescent and electron microscopic study. Virchows Arch A Pathol Pathol Anat. 1973;358(3):205–214. doi: 10.1007/BF00543228. [DOI] [PubMed] [Google Scholar]