Abstract
The cardiac late Na + current is generated by a small fraction of voltage-dependent Na + channels that undergo a conformational change to a burst-gating mode, with repeated openings and closures during the action potential (AP) plateau. Its magnitude can be augmented by inactivation-defective mutations, myocardial ischemia, or prolonged exposure to chemical compounds leading to drug-induced (di)-long QT syndrome, and results in an increased susceptibility to cardiac arrhythmias. Using CytoPatch™ 2 automated patch-clamp equipment, we performed whole-cell recordings in HEK293 cells stably expressing human Nav1.5, and measured the late Na + component as average current over the last 100 ms of 300 ms depolarizing pulses to -10 mV from a holding potential of -100 mV, with a repetition frequency of 0.33 Hz. Averaged values in different steady-state experimental conditions were further corrected by the subtraction of current average during the application of tetrodotoxin (TTX) 30 μM. We show that ranolazine at 10 and 30 μM in 3 min applications reduced the late Na + current to 75.0 ± 2.7% (mean ± SEM, n = 17) and 58.4 ± 3.5% ( n = 18) of initial levels, respectively, while a 5 min application of veratridine 1 μM resulted in a reversible current increase to 269.1 ± 16.1% ( n = 28) of initial values. Using fluctuation analysis, we observed that ranolazine 30 μM decreased mean open probability p from 0.6 to 0.38 without modifying the number of active channels n, while veratridine 1 μM increased n 2.5-fold without changing p. In human iPSC-derived cardiomyocytes, veratridine 1 μM reversibly increased APD90 2.12 ± 0.41-fold (mean ± SEM, n = 6). This effect is attributable to inactivation removal in Nav1.5 channels, since significant inhibitory effects on hERG current were detected at higher concentrations in hERG-expressing HEK293 cells, with a 28.9 ± 6.0% inhibition (mean ± SD, n = 10) with 50 μM veratridine.
Introduction
The late cardiac Na + current can be recorded 10–100 milliseconds after membrane depolarization as a sustained inward current component ( I sus) in cardiomyocytes 1. This late current represents a fraction of voltage-dependent Na + channels that fail to inactivate after the initial opening. Instead, these channels change to a conformation with frequent late re-openings in a so-called burst mode 2. Among other mechanisms, calmodulin kinase II overexpression in chronic heart failure increases the rate of transition to the late bursting mode conformation 2. The phenomenon is also observed in Na v1.5 inactivation-deficient mutants, such as ΔKPQ 3 or 1795InsD 4, 5, leading to a specific form of congenital long QT syndrome, LQT-3 6– 8.
Given its role in cardiac arrhythmogenesis 9, 10, pharmacological inhibition of the late Na + current component ( I Na late) is seen as an anti-arrhythmic strategy. Novel late sodium channel blockers, such as the partial fatty acid beta-oxidation inhibitor, ranolazine 11– 15, are used for both myocardial ischemia and to alleviate neuropathic pain and show a preferential affinity for the burst mode conformation of Na + channels 16– 21. Other compounds, such as veratridine, a steroid-derived alkaloid extracted from rhizomes of Veratrum album or seeds of Schoenocaulon officinale 22, 23, preferentially bind to activated Na + channels, impeding inactivation and leading to increased nerve excitability.
The objectives of this study were to (1) record the veratridine-dependent increase in the late Na + current using a HEK293 cell line stably transfected with human Na v1.5 24, (2) measure the ranolazine-induced inhibition of basal and veratridine-activated I Na late, and (3) asses the effects of veratridine at the same concentrations on action potentials (APs) in hiPSC-cardiomyocyte preparations externally paced in current-clamp mode. In parallel we also tested the inhibitory effects of veratridine on hERG1 current in stably transfected HEK293 cells. All these experiments were performed using CytoPatch™2 automated patch-clamp equipment.
Materials and methods
All experiments were performed using the CytoPatch™2, using standard dual-channel Cytocentrics chips with embedded quartz pipette tips 2 μM in diameter. For whole-cell late Na + current recordings, the voltage-clamp protocol consisted of repeated depolarizing pulses of -10 mV amplitude and 300 ms duration, from a holding potential of -100 mV, to allow a substantial removal from inactivation of Na v1.5 channels. The peak and late Na + current were plotted and monitored over the entire duration of experiment, and extracted from recorded data for further analysis. Pharmacological compounds were applied in a predefined sequence using the dispensing needle of automated equipment. All experiments were performed at room temperature (21–22°C).
For whole-cell hERG current recordings, cells were held at -70 mV. After a brief 100-ms prepulse to -50 mV to determine the current leak, a 2-s depolarizing voltage step to +40 mV was followed by a 2-s step to -50 mV to elicit hERG tail currents every 10 s. Peak tail current amplitude was corrected by the leak current and the corrected peak tail current was averaged over the last three pulses of the control phase ( I ctrl) and the application phase ( I cpd), respectively. From these averaged values the hERG tail current inhibition was calculated as follows:
Inhibition = 1 – ( I cpd/ I ctrl)
iPSC-CM recordings were performed in current-clamp mode, using repeated sweeps consisting of 3 injected current pulses of 2000 pA amplitude, 0.5 ms duration, at 3-s intervals. During solution uptake the system was switched to voltage-clamp mode.
Cell cultures and preparations
HEK293 cells stably expressing either the human Na v1.5 channel or the hERG K + channel were used as ready-to-use frozen Instant cells (product of Cytocentrics). Cells, kept in liquid nitrogen, were quickly thawed, centrifuged, resuspended in extracellular solution at a density of ~10 6 cells/ml, and used for experiments within 3 hours. For other experiments, Nav1.5-expressing cells were cultured in 25 cm 2 flasks in DMEM supplemented with 10% fetal bovine serum and 100 μg/ml zeozin, and kept at 37°C, 8% CO 2 in a humidified incubator. Adherent cell monolayers were detached with Versene (ethylenediaminotetraacetic acid – EDTA 0.02% in phosphate buffered saline - PBS), centrifuged for 2 min at 100 × g, and resuspended in hERG external solution.
hiPSC-derived iCell® Cardiomyocytes were kindly provided by Cellular Dynamics International (Madison, WI) as frozen samples, and cultured in monolayers in 12-well plates coated with 0.1% gelatin, for up to 41 days. The thawing/plating and maintenance media were provided by the cell supplier. For detachment, the monolayers were rinsed twice with calcium and magnesium-free PBS, then incubated for 2 min with trypsin 0.1% at 37°C. 1.5 ml of medium was added per well, the cells were suspended, centrifuged at 180 × g for 5 min, and resuspended in a 1:1 mixture of culture medium and hERG extracellular solution.
Solutions and chemicals
For recordings in Na v1.5-transfected HEK293 cells the external solution had the following composition (in mM): NaCl 130, CsCl 5, CaCl 2 2, MgCl 2 1.2, HEPES 10, D-glucose 5, pH 7.4, osmolality 320 mOsm/kg. The internal solution contained (in mM): Cs-aspartate 70, CsCl 60, CaCl 2 1, MgCl 2 1, Na 2ATP 5, EGTA 11, HEPES 10, pH 7.2 with CsOH, osmolality 290 mOsm/kg. For recordings in iPSC-derived cardiomyocytes and hERG-transfected HEK293 cells the extracellular solution had the following composition (in mM): 140 NaCl, 2.5 KCl, 2 MgCl 2, 2 CaCl 2, 10 HEPES, 10 Glucose, 15 Sucrose. The pH was adjusted to 7.4 with NaOH 1M and the osmolality to 320 (± 5) mOsmol/kg with sucrose 1M, and the storage temperature was 4°C. The intracellular solution contained (in mM): 100 K Gluconate, 20 KCl, 1 CaCl 2, 1 MgCl 2, 10 HEPES, 11 EGTA-KOH, 4 ATP-Mg 2+, 3 Phosphocreatine-Na 2-H 2O, 9 Sucrose. The pH was adjusted to 7.2 with KOH 1M, the osmolality to 295 (± 5) mOsmol/l, and then it was stored in 10-ml aliquots at -20°C, thawed and used within 4 hours. Veratridine (Sigma V5754) working solutions at 1, 5 and 50 μM were prepared from a 50 mM stock solution in ethanol. Ranolazine dihydrochloride (Sigma R6152) 10 and 30 μM was prepared from a 10 mM stock solution in DMSO. TTX (BN0518, Biotrend, Zurich, CH) was prepared from a 1 mM aqueous stock solution.
Data storage and analysis
The software files generated during the recordings were stored on computer hard disks. Patch clamp data were analyzed using the CytoPatch TM software and exported to Microsoft Excel or pClamp10 (Axon Instruments, part of Molecular Devices, Sunnyvale, CA) for further analysis, using a proprietary conversion tool. APD90 analysis for current-clamp recordings in iPSC-derived cardiomyocytes was performed with self-written software routines. APD90 was computed as the duration between the point of maximal AP upstroke speed during phase 0 to recovery of 90% of the difference between peak upstroke potential and resting potential. In the case of veratridine application, if recovery was incomplete during the 3-s interstimulus interval, APD90 was computed over several pacing cycles. For statistical analysis, one-way ANOVA for independent samples with Dunnett’s post-hoc comparison, as well as Student’s t tests where appropriate, were applied, at a level of significance of p ≤ 0.05, using the GraphPad Prism software (La Jolla, CA).
Results
1. Late Nav1.5 sodium current component
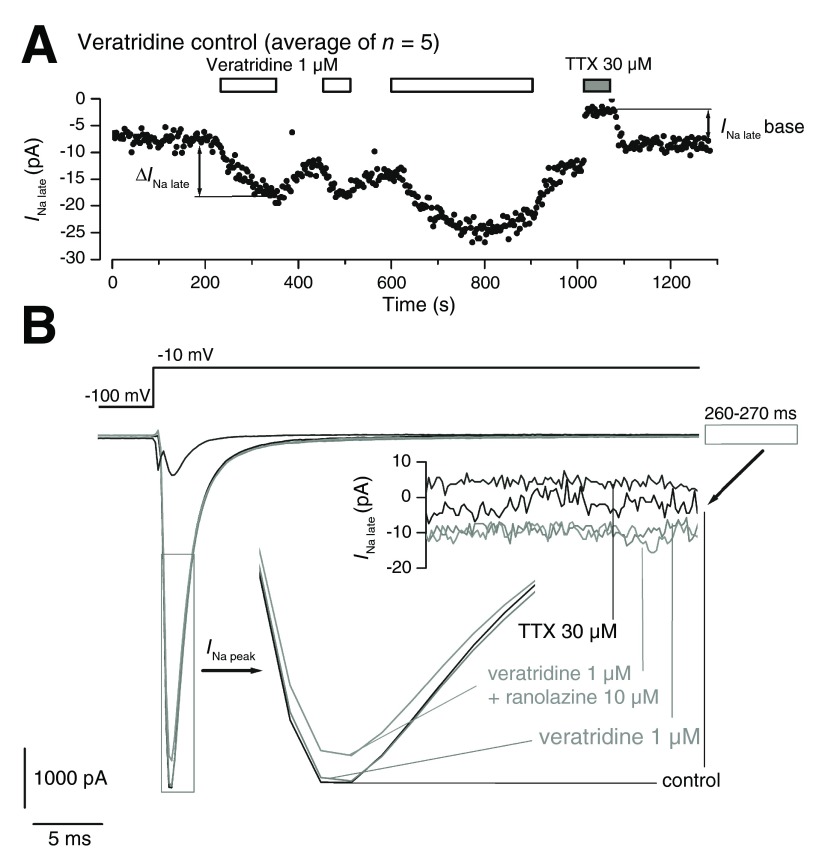
Using automated voltage-clamp protocols applied to human Nav1.5-expressing HEK293 cells in the above mentioned conditions, we routinely recorded, in high-quality seal conditions (both seal and membrane resistance > 1 GΩ), and with stability for at least 20 min, whole-cell Na + currents. These currents were elicited by membrane depolarization to -10 mV from a holding potential of -100 mV, required for the proper removal from inactivation of cardiac Na + channels. The late Na + current component ( I Na late) was automatically computed and plotted as time average over the last 100 ms of the 300-ms depolarizing pulse of each sweep. Figure 1A shows the time course of 5 averaged experiments including repeated applications of veratridine 1 μM with different durations (2 min, 1 min, 5 min), as well as a 1-min application of TTX 30 μM, resulting in the rapid complete block of late Na + current. The average current level during TTX application in each experiment was subsequently subtracted from all other averaged steady-state levels to obtain unbiased estimations of I Na late in different experimental conditions. In general, reversibility was good and rapid upon TTX wash-out.
Figure 1. Activation by veratridine of the late Na + current in Nav1.5-expressing cells.
A. Time course of I Na late (average transmembrane current during the last 100 ms of a 300-ms depolarizing pulse to -10 mV from a holding potential of -100 mV) during a typical experiment with repeated applications of veratridine 1 μM and a final application of TTX 30 μM. B. Overlap of individual sweeps showing the peak and late component of I Na during the application of veratridine 1 μM, the co-application with ranolazine 10 μM, and with TTX 30 μM. The peak and late I Na component are shown separately in inserts using magnified time and voltage scales, respectively.
2. Late sodium current activation by veratridine and block by ranolazine in Nav1.5 cells
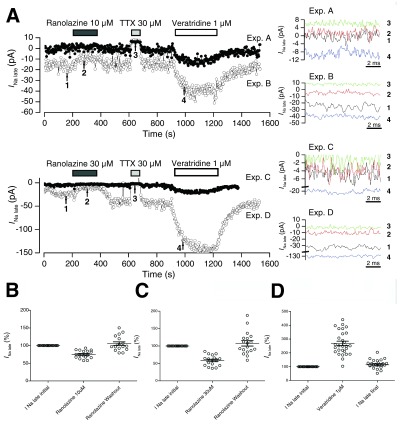
Under control conditions, a 5-min application of veratridine 1 μM induced an increase of the late Na + current by 269.1 ± 16.1% (mean ± SEM, n = 28) of initial values ( Table 1 and Figure 2). Figure 1B shows representative current traces of selected sweeps, as well as magnified inserts of the peak and late phase, within an individual experiment. The application of veratridine 1 μM increased the late current amplitude by more than two-fold relative to the TTX 30 μM reference trace, without any noticeable effect on peak current, while the co-application of ranolazine 10 μM reduced the peak current with apparently no effect on the late component. Systematic experiments with ranolazine applied alone demonstrated I Na late reduction to 75.0 ± 2.7% (mean ± SEM, n = 17) of initial values at 10 μM, and 58.4 ± 3.5% (mean ± SEM, n = 18) at 30 μM ( Table 1 and Figure 2).
Figure 2. Effects of ranolazine, TTX and veratridine on the late Na + current in Nav1.5-expressing cells.
A. Time plots of I Na late in four typical experiments. Intervals of application of ranolazine 10 or 30 μM, TTX 30 μM, and veratridine 1 μM are indicated. Right panels: I Na late traces from 260 to 270 ms at the time points marked with arrows and numbered in the left panels. B and C. Effects of ranolazine 10 and 30 μM, respectively, on relative levels of TTX-sensitive late Na + current. D. Effect of veratridine 1 μM. Mean values are indicated for each condition, and error bars represent SEM.
Table 1. Effects of ranolazine 10 and 30 μM and veratridine 1 μM on TTX-sensitive * late Na + current.
| Experiment | Absolute values
(mean ± SEM) |
Relative values
(mean ± SEM) |
||||||
|---|---|---|---|---|---|---|---|---|
| Drug application
( n = number of cells tested) |
INa late initial
(pA) |
INa late drug
(pA) |
INa late final
(pA) |
INa late initial
(%) |
INa late drug
(%) |
p value
vs. initial § |
INa late final
(%) |
p value
vs. initial § |
| Ranolazine 10 μM 3 min
( n = 17) |
-13.2 ± 2.6 | -9.7 ± 1.8 | -13.2 ± 2.3 | 100 | 75.0 ± 2.7 | 7.8E-8 | 105.6 ± 5.0 | 0.280 |
| Ranolazine 30 μM 3 min
( n = 18) |
-10.6 ± 2.2 | -6.1 ± 1.4 | -11.9 ± 2.8 | 100 | 58.4 ± 3.5 | 1.1E-9 | 107.7 ± 7.7 | 0.330 |
| Veratridine 1 μM 5 min
( n = 28) |
-12.4 ± 1.9 | -36.3 ± 6.7 | -15.8 ± 2.7 | 100 | 269.1 ± 16.1 | 4.7E-11 | 114.2 ± 8.2 | 0.095 |
* I Na late values were computed by subtraction of averaged amplitude in the presence of TTX 30 μM from averaged amplitudes in all other experimental conditions
§ using two-tailed Student’s t test for paired samples
Figure 3 shows the power density spectra of Fourier-transformed traces recorded under different conditions, as numbered in the upper time course of the experiments. Presumably, the opening and closure of Nav1.5 channels in the late gating mode conformation produces a Lorentzian component with corner frequency above 2 KHz. However, due to the reduced number of individual signal sources, the plateau spectral power density was small and difficult to distinguish. A better approach to fluctuation analysis is by the computation of single-channel parameters such as the mean open probability p and the average number of channels n, starting from the well-known estimations of current variance σ 2 and macroscopic current I 25:
Figure 3. The late Na + current presents fluctuations due to the opening and closure of individual channels.
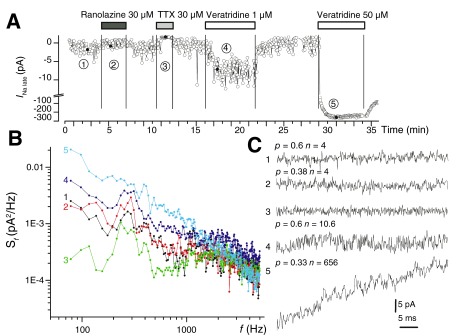
A. Time course of a typical experiment in Nav1.5-expressing cells showing the effects of ranolazine 30 μM, TTX 30 μM, and veratridine 1 μM on I Na late. B. Averaged Fourier transforms of multiple traces recorded in different conditions in the experiment shown in A: 1. initial control; 2. ranolazine 30 μM; 3. TTX 30 μM; 4. veratridine 1 μM; 5. veratridine 50 μM. C. Fluctuation analysis of individual traces recorded during the five distinct periods shown in A allowed the distinction of individual channel gating events and the computation of the average open probability p and the average number of channels n for each condition.
σ 2 = npi 2 – np 2 i 2 = np(1 – p) i 2 and I = npi
We used a unitary Na + current amplitude i at -10 mV of 1.43 pA, based on the unitary current amplitude of 2 pA at -40 mV observed in single-channel recordings of Nav1.5 late currents in HEK293 cells 26 and a reversal potential of +65 mV. In doing so, we could evaluate the mean open probability p and further the average number of Na + channels n open in the late mode in selected traces, over the last 100 ms of activity during the 300-ms depolarizing pulses. It was observed that 30 μM ranolazine application resulted in a decrease of mean open probability from 0.6 to 0.38, without affecting the average number of channels contributing the late Na + current ( n = 4), in agreement with estimates obtained from averaged macroscopic currents. On the other hand, veratridine 1 μM increased the number of channels in late gating mode to n = 10, without influencing the mean open probability.
3. di-APD prolongation in iPSC-CM by application of veratridine
To get a better understanding of the role that the late Na + current may play in arrhythmogenesis and in the complex mechanisms of di-LQT syndrome, we performed a series of automated patch-clamp experiments on hiPSC-derived cardiomyocytes with current-clamp recordings of stimulus-triggered APs. Each recorded sweep contained responses elicited by three injected current stimuli (0.5 ms duration, 2 nA amplitude). As shown in Figure 4, veratridine 1 μM induced a marked prolongation of AP duration, reaching saturation in less than 3 min. Further application of 5 μM veratridine resulted in further APD90 prolongation, exceeding the interstimulus interval. There was a slow trend to reversibility during the wash-out phase. Quantitative results for veratridine 1 μM application are summarized in Table 2. Thus, in n = 6 experiments at room temperature the relative APD90 prolongation induced by veratridine was 2.12 ± 0.41 fold (mean ± SEM), reversible to 1.21 ± 0.50 fold at wash-out (mean ± SEM, n = 4).
Figure 4. AP prolongation by veratridine in human iPSC-derived cardiomyocytes.
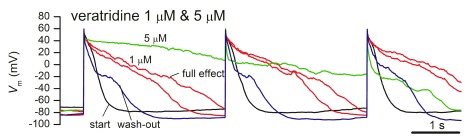
Current-clamp AP recordings using a pacing protocol with injected current stimuli of 0.5-ms duration and 2 nA amplitude repeated at 3-s intervals. The overlap of traces in control conditions at the start of the experiment, with 1 μM veratridine (onset and full effect), with 5 μM veratridine, and during wash-out, shows partial recovery.
Table 2. Changes in APD90 duration (in ms, mean ± SEM) induced by veratridine in hiPSC-derived cardiomyocytes.
| Experimental
condition |
APD90
initial |
APD90
during appl. |
APD90
wash-out |
Relative APD90
during appl. |
Relative APD90
wash-out |
|---|---|---|---|---|---|
| Veratridine 1 μM | 777.5 ± 179.1 | 1429.3 ± 289.8 | 688.0 ± 86.9 | 2.12 ± 0.41 | 1.21 ± 0.50 |
| p | 0.02482 | 0.65037 | |||
| n | 6 | 6 | 4 | 6 | 4 |
| Ethanol 0.1%
control |
893.8 ± 343.8 | 1013.0 ± 408.4 | 917.3 ± 318.3 | 1.08 ± 0.07 | 1.17 ± 0.17 |
| p | 0.16664 | 0.75986 | |||
| n | 4 | 4 | 4 | 4 | 4 |
Relative values are computed taking initial values as reference
p values for Student’s t test for paired samples, two-tailed, for condition tested vs. initial values
n = number of cells included in analysis
4. Weak hERG inhibition by veratridine
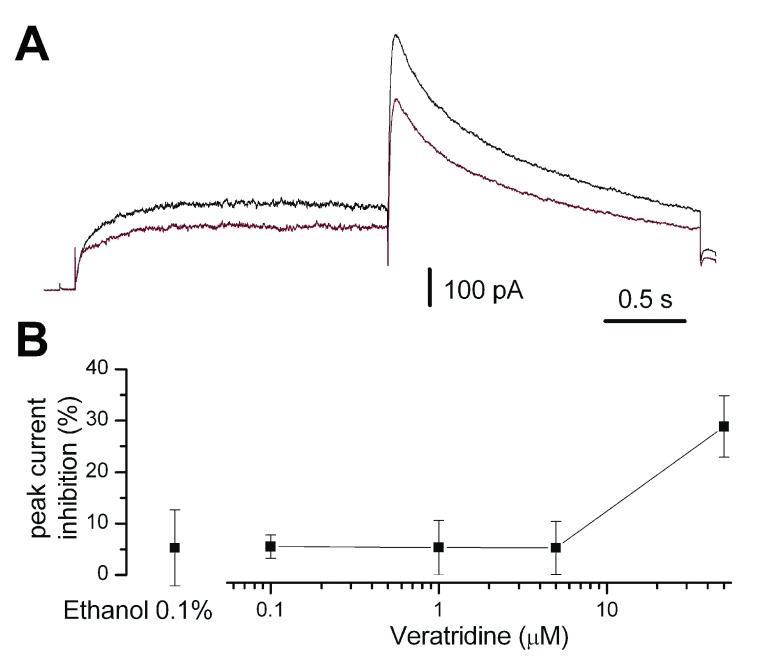
Last, we performed experiments using HEK293 cells stably expressing hERG1 to assess the effects of veratridine at different concentrations on this current component, in order to better understand the complex effects of this compound on the action potential of iPSC-derived cardiomyocytes. As shown in Figure 5, veratridine concentrations up to 5 μM did not produce levels of hERG inhibition significantly different than the diluting vehicle alone (ethanol 0.1%), while at 50 μM there was a 28.9 ± 6.0% (mean ± SD, n = 10) inhibition of peak hERG current, a statistically significant effect ( p < 0.0001, one-way ANOVA for independent samples with Dunnett’s multiple comparison test vs. control).
Figure 5. Effects of veratridine on hERG channels.
A. Current traces in control conditions (upper) and during application of veratridine 50 μM (lower trace). B. Percentages of peak hERG current inhibition by different concentrations of veratridine and control ethanol 0.1% vehicle. Error bars represent SD. Number of experiments: control ( n = 6), veratridine 0.1 μM ( n = 10), 1 μM ( n = 5), 5 μM ( n = 7), 50 μM ( n = 10).
Patch-clamp data showing the effects on late Na + current after veratridine, ranolazine and TTX treatment in Nav1.5-expressing HEK293 cells, the effect of veratridine on hERG currents and on externally paced action potentials in iCell® Cardiomyocytes assessed with CytoPatch™2 automated patch-clamp equipment. For more details, please see the legend file in Dataset 1.
Discussion
The main findings of the present study using the CytoPatch TM2 instrument are the following: (1) inhibition of cardiac late Na + current to 75.0% and 58.4% of initial values by ranolazine 10 and 30 μM, respectively; (2) activation of the same current by veratridine 1 μM to 269.1% of initial values; and (3) prolongation of APD90 by veratridine 1 μM to 212% of initial values in human iPSC-derived cardiomyocytes. We have succeeded in recording and analysing the small (a few picoA) late Na + current component in a human cell line stably transfected with wild-type human SCN5A, using automated patch-clamp technology, with a voltage protocol different from that used in the first electrophysiology characterization of ΔKPQ LQT-3 mutant Na + channels 3. We observed that instrumental noise does not significantly affect the average current value over the last 100 ms of a 300-ms depolarizing pulse from -100 to -10 mV, therefore this value can be computed and plotted in real-time, representing a sensitive indicator for evidencing pharmacological effects in automated patch-clamp assays. Another critical parameter is the frequency of repetition of depolarizing stimuli, because it may reveal use dependency for compounds exerting state-dependent binding effects 27. Although in the present set of experiments we kept a fixed value of 0.33 Hz, inclusion of different pacing rates in future variants of the assay may offer supplementary information of pharmacological relevance. An important tool in the precise assessment of I Na late levels is the application of TTX 30 μM, which results in an almost complete late Na + current inhibition, and offers a current reference used for the correction by subtraction of all other values in different experimental conditions. An excellent seal stability and access resistance, such as those offered by the Cytocentrics microfluidic chips 28, is another prerequisite for successful late Na + pharmacology experiments.
Due to the limited amplitude of late Na + current under basal conditions, we increased it using low concentrations of veratridine, a steroid-derived natural alkaloid known to bind to an intramembrane site of Na + channel pore subunits and, in doing so, stabilize it in the open conformation 29, 30, contributing to the I Na late. Although veratridine at 1 μM resulted in a consistent 2.5 – 3-fold increase in late Na + current, compared to initial levels ( Table 1 and Figure 1– Figure 3), we would not recommend this procedure for pharmacology assays because of possible interactions between veratridine and test compounds. Although we have not systematically explored this phenomenon, preliminary observations, as shown in Figure 1B, suggest a reduced effectiveness of ranolazine 10 μM when co-applied with veratridine, compared to application of ranolazine alone at the same concentration. The value of relative inhibition of the late Na + current by ranolazine 10 μM in the single compound application obtained in our study (75.0% of control levels) is larger than those obtained with the same concentration of ranolazine on late Na + current activated by veratridine 40 μM (89% and 81% for holding potentials of -110 mV and -90 mV, respectively) 31. An elegant method that may offer pharmacologically relevant information concerning the site and mechanism of action of a certain compound is fluctuation analysis. As shown in Figure 3, when applied to selected traces, this method allows the computation of the mean open probability p and the average number of open channels n contributing to current variance. From this analysis, we hypothesize that ranolazine acts as an open pore blocker, reducing the mean open probability at 30 μM to 63% of initial values, from 0.6 to 0.38, without lowering the number of active channels, while veratridine 1 μM increases the number of active channels in late gating mode 2.5-fold, without influencing their mean open probability.
In agreement with the significant activatory effects of veratridine 1 μM on cardiac Nav1.5 channels in late gating mode in stably transfected cell lines, we found an important prolongation of action potential duration when the drug was applied to human iPSC-derived cardiomyocytes in current-clamp configuration, using CytoPatch™2 automated patch-clamp equipment. The APD90 was increased over twofold by veratridine 1 μM, with a good reversibility upon wash-out ( Table 2), and an even higher prolongation with 5 μM ( Figure 4), although in this case we were unable to accurately compute APD90 values for externally paced APs, due to their overlap with the next stimulus. This effect is consistent with the findings of previous studies, showing APD prolongation in bronchial smooth muscle cells upon veratridine application 32, and the enhancement of AP burst generation by veratridine in rat trigeminal sensory neurons 33. Furthermore, we have demonstrated that hERG inhibition does not play any role in the observed APD prolongation by veratridine at 1 or 5 μM, since significant hERG inhibition occurred with higher veratridine concentrations ( Figure 5). A recent combined in vivo and in vitro study proved that the augmentation of the myocardial late Na + current during ventricular remodeling induced by pulmonary arterial hypertension in rats was accompanied by significant APD90 increases, and a beneficial effect of repeated ranolazine administration via late Na + current block was demonstrated with a subsequent alleviation of induced intracellular Ca 2+ overload 34.
In conclusion, the present study demonstrates that automated patch-clamp, as implemented by the CytoPatch™2 equipment using our proprietary CYTOCENTERING technology and quartz pipette tips embedded in silicon microfluidic chips 28, 35, which allow high-quality stable seals, lead to reliable late Na + current pharmacology recordings, with results at least comparable to those obtained in manual patch-clamp experiments. In addition, automation may prove advantageous given the small amplitude of late Na + current, which therefore requires a large number of experiments for the accurate assessment of pharmacological effects.
Data availability
F1000Research: Dataset 1. Experimental data showing the effect of veratridine, ranolazine and TTX on late Na + currents in cultured cells, 10.5256/f1000research.5544.d36993 36
Abbreviations
HEK: human embryo kidney
CHO: Chinese hamster ovary
hERG: human ether-á-go-go related gene
SEM: standard error of the means
SD: standard deviation
DMEM: Dulbecco’s modified Eagle’s medium
DMSO: dimethylsulfoxide
HEPES: N-2-hydroxyethylpiperazine-N´-2-ethansulfonic acid
EGTA: ethylene glycol-bis(ß-aminoethyl ether)-N,N,N,N´-tetraacetic acid
ATP: adenosine triphosphate
TTX: tetrodotoxin
IC: intracellular solution (pipette-filling solution)
EC: extracellular solution
PBS: phosphate-buffered saline
iPSC-CM: induced pluripotent stem cell-derived cardiomyocytes
APD: action potential duration
Acknowledgements
The authors gratefully acknowledge Stefanie Frech, Jörg Eisfeld, Ingrid Rosenkranz, Juliane Böttcher, Christian Scherpeltz, Yassine M. Amarcouch and Jean-Sébastien Rougier for their support, and Cellular Dynamics International for the provision of iCell ® Cardiomyocytes.
Funding Statement
This study was in part funded by a BMBF grant 03WKCC4D, TransCure NCCR Network to HA, and Austrian Science Fund FWF (grant W1232-B11).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
v1; ref status: indexed
References
- 1.Clancy CE, Tateyama M, Liu H, et al. : Non-equilibrium gating in cardiac Na + channels: an original mechanism of arrhythmia. Circulation. 2003;107(17):2233–7. 10.1161/01.CIR.0000069273.51375.BD [DOI] [PubMed] [Google Scholar]
- 2.Grandi E, Puglisi JL, Wagner S, et al. : Simulation of Ca-calmodulin-dependent protein kinase II on rabbit ventricular myocyte ion currents and action potentials. Biophys J. 2007;93(11):3835–47. 10.1529/biophysj.107.114868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett PB, Yazawa K, Makita N, et al. : Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376(6542):683–5. 10.1038/376683a0 [DOI] [PubMed] [Google Scholar]
- 4.Bezzina C, Veldkamp MW, van Den Berg MP, et al. : A single Na(+) channel mutation causing both long-QT and Brugada syndromes. Circ Res. 1999;85(12):1206–13. 10.1161/01.RES.85.12.1206 [DOI] [PubMed] [Google Scholar]
- 5.Veldkamp MW, Viswanathan PC, Bezzina C, et al. : Two distinct congenital arrhythmias evoked by a multidysfunctional Na(+) channel. Circ Res. 2000;86(9):E91–7. 10.1161/01.RES.86.9.e91 [DOI] [PubMed] [Google Scholar]
- 6.Abriel H: Cardiac sodium channel Na(v)1.5 and interacting proteins: Physiology and pathophysiology. J Mol Cell Cardiol. 2010;48(1):2–11. 10.1016/j.yjmcc.2009.08.025 [DOI] [PubMed] [Google Scholar]
- 7.Antzelevitch C, Nesterenko V, Shryock JC, et al. : The role of late I Na in development of cardiac arrhythmias. Handb Exp Pharmacol. 2014;221:137–68. 10.1007/978-3-642-41588-3_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant AO: Cardiac ion channels. Circ Arrhythm Electrophysiol. 2009;2(2):185–94. 10.1161/CIRCEP.108.789081 [DOI] [PubMed] [Google Scholar]
- 9.Clancy CE, Rudy Y: Linking a genetic defect to its cellular phenotype in a cardiac arrhythmia. Nature. 1999;400(6744):566–9. 10.1038/23034 [DOI] [PubMed] [Google Scholar]
- 10.Kleber AG, Rudy Y: Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84(2):431–88. 10.1152/physrev.00025.2003 [DOI] [PubMed] [Google Scholar]
- 11.Antzelevitch C, Belardinelli L, Zygmunt AC, et al. : Electrophysiological effects of ranolazine, a novel antianginal agent with antiarrhythmic properties. Circulation. 2004;110(8):904–10. 10.1161/01.CIR.0000139333.83620.5D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belardinelli L, Shryock JC, Fraser H: Inhibition of the late sodium current as a potential cardioprotective principle: effects of the late sodium current inhibitor ranolazine. Heart. 2006;92(Suppl 4):iv6–iv14. 10.1136/hrt.2005.078790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Undrovinas AI, Belardinelli L, Undrovinas NA, et al. : Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17(Suppl 1):S169–S77. 10.1111/j.1540-8167.2006.00401.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hale SL, Kloner RA: Ranolazine, an inhibitor of the late sodium channel current, reduces postischemic myocardial dysfunction in the rabbit. J Cardiovasc Pharmacol Ther. 2006;11(4):249–55. 10.1177/1074248406294607 [DOI] [PubMed] [Google Scholar]
- 15.Mahesh Kumar KN, Sandhiya S: Ranolazine: A novel partial inhibitor of fatty acid oxidation for angina. Indian J Pharmacol. 2006;38(4):302–4. 10.4103/0253-7613.27040 [DOI] [Google Scholar]
- 16.Fredj S, Sampson KJ, Liu H, et al. : Molecular basis of ranolazine block of LQT-3 mutant sodium channels: evidence for site of action. Br J Pharmacol. 2006;148(1):16–24. 10.1038/sj.bjp.0706709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo A, Ma J, Song Y, et al. : Larger late sodium current density as well as greater sensitivities to ATX II and ranolazine in rabbit left atrial than left ventricular myocytes. Am J Physiol Heart Circ Physiol. 2014;306(3):H455–61. 10.1152/ajpheart.00727.2013 [DOI] [PubMed] [Google Scholar]
- 18.Maltsev VA, Undrovinas AI: A multi-modal composition of the late Na + current in human ventricular cardiomyocytes. Cardiovasc Res. 2006;69(1):116–27. 10.1016/j.cardiores.2005.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno JD, Clancy CE: Pathophysiology of the cardiac late Na current and its potential as a drug target. J Mol Cell Cardiol. 2012;52(3):608–19. 10.1016/j.yjmcc.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreno JD, Yang PC, Bankston JR, et al. : Ranolazine for congenital and acquired late I Na-linked arrhythmias: in silico pharmacological screening. Circ Res. 2014;113(7):e50–61. 10.1161/CIRCRESAHA.113.301971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaza A, Belardinelli L, Shryock JC: Pathophysiology and pharmacology of the cardiac “late sodium current”. Pharmacol Ther. 2008;119(3):326–39. 10.1016/j.pharmthera.2008.06.001 [DOI] [PubMed] [Google Scholar]
- 22.McKinney LC, Chakraverty S, De Weer P: Purification, solubility, and pKa of veratridine. Anal Biochem. 1986;153(1):33–8. 10.1016/0003-2697(86)90056-4 [DOI] [PubMed] [Google Scholar]
- 23.Kupchan SM, Lavie D, Deliwala CV, et al. : Schoenocaulon alkaloids. I. Active principles of Schoenocaulon officinale. Cevacine and protocevine. J Am Chem Soc. 1953;75(22):5519–24. 10.1021/ja01118a017 [DOI] [Google Scholar]
- 24.Dice MS, Kearl T, Ruben PC: Methods for studying voltage-gated sodium channels in heterologous expression systems. Methods Mol Med. 2006;129:163–85. 10.1385/1-59745-213-0:163 [DOI] [PubMed] [Google Scholar]
- 25.Van Driessche W, Lindemann B: Concentration dependence of currents through single sodium-selective pores in frog skin. Nature. 1979;282(5738):519–20. 10.1038/282519a0 [DOI] [PubMed] [Google Scholar]
- 26.Lacerda AE, Kuryshev YA, Chen Y, et al. : Alfuzosin delays cardiac repolarization by a novel mechanism. J Pharmacol Exp Ther. 2008;324(2):427–33. 10.1124/jpet.107.128405 [DOI] [PubMed] [Google Scholar]
- 27.Rajamani S, El-Bizri N, Shryock JC, et al. : Use-dependent block of cardiac late Na(+) current by ranolazine. Heart Rhythm. 2009;6(11):1625–31. 10.1016/j.hrthm.2009.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stett A, Burkhardt C, Weber U, et al. : CYTOCENTERING: a novel technique enabling automated cell-by-cell-patch clamping with the CYTOPATCH chip. Recept Chann. 2003;9(1):59–66. [PubMed] [Google Scholar]
- 29.Catterall WA: Cooperative activation of action potential Na + ionophore by neurotoxins. Proc Natl Acad Sci U S A. 1975;72(5):1782–6. 10.1073/pnas.72.5.1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ulbricht W: Effects of veratridine on sodium currents and fluxes. Rev Physiol Biochem Pharmacol. 1998;133:1–54. 10.1007/Bfb0000612 [DOI] [PubMed] [Google Scholar]
- 31.Le Grand B, Pignier C, Letienne R, et al. : Sodium late current blockers in ischemia reperfusion: is the bullet magic? J Med Chem. 2008;51(13):3856–66. 10.1021/jm800100z [DOI] [PubMed] [Google Scholar]
- 32.Bradley E, Webb TI, Hollywood MA, et al. : The cardiac sodium current Na(v)1.5 is functionally expressed in rabbit bronchial smooth muscle cells. Am J Physiol Cell Physiol. 2013;305(4):C427–35. 10.1152/ajpcell.00034.2013 [DOI] [PubMed] [Google Scholar]
- 33.Tsuruyama K, Hsiao CF, Chandler SH: Participation of a persistent sodium current and calcium-activated nonspecific cationic current to burst generation in trigeminal principal sensory neurons. J Neurophysiol. 2013;110(8):1903–14. 10.1152/jn.00410.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocchetti M, Sala L, Rizzetto R, et al. : Ranolazine prevents I NaL enhancement and blunts myocardial remodelling in a model of pulmonary hypertension. Cardiovasc Res. 2014;104(1):37–48. 10.1093/cvr/cvu188 [DOI] [PubMed] [Google Scholar]
- 35.Scheel O, Himmel H, Rascher-Eggstein G, et al. : Introduction of a modular automated voltage-clamp platform and its correlation with manual human ether-à-go-go related gene voltage-clamp data. Assay Drug Dev Technol. 2011;9(6):600–7. 10.1089/adt.2010.0352 [DOI] [PubMed] [Google Scholar]
- 36.Chevalier M, Amuzescu B, Gawali V, et al. : Experimental data showing the effect of veratridine, ranolazine and TTX on late Na + currents in cultured cells. F1000Research. 2014. Data Source