Abstract
A number of methods have been developed recently that stimulate the human brain non-invasively through the intact scalp. The most common are transcranial magnetic stimulation (TMS), transcranial electric stimulation (TES) and transcranial direct current stimulation (TDCS). They are widely used to probe function and connectivity of brain areas as well as therapeutically in a variety of conditions such as depression or stroke. They are much less focal than conventional invasive methods which use small electrodes placed on or in the brain and are often thought to activate all classes of neurones in the stimulated area. However, this is not true. A large body of evidence from experiments on the motor cortex shows that non-invasive methods of brain stimulation can be surprisingly selective and that adjusting the intensity and direction of stimulation can activate different classes of inhibitory and excitatory inputs to the corticospinal output cells. Here we review data that have elucidated the action of TMS and TES, concentrating mainly on the most direct evidence available from spinal epidural recordings of the descending corticospinal volleys. The results show that it is potentially possible to test and condition specific neural circuits in motor cortex that could be affected differentially by disease, or be used in different forms of natural behaviour. However, there is substantial interindividual variability in the specificity of these protocols. Perhaps in the future it will be possible, with the advances currently being made to model the electrical fields induced in individual brains, to develop forms of stimulation that can reliably target more specific populations of neurones, and open up the internal circuitry of the motor cortex for study in behaving humans.
Vincenzo Di Lazzaro is Professor of Neurology and Chair of the Department of Neurology, University Campus Bio-Medico, Rome, Italy. His main areas of research are human brain plasticity, the physiological bases of recovery in stroke, the use of neurophysiological techniques for the diagnosis of neurological disorders and the evaluation of the effects of drugs on the intact human brain. John C. Rothwell is Professor of Human Neurophysiology at UCL Institute of Neurology, London. His research concentrates on understanding the physiology and pathophysiology of human movement control in health an in neurological disease. He has contributed widely to understanding the physiology of movement disorders and to the development of non-invasive methods of brain stimulation.
 |
Introduction
Stimulation of the brain is far more complex than stimulation of a peripheral nerve. A single electrical stimulus of a nerve produces a single action potential that spreads away from the site of stimulation. In humans, this gives rise to a muscle twitch if the nerve contains motor fibres, and a sensation if it contains sensory fibres. A single stimulus to the brain also initiates an action potential in the axons of nearby neurones, but because these synapse on and activate other neurones, which in turn activate other neurones etc., the single stimulus evokes a complex response that can outlast the stimulus for several milliseconds.
In the motor cortex, some notion of this complex cascade of activity can be obtained by recording its main output, the corticospinal tract. Experimental animal studies have shown that a single electrical stimulus causes a series of high frequency discharges that have a frequency of about 600 Hz (Adrian & Moruzzi, 1939; Patton & Amassian, 1954; Kernell & Chien-Ping, 1967). The earliest wave persists after cortical depression by cooling and after cortical ablation and is thought to originate from direct activation of the axons of fast pyramidal tract neurones (PTNs). It has therefore been termed the ‘D’ wave. The later waves require the integrity of the cortical grey matter, and are thought to originate from indirect, trans-synaptic activation of PTNs and are termed ‘I’ waves.
It was initially proposed that the I waves were the result of high frequency repetitive activity in a neural circuit that had excitatory inputs to corticospinal neurones. The more intensely this circuit was activated the more I waves it would produce and thereby account for gradual recruitment of additional I waves at higher stimulus levels. However, although the basic principle of the model may still hold, data from conscious humans suggests that transcranial stimulation of motor cortex can activate corticospinal neurones via a number of additional mechanisms, probably by activating different subsets of neurones that in turn depend on the precise parameters of stimulation.
In this review we discuss data from experiments in which the motor cortex has been activated with either transcranial magnetic stimulation (TMS; Barker et al. 1985) or transcranial electrical stimulation (TES; Merton & Morton, 1980). TES involves applying a single high voltage pulse of electrical stimulation through scalp electrodes (Merton & Morton, 1980). Since most of the applied current flows between the electrodes on the scalp rather than penetrating the brain, this produces a strong sensation and local scalp muscle contraction. TMS in contrast, employs a rapidly changing magnetic field to ‘carry’ the electrical pulse into the brain across the scalp and skull (for a detailed review see Rothwell, 1991). Although the currents in the brain are of similar magnitude and time course following both forms of stimulation, there is one important difference between them. The currents from TES spread in all directions, both radially and tangentially to the inner skull surface from anode to cathode. In contrast, the currents induced by TMS flow parallel to the surface with little radial component. This means that TMS produces a highly directional current. If TMS is applied using a circular coil, the currents flow in an annulus under the coil. The same occurs in each wing of a figure-of-eight coil, but since these are made such that the two wings overlap at the centre, the currents here summate and are twice as strong as those in the periphery. The outcome is a stimulus current that is aligned along the length of the coil intersection. Finally, it is important to note that TMS does not deliver any net charge to the brain. Effectively, when a current is induced in one direction it always flows back again in the opposite direction. Despite this, stimulators are often classified as ‘monophasic’ or ‘biphasic’ depending on the relative durations of the forwards and backwards currents. ‘Monophasic’ pulses have a high amplitude short flow of current in one direction followed by a smaller but longer duration flow in the backwards direction. ‘Biphasic’ pulses have two phases of equal amplitude and duration.
Both forms of stimulation excite axons rather than cell bodies of cortical neurones. Evidence for this comes from measurement of the strength–duration (S-D) time constant, a measure of how the threshold for stimulation varies with the duration of the stimulus pulse. Burke et al. (1993) estimated that the D wave after TES was initiated at a site that had a S-D time constant of around 400 μs, which is in the same range as simulation of large diameter myelinated axons, and thus presumably represents activation of corticospinal axons. Estimates of the S-D time constant for TMS similarly yield values that are the same as those estimated from TMS of large diameter peripheral nerve axons (Barker et al. 1991; Peterchev et al. 2013).
The direction of the stimulating current is important because axons are activated by differences in potential along their length. Currents that are perpendicular to an axon are far less effective in stimulating than longitudinal currents. This becomes important when we consider TMS. If an axon followed a circular trajectory under the circumference of a round coil, it would be difficult to activate; stimulation would be much more likely if the axon bent out of the circle at any point. Points where an axons bends out of a locally uniform electric field will thus tend to be where the length differential of the field is maximum (Amassian et al. 1992). The outcome is that TMS will preferentially activate different sets of axons depending on their orientation with respect to the induced current (Salvador et al. 2011). One result of this is that the threshold for stimulation of motor cortex depends on the orientation of the induced current pulse, and for the hand area, is usually lowest when the currents flow in a roughly posterior to anterior direction perpendicular to the line of the sulcus (see Fig. 1 for typical stimulus directions and nomenclature).
Figure 1. Descending volleys evoked by electrical and magnetic stimulation and by paired pulse magnetic stimulation.
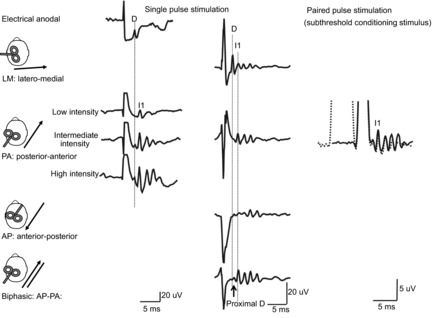
Each trace is the average of the responses to 10–25 cortical stimuli; recordings shown in the three columns have been obtained in three different subjects. Electrical anodal stimulation at threshold intensity evokes the earliest volley, termed D wave. Low intensity magnetic stimulation with a posterior–anterior (PA) induced current in the brain evokes a single descending wave with a latency about 1 ms longer than the D wave evoked by electrical stimulation, termed I1 wave. At intermediate intensity later I waves are evoked and at high intensity, an earlier small wave with the same latency as the D wave evoked by electrical anodal stimulation appears. Magnetic stimulation with a latero-medial (LM) induced current in the brain preferentially evokes D wave activity. With biphasic magnetic stimulation the earliest volley has a latency of about 0.4 ms longer than the D wave evoked by LM magnetic stimulation. Because of its longer latency, it is suggested that the D wave evoked by biphasic stimulation is initiated closer to the cell body of the PTNs than the conventional D wave evoked by LM magnetic stimulation and anodal stimulation and it is termed ‘proximal D wave’. On the right, epidural volleys evoked by test magnetic stimulus alone (continuous trace) and by test magnetic stimulus preceded by a subthreshold conditioning stimulus at 3 ms interstimulus interval (dotted trace). The test stimulus evokes multiple descending waves. There is a clear suppression of the late corticospinal volley when the test magnetic stimulus is preceded by the subthreshold conditioning stimulus.
Transcranial stimulation of motor cortex in humans
Stimulation of the motor cortex can produce twitches in contralateral muscles, which are usually quantified using electromyography to record the resulting motor evoked potential (MEP). However, this is a complex and indirect measure of the motor cortex output since it has been filtered by activity in synaptic connections in the spinal cord. In humans, the most direct way to probe the effects of non-invasive stimulation is to record, as in animal experiments, the activity evoked in the corticospinal output neurones. This can be achieved recording directly from the surface of the spinal cord through electrodes implanted into the epidural space at the high cervical level, typically at C2 level. We will concentrate on this type of evidence below. Unfortunately, since the opportunities to perform such recordings are relatively rare, data often come from a small number of observations on a handful of individuals. However, it is often possible to obtain confirmatory evidence from specialised EMG measurements that can be made in a larger number of volunteers. We will also discuss some of the work done using these approaches.
Epidural spinal recordings have shown that both TMS and TES evoke corticospinal activity as described in animal preparations. Initial studies were performed in anaesthetised patients during surgery (Boyd et al. 1986; Berardelli et al. 1990; Thompson et al. 1991; Burke et al. 1993) and demonstrated that both TES and TMS could produce a series of waves that travelled down the corticospinal tract. However, anaesthesia depressed their recruitment and impeded a full physiological characterisation. A few years later, Kaneko and colleagues (Kaneko et al. 1996) and Nakamura and co-workers (Nakamura et al. 1996) recorded for the first time descending volleys evoked by transcranial stimulation in conscious human subjects from epidural electrodes implanted chronically in the spinal cord for the relief of pain. From 1998 onwards, Di Lazzaro and co-workers performed an extensive series of studies using the same approach (see Di Lazzaro et al. 2012 for a review). The composition of the descending volley depends on the intensity and the nature of the stimulus (electric or magnetic), and, in case of magnetic stimulation, on the direction of the induced current in the brain (Di Lazzaro et al. 2012). This variation suggests that TMS and TES can activate several different circuits or even the same circuits at different sites within the motor cortex.
Direct activation of pyramidal tract neurons
TES at around the threshold for evoking a liminal muscle twitch or MEP evokes a short latency wave (2–2.6 ms; Fig. 1) that descends the cord with a conduction velocity of around 60–70 ms−1 (Boyd et al. 1986). It is not modified in amplitude or latency by changes in motor cortical excitability (such as voluntary contraction) and is therefore believed to originate from direct excitation of corticospinal axons in the subcortical white matter at some distance from the cell body (Di Lazzaro et al. 1998a, 1999). It is thought to be analogous to the D wave described in experimental animal studies. TMS usually evokes I waves (see next section) and only recruits D waves at high intensity. The lowest threshold D wave can usually be evoked by monophasic magnetic stimulation with a focal figure-of-eight stimulating coil and a lateral to medial (LM) induced current in the brain, but the intensity is usually higher than MEP threshold since I waves almost invariably are recruited at lower intensities. It is unclear why this direction is optimal for activating corticospinal axons, but it seems likely to reflect the orientation of the axons as they enter the white matter and turn towards the internal capsule (Amassian et al. 1992). In three individuals, Di Lazzaro et al. (2002d) found that stimulation with a circular coil centred over the vertex with a clockwise inducing current flow, as viewed from above, to preferentially activate the right motor cortex, produced a descending wave with a latency approximately 0.2 ms longer than the D wave elicited by anodal TES or LM magnetic stimulation of the right motor cortex. They argued that since maximum voluntary contraction increases the amplitude of this wave it may be initiated closer to the cell body of the corticospinal neurone, perhaps at the axon hillock region in the grey matter. This would also account for its slightly longer latency than the conventional D wave. Such a proximal D wave was also observed in three individuals using focal TMS with a biphasic current waveform independently of the direction of the forward current (Fig. 1; Di Lazzaro et al. 2001a).
Indirect activation of pyramidal tract neurons
High frequency repetitive discharge
At low intensity, TMS using a focal figure-of-eight stimulating coil and a monophasic posterior to anterior (PA) induced current in the brain evokes a single descending wave with a latency about 1 ms longer than the D wave evoked by TES and LM magnetic stimulation (Fig. 1; Kaneko et al. 1996; Di Lazzaro et al. 1998a,b). It has been suggested that this wave is the result of monosynaptic activation of PTNs originating from presynaptic axons. The sensitivity of this wave to the level of cortical excitability (Di Lazzaro et al. 1998b) supports its presynaptic origin. In analogy with experimental studies in animals it has been termed the I1 wave. At higher stimulus intensities later volleys appear: these are termed late I waves (Fig. 1). A further increase of TMS intensity leads to direct excitation of the corticospinal axons resulting in a D wave with the same latency of the D wave evoked by TES and by LM magnetic stimulation (Fig. 1; Di Lazzaro et al. 1998a). The interpeak interval between I waves is about 1.5 ms, which indicates a discharge frequency of about 600 Hz. Interestingly, high frequency oscillations can be recorded in the EEG following electrical stimulation of the human median nerve (Cracco & Cracco, 1976). Similar bursts in the monkey have been shown to result from bursts of activity at the same frequency in neocortical neurons of S1 (Baker et al. 2003). Neocortical neurones fire once or multiple times at an interval of around 1.5 ms, with spikes tightly locked to electrical stimuli (Baker et al. 2003). If similar neurones are present in M1 and have synaptic connections with PTNs, their activation by magnetic stimuli could in turn evoke bursts of activity that produce the I waves recorded at cervical level.
Epidural recordings show that interventions can selectively influence the amplitude of the I1 or later I waves. Several different studies have shown that the late I waves but not the I1 wave are depressed by a subthreshold conditioning stimulus delivered 1–5 ms before a suprathreshold stimulus (Fig. 1; Nakamura et al. 1997; Di Lazzaro et al. 1998c; Ni et al. 2011b; Weise et al. 2013). The effect is to reduce the amplitude of the MEP evoked by the suprathreshold stimulus in contralateral muscles, an effect known as short interval intracortical inhibition (SICI; Kujirai et al. 1993). SICI is thought to depend on activity in GABAA pathways since it is increased by benzodiazepines such as diazepam and lorazepam (Di Lazzaro et al. 2006a; Teo et al. 2009; Ziemann, 2013). When Di Lazzaro et al. (2000) examined this with simultaneous recordings of the corticospinal volley in one patient they found that the subthreshold conditioning pulse suppressed later I waves more strongly than at baseline (and led to greater inhibition of the MEP).
Late I waves are suppressed in a second protocol, known as short latency afferent inhibition (SAI; Tokimura et al. 2000) in which an electrical stimulus given to the median nerve reduces the amplitude of MEPs evoked 20–25 ms later by a TMS pulse. In five patients, Tokimura et al. (2000) found that the late (but not I1) waves were suppressed coincident with suppression of the MEP. A final protocol that has been studied with epidural recordings is long interval intracortical inhibition. This refers to the interaction between two suprathreshold TMS pulses given about 150 ms apart. Unlike SICI, it is thought to be a GABAB-ergic effect, with the longer lasting inhibition reflecting the longer lasting IPSP that GABAB usually produces (Werhahn et al. 1999). In three and in two patients, respectively, Di Lazzaro et al. (2002c)2002c and Ni et al. (2011b)2011b found that, as with SICI, the first stimulus suppressed late I waves but not the I1 wave.
Two conclusions arise from such observations. First, stimulation at very low intensities can activate inhibitory circuits without evoking sufficient excitatory input to provoke corticospinal discharge and hence recruit an MEP. Thus, the threshold for inhibition is lower than for facilitation. The second conclusion is that I1 and later I waves may have a different origin. One possibility is that TMS activates the axons of neurones with monosynaptic excitatory connections to pyramidal neurones. This input generates the I1 wave. The same or different axons may synapse onto populations of intrinsically bursting neurones that can drive late I wave discharge in pyramidal neurones (Fig. 2). The amount of activity in these oscillating circuits may depend on the level of other excitatory and inhibitory inputs. It will also depend on the intensity of the TMS pulse, since this determines the initial amount of excitatory input they receive.
Figure 2. Schematic representation of motor cortex circuits and possible preferential site of activation using the different techniques of transcranial brain stimulation.
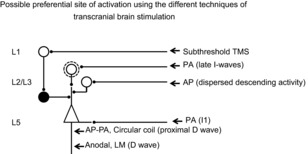
Open circles indicate excitatory neurones while filled circles indicate inhibitory neurones. This model includes a superficial inhibitory circuit composed of layer 1 (L1) neurones that have connections with layer 2 (L2) and 3 (L3) interneurones (filled circle) which inhibit the distal apical dendrites of layer 5 pyramidal neurones (Jiang et al. 2013); L2 and L3 bursting (open circle inside a dotted circle) and non-bursting excitatory interneurones projecting upon the distal apical dendrites of layer 5 pyramidal neurones; cortico-cortical axons projecting upon basal dendrites of layer 5 pyramidal neurones. It is proposed that anodal stimulation and LM magnetic stimulation activate directly the corticospinal axons of pyramidal tract neurones (PTNs) evoking a short latency wave termed D wave. The wave evoked by low intensity magnetic stimulation that appears 1–1.4 ms later than the D wave evoked by electrical anodal stimulation is suggested to be produced by monosynaptic activation of basal dendrites of PTNs by cortico-cortical axons activated by the magnetic stimulus. The late I waves evoked at higher intensities might be produced by a circuit that involves cortico-cortical axons that activate L2 and L3 bursting neurones, and in turn activate PTN apical dendrites. It is proposed that the more dispersed descending activity evoked by AP magnetic stimulation is produced by a different circuit that might include non-bursting L2 and L3 interneurones projecting upon PTN apical dendrites. It is also proposed that the longer latency D wave evoked by biphasic stimulation is initiated closer to the cell body of the PTNs at axon hillock level, rather than at some distance down the axon. It is proposed that inhibitory effects produced by a subthrehsold conditioning stimulus originate from a selective enhancement of the excitability of the GABAergic circuit originating from L1 neurones and projecting upon PTNs apical dendrites, resulting in a selective suppression of the late I waves.
There are two ways in which the conditioning inputs could reduce recruitment of late I waves. They could decrease the excitability of the oscillating neurones which produce the late I waves or, alternatively, they might directly suppress the excitability of the PTNs (Di Lazzaro et al. 2000). Even at this location, there would be relatively little effect on the I1 wave if we postulate that I1 inputs target regions of the cell body that are closer to the site of impulse initiation than most GABAergic inputs. The location of the neurones responsible for inhibition is unknown, although it is likely that they differ between SAI and SICI (Stefan et al. 2002; Di Lazzaro et al. 2006a; Alle et al. 2009; Teo et al. 2009; Udupa et al. 2014). One possibility is that they involve the layer 2/3 interneurones that inhibit the apical dendrites of pyramidal cells. These neurones have reciprocal connections with layer 1 neurogliaform cells (Jiang et al. 2013). Because of their superficial location, the axons of layer 1 neurones might be activated by low intensity magnetic stimulation in the SICI protocol and in turn suppress late I wave input onto pyramidal cell dendrites (Fig. 2).
It should be recognised that this model of I wave origin is one of many possibilities that range from oscillating chains of neurones which repeatedly bombard the corticospinal neurones with excitatory inputs to models in which the bulk of the repetitive activity is driven by the membrane electrical properties of the corticospinal cells (Rusu et al. 2014). The observations here cannot distinguish between these models.
Less synchronised longer latency descending waves
When the direction of the induced current in the brain is reversed from the usual PA direction to AP, the threshold for stimulation increases and the latency of the MEP, particularly in precontracted muscle, is delayed by 2–3 ms in many individuals (e.g. Sakai et al. 1997; Hamada et al. 2013). Di Lazzaro et al. (2001b)2001b found that the corticospinal volleys evoked by AP stimulation had slightly later peak latencies and/or longer durations than those seen after PA stimulation (Fig. 1). In addition, the order of I wave recruitment was not always as clear as with PA stimulation; sometimes later I waves were recruited at lower intensities than the I1 wave, and in these cases the MEP onset was correspondingly delayed.
There are several possible reasons for the delayed I waves. For example, an AP stimulus could activate the same axons as PA stimulation at a site more distant from M1. It might also activate a different set of axons with a slightly longer conduction time. However, neither possibility explains why the I waves are not only later but are also more dispersed and have a more variable recruitment order than those evoked by PA stimulation. Another possibility is that these longer latency I waves are produced by activation of a circuit(s) with a slightly different periodicity that is separate from those evoking the highly synchronised I waves.
A more probable explanation is that AP stimulation activates not only oscillatory networks that produce the highly synchronised I waves, but also other axons of non-bursting cells that can activate PTNs at longer latencies (Fig. 2). This non-oscillatory activity will be more dispersed because the discharge of individual neurones is less time-locked to each stimulus and thus not only it will not summate in discrete peaks, but will also produce a partial phase cancellation of I waves peaks, making the I wave activity less clear and more dispersed. This input could arise from many sources, although it is interesting to note that Shimazu et al. (2004) identified a short latency excitatory input from ventral premotor cortex to M1 in the anaesthetised monkey, and Groppa et al. (2012) described a similar short latency facilitatory input from dorsal premotor cortex in humans. Both inputs summated particularly well when timed to coincide with the peaks of I wave output, but also had smaller effects at less specific intervals, implying the existence of a longer lasting facilitation. AP stimulation, which induces current from anterior to posterior in the brain, might be particularly effective in recruiting inputs from premotor areas to M1. The hypothesis that different ensembles of bursting and non-bursting cortical interneurones can be activated by artificial forms of stimulation is supported by the recordings in S1 after median nerve stimulation that showed that only about 45% of cortical interneurones respond with more than one spike at discrete intervals after the stimulus, while the remaining interneurones give a single spike (Baker et al. 2003).
It is important to note that these epidural recordings were only made in four individuals. The tendency for AP stimulation to recruit preferentially the late I waves rather than the I1 wave is backed up by EMG studies noted below, as is the notion that I waves produced by AP and PA stimulation may involve different sets of neural inputs. However, there is no other evidence to confirm that the I wave peak latencies tend to be slightly later and more dispersed than after PA stimulation.
Finally, we note that the possibility of evoking more dispersed descending activity is also suggested by recordings of the epidural activity after a paired TMS protocol designed to produce intracortical facilitation (ICF). It is a protocol similar to SICI but with a longer interstimulus interval (ISI) of 10–25 ms, between the subthreshold conditioning stimulus and the test stimulus. Conditioning stimuli at these intervals facilitate MEPs but epidural recordings show that there is no significant change in the amplitude or number of descending corticospinal waves (Di Lazzaro et al. 2006b; Ni et al. 2011b). This form of facilitation might result from the recruitment of circuits separate from those involved in I wave generation, producing additional descending activity that is more dispersed and thus not evident in the epidural records.
Confirmatory evidence from EMG recordings: single unit studies
Although epidural recordings have only been performed in a small number of patients, many of the conclusions about recruitment of I waves have been confirmed by recording the response of single motor units (Fig. 3). Each motor unit in a muscle is innervated by a single motorneurone in the spinal cord, so that the discharge recorded in the periphery is a direct measure of the firing of that spinal neurone. TMS/TES is applied while participants voluntarily maintain the discharge of the unit at around 10 Hz. One hundred or so stimuli are usually given so that a histogram can be constructed of the times at which the unit discharges after the pulse (a peri-stimulus time histogram, PSTH; see papers by Day et al. 1989; Hanajima et al. 1998a). A just-suprathreshold pulse of TES increases the probability that the unit will fire some 25 ms later (the exact latency depends on the peripheral conduction time from spinal cord to muscle). The peak of increased probability lasts only 1–2 ms, and reflects the rise time of the EPSP released at the motorneurone by a single descending volley (the D wave) evoked by the TES pulse. Higher intensity TES pulses recruit further peaks in the PSTH, separated by about 1.5 ms intervals, consistent with arrival of multiple later I waves. TMS with a just-suprathreshold PA oriented pulse usually evokes a single increase in firing probability that starts about 1.5 ms after the earliest peak evoked by TES. This is presumed to be the EPSP released by the I1 wave. As the intensity is increased, later peaks occur, corresponding to the later I waves. Finally, AP stimulation often recruits only later peaks in the PSTH, consistent with activation of later I waves.
Figure 3. .
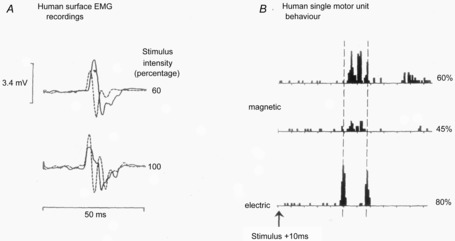
A, surface EMG responses in the preactivated first dorsal interosseous muscle after two different intensities of TMS (continuous lines) and TES (dashed lines). At an intensity of 60%, the MEP to TMS has a longer latency than the response to TES; however, this difference disappears at 100%. B, comparison of TMS and TES activation of a single motor unit in the FDI muscle (same unit all traces, x-axis calibration intervals are 2.5 ms). The histograms show the number of times the unit discharged at each interval after a single TES or TMS pulse. Note that TES evokes a very early peak of increased firing plus one about 5 ms later. In contrast the earliest increase in discharge after TMS occurs about 1.5 ms later than after TES. It is followed by one or two other peaks as the intensity of TMS increases from 45–60%. These increases in firing probability are thought to be caused by arrival of EPSPs at the spinal motoneurone released by D (TES only) and I wave (both TES and TMS) volleys in the corticospinal tract. (A from Day et al. 1989; B from Rothwell et al. 1991).
It is difficult to study single unit responses to high intensity TMS pulses that appear to recruit D waves in the epidural recordings. However, evidence that such recruitment does happen comes from examining the latency of the surface EMG response in pre-activated muscle. The latency of MEPs to TES is usually about 1.5 ms earlier than to PA TMS (Day et al. 1989). However, at higher intensities, the TMS evoked MEP eventually matches that of TES (see Fig. 3). The same argument can be used to confirm that LM pulses usually recruit D waves at relatively lower thresholds than other orientations of TMS.
Hanajima et al. (1998b)1998b also examined how SICI affected recruitment of PSTH peaks. They found that the later peaks were suppressed during SICI, but that the earliest peaks to PA stimulation were unaffected. Again, this appears to confirm the epidural data showing a preferential suppression of late I waves by SICI. The same group also pointed out that because SICI selectively targets late I waves, it is difficult to interpret measurement of SICI in different patient populations (Hanajima & Ugawa, 1998; Hanajima et al. 1999, 2007). If SICI is reduced, is it because the GABAergic circuits are less excitable, or is it because the test pulse recruits proportionately more early than late I waves or more D than I waves (Hanajima et al. 2008)? We return to this topic in the section below.
Implications for interpreting MEPs
Even though we have no validated neural model of I wave generation, the results above have important implications for how we interpret MEP differences between individuals (e.g. with and without a certain disease). They show that: (1) different types of stimulation (electrical, magnetic with round coil or figure-of-eight coil with PA, AP or LM oriented current) activate different combinations of I waves; (2) longer latency descending waves evoked with one form of stimulation (PA) may differ from those evoked by another form of stimulation (AP); and (3) stimulation may activate corticospinal neurones outside of the I wave pattern (non-synchronised discharges). The outcome is that an MEP of a given amplitude could be the result of any pattern of D and I wave inputs.
On a practical basis, these points help us to understand why paired pulse conditioning protocols often have different effects on MEPs evoked by different forms of stimulation. For example, it is well known that MEPs elicited by TMS are suppressed by the SICI protocol whereas those evoked by TES are not (Kujirai et al. 1993). Indeed, the fact that MEPs evoked by TES have a much higher proportion of D wave activity than I wave activity is often used to indicate that a conditioning effect on MEP is due to an action on cortical circuitry rather than spinal circuits since if the latter were correct then MEPs evoked by TMS and TES would be more likely to be equally affected.
Less well known is the fact that I waves evoked by PA and AP stimulation are also differentially affected by conditioning protocols. Epidural recordings show that SAI produced by stimulation of the median nerve at the wrist (see next section) suppresses late I waves and MEPs evoked by PA TMS. However, in healthy volunteers, SAI is less effective in suppressing MEPs evoked by AP than PA stimulation (Ni et al. 2011a), implying that the late I waves evoked by the two directions of stimulation differ in some way.
The conclusion is that different forms of TES and TMS activate different inputs to corticospinal neurones. However, both epidural (Di Lazzaro et al. 2001b) and MEP recordings (Hamada et al. 2013) show that each individual may respond in a specific way to certain types of transcranial stimulation, particularly AP TMS, suggesting that the efficiency of late I wave recruitment varies between subjects (Hamada et al. 2013). If this is the case, then there is one highly important implication. Individual differences in I wave inputs that are evoked by TMS will necessarily contribute to interindividual differences in response to conditioning protocols. An individual may respond poorly to SICI not because of reduced excitability of the GABA system but because of the composition of the I waves recruited by the test pulse is less sensitive to the SICI input. Thus, the data may be telling us as much about the mechanism of the test MEP as it is about the sensitivity of the conditioning protocol being tested. These considerations have clear implications for how we interpret correlations between SICI, or SAI etc., and behavioural measures. It is equally relevant for comparisons between patient populations.
Modulating corticospinal activity using protocols of prolonged non-invasive brain stimulation
Recently, several techniques of repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (TDCS) have been introduced that can induce long-lasting changes in motor cortex excitability in a manner which is biologically similar to long term potentiation/depression (LTP/LTD) described at cellular level. Most of the after-effects have been described in terms of the changes that are produced in threshold or size of evoked MEPs. Nevertheless a few studies have examined accompanying effects on the composition of descending corticospinal volleys in order to understand more about their mechanism and site of action (see Di Lazzaro et al. 2010 for a review). Interestingly, it appears that although most protocols selectively modulate late I waves some of them can also modulate selectively the D and I1 waves (Di Lazzaro et al. 2010; Fig. 4).
Figure 4. Epidural volleys recorded in baseline conditions (black trace) and after several excitatory (red traces) and inhibitory (green traces) neuromodulation protocols.
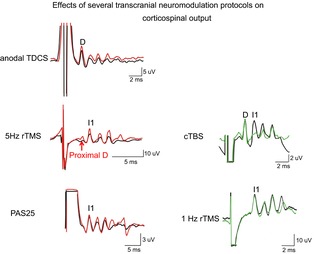
Each trace is the average of the responses to 10–25 cortical magnetic stimuli. After anodal transcranial direct current stimulation (TDCS) a D and I wave enhancement is observed. 5 Hz repetitive transcranial magnetic stimulation (rTMS) increases the amplitude of the proximal D wave and all I waves. Paired associative stimulation at 25 ms interstimulus interval (PAS25) produces a selective facilitation of late I waves with no change in I1 wave. After continuous theta burst stimulation (cTBS) the amplitude of the I1 wave is suppressed. 1 Hz rTMS produces a selective suppression of late I waves with no change in I1 wave.
The protocols that have been studied in terms of effects on corticospinal volleys are:
Paired associative stimulation (PAS) is thought to engage a form of spike timing dependent plasticity, in which an electrical stimulus to the median nerve is applied about 10 (PAS10) or 25 ms (PAS25) prior to a TMS pulse to the hand area of motor cortex. After 100 or more pairings, MEPs are reduced after PAS10 and increased after PAS25 for about 30 min after the end of the PAS (Stefan et al. 2000; Wolters et al. 2003).
Theta burst stimulation (TBS) is based on the theta burst stimulation protocol used to induce LTP in experimental preparations. The protocol uses short bursts of low intensity (80% of active motor threshold), high frequency (50 Hz) pulses, repeated at 5 Hz, the frequency of the theta rhythm in the EEG. Different patterns of delivery of TBS (continuous versus intermittent) produce opposite effects on synaptic efficiency of the stimulated motor cortex (Huang et al. 2005). The protocol named continuous theta-burst stimulation (cTBS) produces a consistent LTD-like effect, causing a prolonged decrease of motor cortex excitability while the protocol named intermittent theta-burst stimulation (iTBS) produces a consistent LTP-like effect (Huang et al. 2005).
Paired pulse repetitive TMS (PprTMS) is another protocol of repetitive stimulation is based on repetitive paired pulse stimulation at I wave periodicity (Thickbroom et al. 2006). This protocol uses pairs of TMS stimuli of equal intensity with a 1.5 ms interstimulus interval, delivered for several minutes at a rate of 0.2 Hz (Thickbroom et al. 2006).
Regular frequency rTMS can be applied at any rate. 1 Hz and 5 Hz protocols have been examined with epidural recordings. The 1 Hz protocol involves applying one thousand or more pulses at a rate of one per second with a suprathreshold stimulus pulse (Chen et al. 1997). It produces a long lasting suppression of excitability. In contrast, the 5 Hz protocol that has been tested examines short term facilitation. Twenty suprathreshold stimuli are given at 5 Hz: the MEP evoked by each pulse gradually increases, with an after-effect lasting only a second or so (Pascual-Leone et al. 1994; Berardelli et al. 1998).
Transcranial direct current stimulation (TDCS) modulates cortical excitability in a polarity-specific manner when assessed using transcranial magnetic stimulation (TMS): motor evoked potentials (MEPs) in contralateral hand muscles are facilitated by anodal TDCS and suppressed by cathodal TDCS of primary motor cortex (Nitsche & Paulus, 2000). Consistent with animal data, these changes in excitability persist beyond the time of stimulation if TDCS is given for several minutes and can remain stable for an hour or more if TDCS is applied for 9 min or longer (Nitsche & Paulus, 2001; Nitsche et al. 2003; Lang et al. 2004).
The effects of these different protocols on epidural volleys are not homogeneous because each protocol can modulate specific neural elements in different layers of the cortex (Fig. 4). At least six patterns can be identified. Unfortunately there are no epidural recordings of the effects of quadripulse stimulation (Hamada et al. 2008), which is a highly effective method that produces long lasting suppression or facilitation of MEPs depending on the interval between the pulses.
Pattern 1: modulation of the D wave and all I waves (changes in excitability of corticospinal axons and cortico-cortical axons/synapses)
Twenty minutes of anodal TDCS of primary motor cortex increases both D and I wave responses to TMS pulses. The amplitude of the D wave increases by about 25% for at least 15 min after the end of TDCS (Fig. 4; Lang et al. 2011; Di Lazzaro et al. 2013). The increase in I wave activity lasts for a shorter time, no longer being present at 15 min post TDCS (Lang et al. 2011). This would be consistent with an effect of TDCS on the excitability of both corticospinal axons (D wave initiation; Fig. 5) and the I wave generating circuitry. The latter could involve effects on the threshold for stimulating the axons of input into fast-spiking neurones, the efficacy of synaptic transmission or the intrinsic properties of fast spiking interneurones.
Figure 5. Possible layer specific modulation of cortical circuits by different protocols of transcranial stimulation.
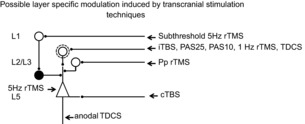
It is proposed that 5 Hz subthreshold rTMS leads to a suppression of the excitability of the superficial inhibitory circuits, including superficial (L1) neurons, and that most of the protocols (iTBS, PAS25, PAS10, 1 Hz rTMS, TDCS) selectively modulate bursting cells of layer 2 and 3 that project upon PTNs and generate the late I waves. We suggest that paired pulse (Pp) rTMS might modulate the excitability of non-bursting layer 2 and 3 interneurones enhancing the non-synchronous activity that cannot be seen as a change in I wave activity. It is proposed that cTBS selectively suppresses the excitability of monosynaptic connections to PTNs. 5 Hz rTMS may produce its effects by enhancing the excitability of PTNs. Anodal TDCS together with a short lived enhancement of late I waves produces a strong and prolonged enhancement in the amplitude of the D wave, perhaps by enhancing the excitability of corticospinal axons.
Pattern 2: modulation of the proximal D wave and I waves (changes in excitability of PTNs)
An increase in amplitude of D, I1 and late I waves has been observed after 5 Hz rTMS (Di Lazzaro et al. 2002a; Fig. 4). In contrast with TDCS, the D wave that is increased after 5 Hz rTMS is the proximal D wave that is believed to originate at the level of the axon hillock of PTNs (Di Lazzaro et al. 2002a). The increase in the amplitude of this proximal D wave suggests that the excitability of pyramidal neurones is enhanced compared to their resting state (Fig. 5). The enhancement in the amplitude of all the descending waves might also be related to an increase in excitability of PTNs.
Pattern 3: selective modulation of I1 wave (changes in excitability of the initial monosynaptic input to PTNs)
The cTBS protocol suppresses the I1 wave, whilst later I waves are much less affected (Fig. 4; Di Lazzaro et al. 2005). This suggests that cTBS has its major effect on the synapse between the inputs responsible for the I1 wave and the PTNs (Fig. 5).
Pattern 4: selective modulation of late I waves (changes in excitability of the bursting interneurones connected to PTNs)
This is the most commonly observed change after repetitive brain stimulation. A selective suppression of late I waves with no change in the amplitude of the I1 wave is observed after 1 Hz rTMS (Di Lazzaro et al. 2008b) and PAS10 (Di Lazzaro et al. 2009b), while a selective enhancement of late I waves with no change in the amplitude of the I1 wave is observed after iTBS (Di Lazzaro et al. 2008a) and PAS25 (Di Lazzaro et al. 2009a; Figs 4 and 5).
Pattern 5: changes in MEPs with no change in D or I waves (changes in excitability of the non-bursting interneurones connected to PTNs)
PprTMS increases the amplitude of MEPs and is considered to take place at cortical level because MEPs evoked by cervico-medullary junction stimulation are not affected by PprTMS (Hamada et al. 2007). The effects of PprTMS on epidural activity were evaluated in a single patient with high cervical epidural electrode (Di Lazzaro et al. 2007). In this patient a pronounced increase in MEP amplitude was paralleled by only a slight increase in the amplitude of epidural volleys (Di Lazzaro et al. 2007). As speculated for ICF, it is suggested that in addition to the I waves there might be additional activity, possibly produced by non-bursting cells connected to PTNs that produce a more dispersed excitatory output that is not evident in the epidural recordings. It is this that may be selectively increased by PprTMS (Fig. 5).
Pattern 6: changes in short interval intracortical inhibition with no change in the output produced by single pulse TMS
Several authors investigated the effects of rTMS protocols on intracortical inhibitory activity as evaluated with SICI protocol (Daskalakis et al. 2006). It was shown that 5 Hz rTMS may reduce SICI (Peinemann et al. 2000; Wu et al. 2000; Quartarone et al. 2005). The effects of 5 Hz rTMS on intracortical inhibitory activity was evaluated by epidural recording in two patients (Di Lazzaro et al. 2002b). Because the threshold for activating intracortical inhibitory circuits is lower than the MEP-threshold (Kujirai et al. 1993), in the study by Di Lazzaro and co-workers (Di Lazzaro et al. 2002b) a very low intensity rTMS was used. This was done in order to evaluate whether a low intensity rTMS protocol at 5 Hz might have effects limited to intracortical inhibitory circuits. The study showed that subthreshold 5 Hz rTMS (total of 50 stimuli was given at an intensity of active motor threshold) has no effect on MEP amplitude but reduced SICI (Di Lazzaro et al. 2002b), suggesting that low-intensity rTMS at 5 Hz can selectively modify the excitability of GABAergic inhibitory networks in the motor cortex. The inhibitory circuit described by Jang and co-workers involving the layer 1 neurones (Jiang et al. 2013) might be modulated by low intensity rTMS resulting in less pronounced SICI.
Cumulatively, the above findings confirm that the main changes produced by protocols of neuromodulation take place at the cortical level. In addition, they show that different protocols have selective effects on specific circuits of the human motor cortex. Thus, as observed using paired pulse stimulation protocols, cortical circuits generating I1 and later I waves can be modulated independently. This means that protocols that appear to have similar effects when evaluated using MEPs actually have quite different effects on cortical excitability. Finally, it is important to note that MEP data show that there is substantial variation between individuals in the response to all these protocols (Hamada et al. 2013; Wiethoff et al. 2014). It may be that part of the reason for this variation relates to individual differences in the recruitment of each network during application of the protocol.
Implications
In the first half of this review we pointed out that not only do different forms of TES and TMS evoke different patterns of I wave input to corticospinal neurones, but also that different individuals respond in a characteristic way to a given form of TMS/TES stimulation. The results reviewed above show that different ‘plasticity’ protocols also affect particular subsets of inputs onto corticospinal neurones. The implications are the same as for paired pulse conditioning protocols: the response of any individual to any protocol may depend on either the ‘amount of plasticity’ or on the composition of the MEP used to test the effect. If a person responds poorly to a certain plasticity protocol it may because the TMS test pulse fails to recruit in that individual the I-inputs affected by plasticity, rather than a poor ‘plasticity’ response. The argument holds as well for differences in healthy populations as in patient groups.
Is there a way out of this dilemma without having to record the composition of the corticospinal volley in each individual? One possibility is to try to infer the composition of the volleys by indirect measures. One rough way is to compare onset latencies of threshold MEPs in activated muscle with the minimum D wave latency to determine whether stimulus is preferentially recruiting early or late I waves. Another example might work as follows. A group of patients has a smaller response to PAS25 than a control group. Is this because there is less ‘plasticity’ in the patients, or because the I-inputs relevant for PAS25 are not being recruited by the test pulse in the patient group? We know that the I waves that are targeted by PAS25 are the same as those that respond to SICI. Thus, if the patient population had good SICI, we can assume that the test pulse is at least capable of recruiting that population of I-inputs so that the lack of response to PAS25 may well indicate ‘reduced plasticity’.
However, such arguments are tenuous and indirect. Perhaps in the future it will be possible by coupling the advances currently being made to model the electrical fields induced in individual brains (e.g. Thielscher et al. 2011) with the knowledge of cortical circuits to develop forms of stimulation that can reliably target more specific populations of neurones (Nummenmaa et al. 2014). Recent developments suggest that this may indeed be possible. Opitz et al. (2014) have shown that the optimal coil orientation for stimulation of the motor cortex hand area with a biphasic TMS pulse is related in individual volunteers to the curvature of the anatomical ‘hand knob’. Individuals in whom the hand knob has the usual shape of an inverted omega respond best with the usual AP orientation of the coil (as shown in Fig. 1, this is approximately 45 deg clockwise from a true parasaggital direction), whereas an individual in whom the hand knob was shaped like an epsilon responded best at 90 deg to the parasaggital direction. If this could be extended to predict what proportion of late or early I waves would be recruited in each individual it would allow us to understand much more clearly the individual differences in response to non-invasive brain stimulation protocols.
Glossary
- AP
anterior to posterior
- ICF
intracortical facilitation
- ISI
interstimulus interval
- LM
lateral to medial
- LTP/LTD
long term potentiation/depression
- MEP
motor evoked potential
- PA
posterior to anterior
- PAS
paired associative stimulation
- PprTMS
paired pulse repetitive TMS
- PSTH
peri-stimulus time histogram
- PTN
pyramidal tract neurone
- rTMS
repetitive transcranial magnetic stimulation
- SAI
short latency afferent inhibition
- SICI
short interval intracortical inhibition
- TBS
theta burst stimulation
- TDCS
transcranial direct current stimulation
- TES
transcranial electric stimulation
- TMS
transcranial magnetic stimulation
Additional information
Competing interests
The authors have no conflict of interests.
Funding
No funding was received.
References
- Adrian ED, Moruzzi G. Impulses in the pyramidal tract. J Physiol. 1939;97:153–199. doi: 10.1113/jphysiol.1939.sp003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H, Heidegger T, Krivanekova L, Ziemann U. Interactions between short-interval intracortical inhibition and short-latency afferent inhibition in human motor cortex. J Physiol. 2009;587:5163–5176. doi: 10.1113/jphysiol.2009.179820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amassian VE, Eberle L, Maccabee PJ, Cracco RQ. Modelling magnetic coil excitation of human cerebral cortex with a peripheral nerve immersed in a brain-shaped volume conductor: the significance of fiber bending in excitation. Electroencephalogr Clin Neurophysiol. 1992;85:291–301. doi: 10.1016/0168-5597(92)90105-k. [DOI] [PubMed] [Google Scholar]
- Baker SN, Curio G, Lemon RN. EEG oscillations at 600 Hz are macroscopic markers for cortical spike bursts. J Physiol. 2003;550:529–534. doi: 10.1113/jphysiol.2003.045674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AT, Garnham CW, Freeston IL. Magnetic nerve stimulation: the effect of waveform on efficiency, determination of neural membrane time constants and the measurement of stimulator output. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:227–237. [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Cruccu G, Manfredi M. Descending volley after electrical and magnetic transcranial stimulation in man. Neurosci Lett. 1990;112:54–58. doi: 10.1016/0304-3940(90)90321-y. [DOI] [PubMed] [Google Scholar]
- Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Curra A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122:79–84. doi: 10.1007/s002210050493. [DOI] [PubMed] [Google Scholar]
- Boyd SG, Rothwell JC, Cowan JM, Webb PJ, Morley T, Asselman P, Marsden CD. A method of monitoring function in corticospinal pathways during scoliosis surgery with a note on motor conduction velocities. J Neurol Neurosurg Psychiatry. 1986;49:251–257. doi: 10.1136/jnnp.49.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M. Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol. 1993;470:383–393. doi: 10.1113/jphysiol.1993.sp019864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Cracco RQ, Cracco JB. Somatosensory evoked potential in man: far field potentials. Electroencephalogr Clin Neurophysiol. 1976;41:460–466. doi: 10.1016/0013-4694(76)90057-2. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Moller B, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp Brain Res. 2006;174:403–412. doi: 10.1007/s00221-006-0472-0. [DOI] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens De Noordhout A, Marsden CD, Nakashima K, Rothwell JC, Thompson PD. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Pilato F, Profice P, Oliviero A, Mazzone P, Insola A, Capone F, Ranieri F, Tonali PA. Associative motor cortex plasticity: direct evidence in humans. Cereb Cortex. 2009a;19:2326–2330. doi: 10.1093/cercor/bhn255. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Dileone M, Profice P, Pilato F, Oliviero A, Mazzone P, Di Iorio R, Capone F, Ranieri F, Florio L, Tonali PA. LTD-like plasticity induced by paired associative stimulation: direct evidence in humans. Exp Brain Res. 2009b;194:661–664. doi: 10.1007/s00221-009-1774-9. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Berardelli A, Mazzone P, Insola A, Pilato F, Saturno E, Dileone M, Tonali PA, Rothwell JC. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002a;144:549–553. doi: 10.1007/s00221-002-1106-9. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Insola A, Pilato F, Saturno E, Accurso A, Tonali P, Rothwell JC. Comparison of descending volleys evoked by monophasic and biphasic magnetic stimulation of the motor cortex in conscious humans. Exp Brain Res. 2001a;141:121–127. doi: 10.1007/s002210100863. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Dileone M, Insola A, Tonali PA, Rothwell JC. Short-term reduction of intracortical inhibition in the human motor cortex induced by repetitive transcranial magnetic stimulation. Exp Brain Res. 2002b;147:108–113. doi: 10.1007/s00221-002-1223-5. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Insola A, Visocchi M, Colosimo C, Tonali PA, Rothwell JC. Direct demonstration of long latency cortico-cortical inhibition in normal subjects and in a patient with vascular parkinsonism. Clin Neurophysiol. 2002c;113:1673–1679. doi: 10.1016/s1388-2457(02)00264-x. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Insola A, Mazzone P, Tonali PA, Rothwell JC. Descending volleys evoked by transcranial magnetic stimulation of the brain in conscious humans: effects of coil shape. Clin Neurophysiol. 2002d;113:114–119. doi: 10.1016/s1388-2457(01)00696-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial electrical stimulation over the motor cortex hand area in conscious humans. Exp Brain Res. 1999;124:525–528. doi: 10.1007/s002210050649. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol. 1998a;109:397–401. doi: 10.1016/s0924-980x(98)00038-1. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Pilato F, Insola A, Mazzone P, Profice P, Tonali P, Rothwell JC. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp Brain Res. 2001b;138:268–273. doi: 10.1007/s002210100722. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Meglio M, Tonali PA, Rothwell JC. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J Physiol. 2008a;586:3871–3879. doi: 10.1113/jphysiol.2008.152736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Tonali PA, Rothwell JC. Low-frequency repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2008b;586:4481–4487. doi: 10.1113/jphysiol.2008.159558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Ranieri F, Ricci V, Profice P, Bria P, Tonali PA, Ziemann U. GABAA receptor subtype specific enhancement of inhibition in human motor cortex. J Physiol. 2006a;575:721–726. doi: 10.1113/jphysiol.2006.114694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Oliviero A, Dileone M, Saturno E, Mazzone P, Insola A, Profice P, Ranieri F, Capone F, Tonali PA, Rothwell JC. Origin of facilitation of motor-evoked potentials after paired magnetic stimulation: direct recording of epidural activity in conscious humans. J Neurophysiol. 2006b;96:1765–1771. doi: 10.1152/jn.00360.2006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, Insola A, Tonali PA, Ranieri F, Huang YZ, Rothwell JC. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005;565:945–950. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Pilato F, Dileone M, Oliviero A, Ziemann U. The effects of motor cortex rTMS on corticospinal descending activity. Clin Neurophysiol. 2010;121:464–473. doi: 10.1016/j.clinph.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Profice P, Ranieri F, Capone F, Dileone M, Oliviero A, Pilato F. I wave origin and modulation. Brain Stimul. 2012;5:512–525. doi: 10.1016/j.brs.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Ranieri F, Profice P, Pilato F, Mazzone P, Capone F, Insola A, Oliviero A. Transcranial direct current stimulation effects on the excitability of corticospinal axons of the human cerebral cortex. Brain Stimul. 2013;6:641–643. doi: 10.1016/j.brs.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J Physiol. 1998b;508:625–633. doi: 10.1111/j.1469-7793.1998.625bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998c;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Thickbroom GW, Pilato F, Profice P, Dileone M, Mazzone P, Insola A, Ranieri F, Tonali PA, Rothwell JC. Direct demonstration of the effects of repetitive paired-pulse transcranial magnetic stimulation at I wave periodicity. Clin Neurophysiol. 2007;118:1193–1197. doi: 10.1016/j.clinph.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Groppa S, Werner-Petroll N, Munchau A, Deuschl G, Ruschworth MF, Siebner HR. A novel dual-site transcranial magnetic stimulation paradigm to probe fast facilitatory inputs from ipsilateral dorsal premotor cortex to primary motor cortex. Neuroimage. 2012;62:500–509. doi: 10.1016/j.neuroimage.2012.05.023. [DOI] [PubMed] [Google Scholar]
- Hamada M, Hanajima R, Terao Y, Arai N, Furubayashi T, Inomata-Terada S, Yugeta A, Matsumoto H, Shirota Y, Ugawa Y. Origin of facilitation in repetitive, 1.5 ms interval, paired pulse transcranial magnetic stimulation (rPPS) of the human motor cortex. Clin Neurophysiol. 2007;118:1596–1601. doi: 10.1016/j.clinph.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Hamada M, Murase N, Hasan A, Balaratnam M, Rothwell JC. The role of interneuron networks in driving human motor cortical plasticity. Cereb Cortex. 2013;23:1593–1605. doi: 10.1093/cercor/bhs147. [DOI] [PubMed] [Google Scholar]
- Hamada M, Terao Y, Hanajima R, Shirota Y, Nakatani-Enomoto S, Furubayashi T, Matsumoto H, Ugawa Y. Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J Physiol. 2008;586:3927–3947. doi: 10.1113/jphysiol.2008.152793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Nomura Y, Segawa M, Ugawa Y. Intracortical inhibition of the motor cortex in Segawa disease (DYT5) Neurology. 2007;68:1039–1044. doi: 10.1212/01.wnl.0000257816.92101.54. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Okabe S, Terao Y, Furubayashi T, Arai N, Inomata-Terada S, Hamada M, Yugeta A, Ugawa Y. Difference in intracortical inhibition of the motor cortex between cortical myoclonus and focal hand dystonia. Clin Neurophysiol. 2008;119:1400–1407. doi: 10.1016/j.clinph.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y. Impaired motor cortex inhibition in patients with ALS: evidence from paired transcranial magnetic stimulation. Neurology. 1998;51:1771–1772. doi: 10.1212/wnl.51.6.1771. [DOI] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Furubayashi T, Machii K, Shiio Y, Enomoto H, Uesugi H, Mochizuki H, Kanazawa I. Intracortical inhibition of the motor cortex is normal in chorea. J Neurol Neurosurg Psychiatry. 1999;66:783–786. doi: 10.1136/jnnp.66.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998a;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanajima R, Ugawa Y, Terao Y, Sakai K, Furubayashi T, Machii K, Kanazawa I. Paired-pulse magnetic stimulation of the human motor cortex: differences among I waves. J Physiol. 1998b;509:607–618. doi: 10.1111/j.1469-7793.1998.607bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Jiang X, Wang G, Lee AJ, Stornetta RL, Zhu JJ. The organization of two new cortical interneuronal circuits. Nat Neurosci. 2013;16:210–218. doi: 10.1038/nn.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K, Kawai S, Fuchigami Y, Morita H, Ofuji A. The effect of current direction induced by transcranial magnetic stimulation on the corticospinal excitability in human brain. Electroencephalogr Clin Neurophysiol. 1996;101:478–482. doi: 10.1016/s0013-4694(96)96021-x. [DOI] [PubMed] [Google Scholar]
- Kernell D, Chien-Ping WU. Responses of the pyramidal tract to stimulation of the baboon's motor cortex. J Physiol. 1967;191:653–672. doi: 10.1113/jphysiol.1967.sp008273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N, Nitsche MA, Dileone M, Mazzone P, De Andres-Ares J, Diaz-Jara L, Paulus W, Di Lazzaro V, Oliviero A. Transcranial direct current stimulation effects on I wave activity in humans. J Neurophysiol. 2011;105:2802–2810. doi: 10.1152/jn.00617.2010. [DOI] [PubMed] [Google Scholar]
- Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res. 2004;156:439–443. doi: 10.1007/s00221-003-1800-2. [DOI] [PubMed] [Google Scholar]
- Merton PA, Morton HB. Stimulation of the cerebral cortex in the intact human subject. Nature. 1980;285:227. doi: 10.1038/285227a0. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Direct and indirect activation of human corticospinal neurons by transcranial magnetic and electrical stimulation. Neurosci Lett. 1996;210:45–48. doi: 10.1016/0304-3940(96)12659-8. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Kitagawa H, Kawaguchi Y, Tsuji H. Intracortical facilitation and inhibition after transcranial magnetic stimulation in conscious humans. J Physiol. 1997;498:817–823. doi: 10.1113/jphysiol.1997.sp021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Charab S, Gunraj C, Nelson AJ, Udupa K, Yeh IJ, Chen R. Transcranial magnetic stimulation in different current directions activates separate cortical circuits. J Neurophysiol. 2011a;105:749–756. doi: 10.1152/jn.00640.2010. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C, Wagle-Shukla A, Udupa K, Mazzella F, Lozano AM, Chen R. Direct demonstration of inhibitory interactions between long interval intracortical inhibition and short interval intracortical inhibition. J Physiol. 2011b;589:2955–2962. doi: 10.1113/jphysiol.2011.207928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial direct current stimulation in humans. J Physiol. 2003;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nummenmaa A, McNab JA, Savadjiev P, Okada Y, Hamalainen MS, Wang R, Wald LL, Pascual-Leone A, Wedeen VJ, Raij T. Targeting of white matter tracts with transcranial magnetic stimulation. Brain Stimul. 2014;7:80–84. doi: 10.1016/j.brs.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz A, Zafar N, Bockermann V, Rohde V, Paulus W. Validating computationally predicted TMS stimulation areas using direct electrical stimulation in patients with brain tumors near precentral regions. Neuroimage Clin. 2014;4:500–507. doi: 10.1016/j.nicl.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Patton HD, Amassian VE. Single- and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Mentschel C, Munchau A, Conrad B, Siebner HR. Subthreshold 5-Hz repetitive transcranial magnetic stimulation of the human primary motor cortex reduces intracortical paired-pulse inhibition. Neurosci Lett. 2000;296:21–24. doi: 10.1016/s0304-3940(00)01616-5. [DOI] [PubMed] [Google Scholar]
- Peterchev AV, Goetz SM, Westin GG, Luber B, Lisanby SH. Pulse width dependence of motor threshold and input–output curve characterized with controllable pulse parameter transcranial magnetic stimulation. Clin Neurophysiol. 2013;124:1364–1372. doi: 10.1016/j.clinph.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant’Angelo A, Battaglia F, Messina C, Siebner HR, Girlanda P. Distinct changes in cortical and spinal excitability following high-frequency repetitive TMS to the human motor cortex. Exp Brain Res. 2005;161:114–124. doi: 10.1007/s00221-004-2052-5. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Physiological studies of electric and magnetic stimulation of the human brain. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:29–35. [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Exp Physiol. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Rusu CV, Murakami M, Ziemann U, Triesch J. A model of TMS-induced I waves in motor cortex. Brain Stimul. 2014;7:401–414. doi: 10.1016/j.brs.2014.02.009. [DOI] [PubMed] [Google Scholar]
- Sakai K, Ugawa Y, Terao Y, Hanajima R, Furabayashi T, Kanazawa I. Preferential activation of different I waves by transcranial magnetic stimulation with a figure-of -eight shaped coil. Exp Brain Res. 1997;113:24–32. doi: 10.1007/BF02454139. [DOI] [PubMed] [Google Scholar]
- Salvador R, Silva S, Basser PJ, Miranda PC. Determining which mechanisms lead to activation in the motor cortex: a modeling study of transcranial magnetic stimulation using realistic stimulus waveforms and sulcal geometry. Clin Neurophysiol. 2011;122:748–758. doi: 10.1016/j.clinph.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu H, Maier MA, Cerri G, Kirkwood PA, Lemon RN. Macaque ventral premotor cortex exerts powerful facilitation of motor cortex outputs to upper limb motoneurons. J Neurosci. 2004;24:1200–1211. doi: 10.1523/JNEUROSCI.4731-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Teo JT, Terranova C, Swayne O, Greenwood RJ, Rothwell JC. Differing effects of intracortical circuits on plasticity. Exp Brain Res. 2009;193:555–563. doi: 10.1007/s00221-008-1658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Edwards DJ, Mastaglia FL. Repetitive paired-pulse TMS at I wave periodicity markedly increases corticospinal excitability: a new technique for modulating synaptic plasticity. Clin Neurophysiol. 2006;117:61–66. doi: 10.1016/j.clinph.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Thielscher A, Opitz A, Windhoff M. Impact of the gyral geometry on the electric field induced by transcranial magnetic stimulation. Neuroimage. 2011;54:234–243. doi: 10.1016/j.neuroimage.2010.07.061. [DOI] [PubMed] [Google Scholar]
- Thompson PD, Day BL, Rothwell JC, Dressler D, Maertens De Noordhout A, Marsden CD. Further observations on the facilitation of muscle responses to cortical stimulation by voluntary contraction. Electroencephalogr Clin Neurophysiol. 1991;81:397–402. doi: 10.1016/0168-5597(91)90029-w. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udupa K, Ni Z, Gunraj C, Chen R. Effects of short-latency afferent inhibition on short-interval intracortical inhibition. J Neurophysiol. 2014;111:1350–1361. doi: 10.1152/jn.00613.2013. [DOI] [PubMed] [Google Scholar]
- Weise D, Mann J, Ridding M, Eskandar K, Huss M, Rumpf JJ, Di Lazzaro V, Mazzone P, Ranieri F, Classen J. Microcircuit mechanisms involved in paired associative stimulation-induced depression of corticospinal excitability. J Physiol. 2013;591:4903–4920. doi: 10.1113/jphysiol.2013.253989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiethoff S, Hamada M, Rothwell JC. Variability in response to transcranial direct current stimulation of the motor cortex. Brain Stimul. 2014;7:468–4675. doi: 10.1016/j.brs.2014.02.003. [DOI] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Wu T, Sommer M, Tergau F, Paulus W. Lasting influence of repetitive transcranial magnetic stimulation on intracortical excitability in human subjects. Neurosci Lett. 2000;287:37–40. doi: 10.1016/s0304-3940(00)01132-0. [DOI] [PubMed] [Google Scholar]
- Ziemann U. Pharmaco-transcranial magnetic stimulation studies of motor excitability. Handb Clin Neurol. 2013;116:387–397. doi: 10.1016/B978-0-444-53497-2.00032-2. [DOI] [PubMed] [Google Scholar]


