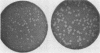
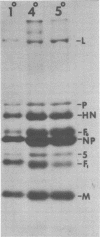
Abstract
Primary and secondary cultures of rhesus monkey kidney cells supported multiple-cycle replication of Sendai virus, but later passages lost this ability, and this was reflected in decreased plaque formation. Multiple-cycle replication also did not occur in LLC-MK2 cells, a continuous line of RMK cells. Failure of replication in serially passed cells was correlated with a decrease in proteolytic cleavage of a viral surface glycoprotein (Fo), and the ability of cells to support multiple-cycle replication and plaque formation could be restored by the addition of trypsin (0.3 microgram/ml) to the overlay medium. The use of wild-type virus, which requires trypsin, and protease activation mutants that require chymotrypsin or elastase for activation has provided evidence that the activating protease supplied by primary or secondary cells has trypsin-like activity. Inactive virus, with uncleaved Fo glycoprotein, absorbed to primary or secondary cells but did not infect them, even though such cells possess the enzyme that is capable of cleaving the Fo glycoprotein of virus synthesized in these cells. The inability of these cells to activate adsorbed virus indicates that the activating protease that they possess is inacessible to adsorbed virus, although it can act on the Fo glycoprotein during virus maturation in these cells. These data provide a biochemical explanation for the failure of later passages of a cell strain or a continuous cell line to support the replication of a paramyxovirus.
Full text
PDF

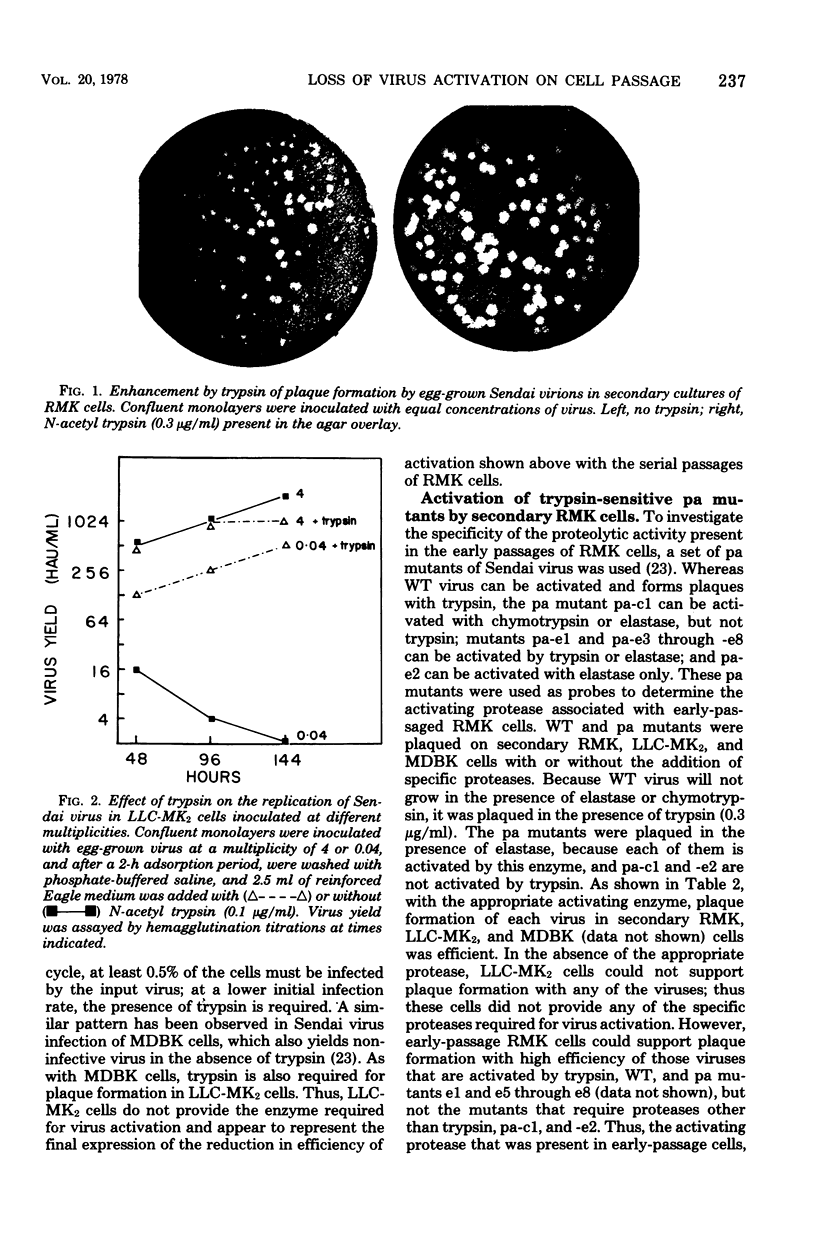




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- BEEUWKES H. Cytochemistry and sensitivity of a human amnion cell-line to Coxsackie A23 (ECHO9) virus. Antonie Van Leeuwenhoek. 1960;26:331–339. doi: 10.1007/BF02539022. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B., Guthell R. L., Jr, Case K. R. Loss of a critical neutral protease in ageing WI-38 cells. Nature. 1976 Jun 10;261(5560):499–501. doi: 10.1038/261499a0. [DOI] [PubMed] [Google Scholar]
- CHOPPIN P. W., PHILIPSON L. The inactivation of enterovirus infectivity by the sulfhydryl reagent p-chloromercuribenzoate. J Exp Med. 1961 Apr 1;113:713–734. doi: 10.1084/jem.113.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOPPIN P. W. Plaque formation by influenza A2 virus in monkey kidney cells. Virology. 1962 Oct;18:332–334. doi: 10.1016/0042-6822(62)90023-5. [DOI] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., Portner A., Kingsbury D. W. Sendai virus replication: an ultrastructural comparison of productive and abortive infections in avian cells. J Gen Virol. 1970 Dec;9(3):169–177. doi: 10.1099/0022-1317-9-3-169. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Homma M., Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973 Dec;12(6):1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H. Plaque assay of Sendai virus in monolayers of a clonal line of porcine kidney cells. J Clin Microbiol. 1976 Feb;3(2):91–95. doi: 10.1128/jcm.3.2.91-95.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Mahy B. W., Choppin P. W. The synthesis of sendai virus polypeptides in infected cells. Virology. 1976 Jan;69(1):116–131. doi: 10.1016/0042-6822(76)90199-9. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Choppin P. W. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975 Dec;68(2):440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Klenk H. D., Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976 Jul 15;72(2):494–508. doi: 10.1016/0042-6822(76)90178-1. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Ogura H., Klenk H. Studies on the assembly of the envelope of Newcastle disease virus. Virology. 1976 Feb;69(2):523–538. doi: 10.1016/0042-6822(76)90482-7. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT R. J., DAVIES J. R., WEISS L., WILKINSON J. H. Studies on tissue cultures of human pathological thyroids. J Pathol Bacteriol. 1959 Jan;77(1):19–32. doi: 10.1002/path.1700770103. [DOI] [PubMed] [Google Scholar]
- Peluso R. W., Lamb R. A., Choppin P. W. Polypeptide synthesis in simian virus 5-infected cells. J Virol. 1977 Jul;23(1):177–187. doi: 10.1128/jvi.23.1.177-187.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Protease activation mutants of sendai virus. Activation of biological properties by specific proteases. Virology. 1976 Jan;69(1):265–277. doi: 10.1016/0042-6822(76)90213-0. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Two disulfide-linked polypeptide chains constitute the active F protein of paramyxoviruses. Virology. 1977 Jul 1;80(1):54–66. doi: 10.1016/0042-6822(77)90380-4. [DOI] [PubMed] [Google Scholar]
- Shibuta H., Akami M., Matumoto M. Plaque formation by sendai virus of parainfluenza virus group, type 1 on monkey, calf kidney and chick embryo cell monolayers. Jpn J Microbiol. 1971 Mar;15(2):175–183. doi: 10.1111/j.1348-0421.1971.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Shibuta H. Effect of trypsin on the infectivity of Sendai virus grown in several host cells. Jpn J Microbiol. 1972 May;16(3):193–198. doi: 10.1111/j.1348-0421.1972.tb00648.x. [DOI] [PubMed] [Google Scholar]
- Sugita K., Maru M., Sato K. A sensitive plaque assay for Sendai virus in an established line of monkey kidney cells. Jpn J Microbiol. 1974 May;18(3):262–264. doi: 10.1111/j.1348-0421.1974.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Sun A. S., Aggarwal B. B., Packer L. Enzyme levels of normal human cells: aging in culture. Arch Biochem Biophys. 1975 Sep;170(1):1–11. doi: 10.1016/0003-9861(75)90092-2. [DOI] [PubMed] [Google Scholar]
- Tozawa H., Watanabe M., Ishida N. Structural components of Sendai virus. Serological and physicochemical characterization of hemagglutinin subunit associated with neuraminidase activity. Virology. 1973 Sep;55(1):242–253. doi: 10.1016/s0042-6822(73)81027-x. [DOI] [PubMed] [Google Scholar]