Abstract
Rhythmic cortical neuronal oscillations in the gamma frequency band (30–80 Hz, gamma oscillations) have been associated with cognitive processes such as sensory perception and integration, attention, learning, and memory. Gamma oscillations are disrupted in disorders for which cognitive deficits are hallmark symptoms such as schizophrenia and Alzheimer's disease. In vitro, various neurotransmitters have been found to modulate gamma oscillations. Serotonin (5-HT) has long been known to be important for both behavioural and cognitive functions such as learning and memory. Multiple 5-HT receptor subtypes are expressed in the CA3 region of the hippocampus and high doses of 5-HT reduce the power of induced gamma oscillations. Hypothesizing that 5-HT may have cell- and receptor subtype-specific modulatory effects, we investigated the receptor subtypes, cell types and cellular mechanisms engaged by 5-HT in the modulation of gamma oscillations in mice and rats. We found that 5-HT decreases the power of kainate-induced hippocampal gamma oscillations in both species via the 5-HT1A receptor subtype. Whole-cell patch clamp recordings demonstrated that this decrease was caused by a hyperpolarization of CA3 pyramidal cells and a reduction of their firing frequency, but not by alteration of inhibitory neurotransmission. Finally, our results show that the effect on pyramidal cells is mediated via the G protein-coupled receptor inwardly rectifying potassium channel Kir3. Our findings suggest this novel cellular mechanism as a potential target for therapies that are aimed at alleviating cognitive decline by helping the brain to maintain or re-establish normal gamma oscillation levels in neuropsychiatric and neurodegenerative disorders.
Key points
Rhythmic activity in the gamma frequency range (30–80 Hz) in the hippocampus is modulated by neurotransmitters such as serotonin, dopamine and histamine.
Multiple different serotonin receptors are expressed in the hippocampus.
Here we show that serotonin suppresses hippocampal gamma oscillations via activation of the receptor 5-HT1A.
The cellular cause for suppressed gamma oscillations is the 5-HT1A receptor-induced hyperpolarization and afterpolarization frequency reduction of pyramidal cells, but not of parvalbumin-positive interneurons.
The pyramidal cell hyperpolarization is mediated by an inwardly rectifying current mediated by the Kir3 potassium channel known to couple to 5-HT1A, which also results in decreased firing frequency.
Introduction
Rhythmic cortical activity in the gamma frequency range (30–80 Hz) has long been associated with cognitive processes including sensory perception, selective attention and working memory (Womelsdorf & Fries, 2007). Gamma oscillations are modulated according to memory load (van Vugt et al. 2010), and are disrupted in brain disorders with cognitive deficits as hallmark symptoms such as schizophrenia and Alzheimer's disease (Spencer et al. 2008) with the degree of disruption correlating with the severity of cognitive decline. Often, the development of cognitive deficits occurs in the hippocampus, where gamma oscillations are a product of recurrent excitation among CA3 pyramidal cells (Fisahn et al. 1998; Csicsvari et al. 2003), a balance between excitation and inhibition (Atallah & Scanziani, 2009), and rhythmic pacing by perisomatic-targeting fast-spiking GABAergic basket cells (Cardin et al. 2009; Sohal et al. 2009). In vitro hippocampal gamma oscillations are mostly decreased by aminergic neurotransmission (Krause & Jia, 2005; Wójtowicz et al. 2009; Andersson et al. 2010; Schulz et al. 2012), but the direct roles of specific receptor subtypes and the underlying cellular mechanisms are unknown in most cases. Recently it has been shown that specific activation of the dopamine D4 receptor actually increases the power of gamma oscillations (Andersson et al. 2012), suggesting that amine receptor activation has differing effects based on receptor subtype differences and G-protein coupling. Given that serotonin (5-HT) decreases the power of induced gamma oscillations (Wójtowicz et al. 2009) we sought to elucidate the receptor subtype specificity and cellular mechanisms responsible for this effect.
5-HT receptors (5-HTRs) are coupled to a number of different G-proteins, thus having a variety of downstream effects. The exception is 5-HT3, which is a non-selective cation channel. 5-HT1 and 5-HT5 are negatively coupled to adenylate cylcase via Gαi/o, thus down-regulating adenylyl cyclase and cAMP, whereas 5-HT4, 5-HT6 and 5-HT7 are positively coupled to Gαs, up-regulating cAMP (Hurley et al. 1998; Chapin et al. 2002; Lin et al. 2002). Additionally, 5-HT2 activates phospholipase C via Gαq/11, regulating inositol trisphosphate (IP3) and diacyl-glycerol (DAG) via a separate mechanism (Day et al. 2002). In addition to the Gαi/o effects, 5-HT1A is also known to couple to the inwardly rectifying potassium channel Kir3 (Andrade et al. 1986; Lüscher et al. 1997) and hyperpolarize hippocampal pyramidal cells when activated (Whittington et al. 2001).
Due to this heterogeneity in downstream effector pathways, different classes of neurons exhibit myriad responses to 5-HT, depending on the type of receptor expressed, and their localization on the neuron (Costa et al. 2012). Multiple 5-HT receptors are expressed in the CA3 region of the hippocampus (Tanaka et al. 2012), and are thus positioned to potentially modulate network activity, including gamma oscillations (Wójtowicz et al. 2009). However, the cell type-specific localization of postsynaptic receptor subtypes other than 5-HT3 and 5-HT1B (Winterer et al. 2011) in CA3 remains unknown.
The aim of this study was to investigate the receptor subtype specificity and the cellular mechanism(s) of action for the 5-HT-induced modulation of gamma oscillations and thereby provide a novel target for therapies that are aimed at alleviating cognitive decline by helping the brain to maintain or re-establish normal gamma oscillation levels. Here we demonstrate a suppressing effect of 5-HT on kainate (KA)-induced gamma oscillations in a hippocampal slice preparation. By selectively activating distinct 5-HTR subtypes we show this effect to be mediated by 5-HT1A, which is coupled to Kir3 channels. Whole-cell patch clamp experiments suggest that this receptor subtype is located on pyramidal cells, but not parvalbumin-positive (PV) interneurons, and that activation of 5-HT1A hyperpolarizes pyramidal cells sufficiently to disrupt the balance of inhibition and excitation, resulting in suppressed gamma oscillations.
Methods
Ethical approval
Experiments were carried out in accordance with the ethical permit granted by Norra Stockholms Djurförsöksetiska Nämnd to A.F. (N45/13) and animal protocols approved by the National Institutes of Health.
Animals
Sprague-Dawley rats (Charles River, Cologne, Germany), PV-GFP mice (gift from Professor Hannah Monyer) and 5-HT1A−/− mice and their wild-type littermates (gift from Professor René Hen, Ramboz et al. 1998) were used in all experiments at postnatal days (P)15–30 (rats) and P14–27 (mice). Green fluorescent protein (GFP)-positive PV-GFP mice were identified using a blue fluorescent light and goggles fitted with a GFP filter (BlueStar flashlight package, Nightsea, Bedford, MA, USA). To genotype 5-HT1A−/– mice, tissue samples were collected by tail-snip, DNA was isolated (Qiagen DNEasy kit) and standard PCR was performed. The primers used to identify 5-HT1A+/+ (∼600 bp) vs. 5-HT1A−/− (∼400 bp) genotypes were as follows: CAGTCTCTAGATCCCCTCCCTT, AAGGGCAAAAGTGAGTATGGTG and GGGCGTCCTCTTGTTCACGTAG. Only full homozygotes for both genes were used for experiments, while heterozygotes were retained for breeding.
Tissue preparation
Animals were deeply anaesthetized with isofluorane before being killed by decapitation. The brain was dissected out and sectioned in ice-cold cutting solution containing (in mm) 80 NaCl, 24 NaHCO3, 25 glucose, 1.25 NaH2PO4, 1 ascorbic acid, 3 sodium pyruvate, 2.5 KCl, 4 MgCl2, 0.5 CaCl2 and 75 sucrose. Horizontal sections (300 μm) of the ventral hippocampi were prepared with a Leica VT 1200S vibratome (Leica Microsystems AG, Wetzlar, Germany). The ventral hippocampus was chosen both because of enriched 5-HT1A expression (Tanaka et al. 2012) and ease of horizontal slice preparation. Immediately after slicing, sections were transferred to a 32–34°C humidified, oxygenated interface-type holding chamber with artificial cerebrospinal fluid (ACSF) containing (in mm) 124 NaCl, 30 NaHCO3, 10 glucose, 1.25 NaH2PO4, 3.5 KCl, 1.5 MgCl2 and 1.5 CaCl2 for rats, which was slightly modified for mice, containing 126 NaCl, 2.5 KCl and 26 NaHCO3, while other concentrations were identical. The slices were incubated for 30 min at 34°C before being cooled to room temperature, for a total recovery time of at least 1 h before any recordings were made.
Electrophysiology
For recording, slices were anchored in a submerged chamber, perfused at a rate of 3–5 ml min−1 with ACSF saturated with carbogen gas (95% O2, 5% CO2), and heated with an inline heater to 32°C. Recording electrodes with a resistance of 3–7 MΩ were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL, USA) with a vertical electrode puller (Narishige, Tokyo, Japan). Local field potentials (LFPs) were recorded with ACSF-filled electrodes. The tip of the electrode was placed in the stratum pyramidale of hippocampal area CA3. Gamma oscillations were elicited by bath application of 100 nm KA and recordings were made after 20 min, when the oscillation power had stabilized. All additional drugs were also bath applied. Intracellular whole-cell recordings were made with electrodes filled with a solution containing (in mm) 122.5 potassium gluconate, 8 KCl, 4 Mg2+ ATP, 10 diNa+ phosphocreatine, 0.3 Na+ GTP, 10 Hepes, 0.2 EGTA, 4 MgCl for most recordings, or (in mm) 132 CsMetSO4, 8 CsCl, 4 Mg2+ ATP, 10 diNa+ phosphocreatine, 0.3 Na+ GTP, 10 Hepes, and 0.6 EGTA for inhibitory postsynaptic current (IPSC) recordings, both with pH set to 7.2–7.3 and osmolarity to 270–280 mosmol l−1. Cells were visualized with an upright microscope using IR differential interference contrast microscopy (Axioskop, Carl Zeis AG, Göttingen, Germany), and pyramidal cells were selected based on location in the hippocampus and morphology. For interneuron experiments, fluorescence microscopy was used to identify GFP+ cells. For cell attached firing frequency experiments, electrodes were filled with filtered ACSF and recordings were made once a GΩ seal was made, and were discarded if the seal ruptured. Current-clamp and voltage-clamp signals were amplified with a Multiclamp 700B amplifier (Axon Instruments, Foster City, CA, USA). Data were sampled at 10 kHz, and digitized and stored with a Digidata 1322A and Clampex 9 software (Molecular Devices, CA, USA); if necessary, a 50 Hz filter was used to remove background electrical noise in extracellular field recordings (HumBug, Quest Scientific, North Vancouver, BC, Canada).
During voltage-clamp experiments, whole-cell compensation and series resistance compensation were used. Recordings were only retained for analysis if the access resistance was stable (less than 30% change). To create I–V curves, cells were held at −70 mV in voltage clamp and were given a series of steps holding the cell at 10 mV increments between −110 and −30 mV.
Drugs
Receptor agonists and antagonists were obtained from Sigma-Aldrich (St Louis, MO, USA) (KA, 5-HT), Tocris Bioscience (Ellisville, MO, USA) (8OH-dPAT, tertiapin-Q, ritanserin, tropisetron, AP-5, DNQX, LY-367385, SR-95531, CGP 55845), Calbiochem (Billerica, MA, USA) (TTX) or as a gift from Professor Sven-Ove Ögren (NAD 299). All drugs were dissolved in water except ritanserin (ethanol), LY-367385 (NaOH) and CGP55845 (DMSO). Full drug names are given in the abbreviations list.
Data analysis
To minimize variation, a paired experimental design was applied when possible, and drugs were washed on sequentially. Because of this and the non-parametric nature of most of the data sets, the Wilcoxon's signed rank sum test was used to report statistical differences unless otherwise stated. Data are reported as median, 1st quartile and 3rd quartile unless otherwise stated. To analyse oscillatory activity in the LFP and whole cell recordings, a fast Fourier transform was applied to 60 s recordings (segment length 8192 points) in Axograph X (AxoGraph, Berkeley, CA, USA). The power of the oscillation was determined by integrating the area under the curve of the power spectrum in the gamma range (20–60 Hz). A frequency range lower than as reported in vivo is used because the oscillations are recorded at slightly below physiological temperature, and their frequency has been shown to be linearly dependent on temperature (Fisahn et al. 2004).
To detect postsynaptic currents, an event detection plug-in using a sliding template function in Axograph was used. A standard event from each raw data trace was chosen as the template and then used to extract other events. Extracted events were visually confirmed for consistency and templates that did not satisfactorily capture events were discarded. The coherence between the voltage clamp recording and the field was analysed using a custom built MatLAB script (MathWorks, Natick, MA, USA). In current clamp experiments, the membrane potential was calculated using another MatLAB script, which calculated an average potential from the recorded trace, excluding action potentials.
To estimate the membrane potential of an actively firing cell, a script was written (MatLAB) to take the average membrane potential, excluding the action potentials. The points that were excluded were any that were more than two standard deviations away from the median voltage in the trace, and the rest of the points were averaged. To determine the membrane potential in non-active cells, an average was made from at least 10 s recordings.
For spike phase analysis, a custom script in MatLAB was implemented that performs a Hilbert transform of the filtered field recording to determine the instantaneous gamma frequency. The peak of an action potential was then detected and the instantaneous frequency of the field at the time of this peak was recorded. These frequencies of each action potential were then incorporated into a cumulative vector showing the preferred phase, and the strength of the preference (1 when the phase was always the same, 0 when it was never the same). Only recordings where the control condition had a vector length of at least 0.2 and where the cell continued to fire in the experimental condition were used. For analysis of firing frequency in cell-attached recordings, threshold event detection was implemented in Axograph X over a period of 2 min.
Results
Serotonin suppresses gamma oscillations via 5-HT1A receptor activation
KA (100 nm) can be used to reliably elicit gamma oscillation in CA3 of the hippocampus (Fisahn et al. 2004). To determine the modulatory effect of 5-HT on gamma oscillations, increasing concentrations of 5-HT were bath applied and the local field potential was recorded from the pyramidal cell layer in rat hippocampal slices. Five different concentrations of 5-HT were tested (10 nm, 100 nm, 1 μm, 10 μm, 100 μm) and significant decreases of gamma oscillation power was observed for two of them: 10 μm (Fig. 1A, B, KA: 4.97 × 10−10, 8.6 × 10−10, 1.2 × 10−9 V2, +5-HT: 6.3 × 10−11, 2.1 × 10−11, 1.1 × 10−11 V2, P < 0.01, n = 10) and 100 μm (KA: 8.4 × 10−11, 4.9 × 10−11, 1.4 × 10−9 V2, +5-HT: 3.4 × 10−11, 1.7 × 10−11, 1.1 × 10−10 V2, P < 0.05, n = 6). The 5-HT concentration causing maximal decrease in gamma oscillation power – 10 μm – was used in the remainder of the study. The percentage change of gamma oscillation power caused by all tested 5-HT concentrations is as follows (mean ± SEM): 10 nm: −15.9 ± 14.1%, 100 nm: −12.7 ± 4.8%, 1 μm 5.5 ± 5.9%, 10 μm −82.4 ± 4.4%, 100 μm: −64.1 ± 11.3%. The observed reduction of KA-induced gamma oscillations by 5-HT, which recovered after 5-HT wash-out, parallels what has been previously reported for 5-HT modulation of carbachol-induced gamma oscillations (Krause & Jia, 2005).
Figure 1. 5-HT suppresses rat hippocampal gamma oscillations via 5-HT1A.
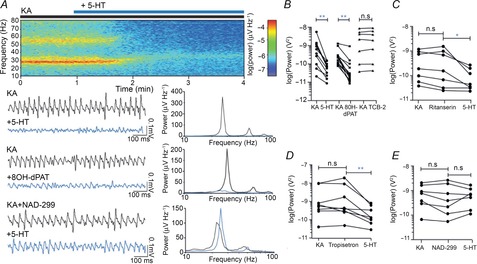
A, top: averaged spectrograms of raw data. Lower left: raw example traces of LFP after 20 min of KA application (black) and after a 5 min (5-HT) or 20 min (8OH-dPAT) additional application of the agonist (blue), 5-HT reduces gamma-band power (top), as does 8OH-dPAT (middle). This effect is blocked with NAD 299 (bottom). Lower right: power spectra showing gamma power at the same time points as in the raw traces, before (black) and after (blue) application of the corresponding agonist. B, population data showing effects of 5-HT agonists. C, population data showing that the 5-HT2 antagonist ritanserin fails to block 5-HT-induced reduction in gamma power. D, population data showing that the 5-HT3 antagonist tropisetron fails to block 5-HT-induced reduction in gamma power. E, population data showing that the 5-HT1A antagonist NAD 299 blocks 5-HT-induced decrease in gamma power. *P < 0.05, **P < 0.01.
The effect of 5-HT on gamma oscillations was replicated with a 5-HT1A agonist, 8OH-dPAT, which caused a power change of −78.6 ± 4.7% (Fig. 1A, B, KA: 2.1 × 10−10, 9.6 × 10−11, 2.8 × 10−10 V2, +50 μm 8OH: 2.3 × 10−11, 1.6 × 10−11, 6.9 × 10−11 V2, P < 0.01, n = 14), although the onset of the effect was slower than with 5-HT: the 5-HT-induced power reduction was stable after 5 min whereas the 8OH-dPAT-induced reduction reached stability after only 20 min. Contrary to previous reports (Krause & Jia, 2005), an agonist of 5-HT2A, TCB-2, had no effect on the power of gamma oscillations, changing the power by a non-significant +6.6 ± 4.7% (Fig. 1B, KA: 2.2 × 10−9, 9.9 × 10−11, 5.5 × 10−9 V2, +500 nm TCB: 2.0 × 10−9, 1.0 × 10−10, 6.3 × 10−9 V2, P = 0.47, n = 7).
We also investigated the effects of 5-HT on different receptor subtypes using specific antagonists of 5-HT1A, 5-HT2A and 5-HT3, as all of these receptors are present in CA3 (Tanaka et al. 2012). A 5-HT-induced decrease of gamma oscillation power of 49.9 ± 13.9% persisted in the presence of the 5-HT2A antagonist ritanserin (Fig. 1C, 10 μm Rit: 1.6 × 10−10, 3.2 × 10−11, 9.9 × 10−10 V2, +5-HT: 4.58 × 10−11, 3.2 × 10−11, 2.0 × 10−9 V2, P < 0.05, n = 7). In the presence of the 5-HT3 antagonist tropisetron, the power change was −66.7 ± 10.6 % (Fig. 1D, 1 μm Trop: 4.1 × 10−10, 2.8 × 10−10, 7.2 × 10−9 V2, +5-HT: 1.4 × 10−10, 7.7 × 10−11, 6.0 × 10−10 V2, P < 0.01, n = 8). In contrast, the 5-HT1A antagonist, NAD 299 (1 μm), blocked the 5-HT-induced decrease in gamma oscillation power, resulting in a non-significant change of 44.1 ± 35.4% (Fig. 1A, E, NAD: 4.1 × 10−10, 1.2 × 10−10, 2.0 × 10−9 V2, +5-HT: 7.0 × 10−10, 1.6 × 10−10, 1.2 × 10−9 V2, P = 0.81, n = 7). The large variance in 5-HT effect in the presence of NAD 299 is probably due to the fact that when 5-HT1A receptors are blocked, the effects of 5-HT on other serotonin receptor subtypes become more prominent. Together with the differing expression profiles of serotonin receptor subtypes other than 5-HT1A, these secondary effects may introduce a variance to gamma oscillation power in the presence of NAD 299 that is absent in naïve gamma oscillations. None of the antagonists alone had a significant effect on either the frequency or the power of gamma oscillations (Fig. 1C–E).
Serotonin differentially affects excitatory drive over inhibitory drive
To determine how 5-HT modulates network contributions of both inhibitory and excitatory drive during KA-induced gamma oscillations, we concomitantly recorded the local field and postsynaptic currents in CA3 pyramidal cells held either near the reversal potential for chloride (−70 mV) to isolate excitatory postsynaptic currents (EPSCs) (Fig. 2A) or near the reversal potential for AMPA receptors (0 mV) to isolate IPSCs (Fig. 2B). There was a decrease in both the amplitude of KA-elicited events (Fig. 2C, EPSCs KA: 51.9, 45.3, 74.3 pA, +5-HT: 24.1, 21.5, 35.7 pA, P < 0.05, n = 7; IPSCs KA: 218.0, 134.1, 303.4 pA, +5-HT: 126.0, 815.9, 142.0 pA, P < 0.05, n = 6) and their coherence with the field (Fig. 2C, EPSCs KA: 0.83, 0.67, 0.93, +5-HT: 0.28, 0.08, 0.44, P < 0.05, n = 7; IPSCs KA: 0.91, 0.80, 0.92, +5-HT: 0.44, 0.24, 0.66, P < 0.05, n = 6) in both experiments with application of 5-HT. Along with the power of the field gamma oscillation, fluctuations in the holding current of the whole-cell recording were also analysed spectrally and the power was computed. This acts as another measurement of the synchrony of the local network by summating synaptic currents onto the recorded cell. The gamma-band power of the whole-cell current also decreased at both holding levels (Fig. 2C, EPSCs KA: 1.5 × 10−22, 1.4 × 10−22, 4.4 × 10−22 V2, +5-HT: 2.9 × 10−23, 1.2 × 10−23, 5.2 × 10−23 V2, P < 0.05, n = 7, IPSCs KA: 2.3 × 10−21, 1.3 × 10−21, 4.8 × 10−21 V2, +5-HT: 8.7 × 10−22, 5.5 × 10−22, 1.2 × 10−21 V2, P < 0.05, n = 6). However, while the frequency of events was decreased for EPSCs (Fig. 2C, KA: 22.8, 16.9, 23.8 Hz, +5-HT: 11.6, 7.8, 16.9 Hz, P < 0.05, n = 7), it remained unchanged for IPSCs (Fig. 2C, KA: 22.16, 20.75, 24.89 Hz, +5-HT: 21.1, 20.3, 21.6 Hz, P = 0.22, n = 6). This indicates that 5-HT modulation may differentially affect excitatory transmission versus inhibitory transmission during gamma oscillations.
Figure 2. 5-HT decreases the number of EPSCs, but not IPSCs, onto rat pyramidal cells.
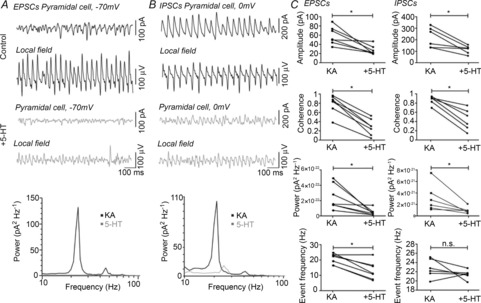
A, example EPSCs (top trace) and concomitant field (bottom trace) both under KA-induced oscillations (black) and with the addition of 5-HT (grey). Power spectrum of the holding current showing a decrease in gamma band response (bottom panel). B, example IPSCs (top trace) and concomitant field (bottom trace) both under KA-induced oscillations (black) and with the addition of 5-HT (grey). Power spectrum of the holding current showing a decrease in gamma band response (bottom panel). C, population data for EPSCs (left panels) and IPSCs (right panels) showing a decrease in amplitude (top), coherence with the field (second) and power of the membrane oscillation (third). The number of EPSCs is decreased, but the number of IPSCs is not (bottom). *P < 0.05.
To confirm that modulation is acting on excitatory rather than inhibitory currents, a transgenic mouse in which GFP was expressed in parvalbumin-positive cells (PV-GFP) was used, as PV+ fast spiking interneurons are known to play a vital role in the generation and maintenance of gamma oscillations (Cardin et al. 2009; Sohal et al. 2009). These cells were identified by both their fluorescence and their firing profile (Andersson et al. 2012), and were recorded during ongoing field oscillations, before and after the application of 5-HT (Fig. 3A). Despite some cell-to-cell variance the average membrane potential was unchanged (Fig. 3B, KA: −47.9, −51.0, −41.2 mV, +5-HT: −42.7, −46.5, −40.2 mV, P = 0.44, n = 6), as was the average action potential frequency (Fig. 3C, KA: 14.9, 9.3, 17.4 Hz, +5-HT: 8.9, 2.4, 15.5 Hz, P = 0.44, n = 6). This suggests that modulation of gamma oscillations via serotonin receptors is probably not occurring via PV+ fast-spiking interneurons.
Figure 3. PV+ interneuron firing is unaffected by 5-HT application in mice.
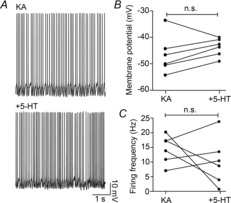
A, raw traces of fast-spiking PV+ interneuron firing. B, PV+ interneurons do not hyperpolarize. C, PV+ interneuron firing frequency is unchanged.
Serotonin-induced field and cellular effects are absent in 5-HT1A knockout mice
To further confirm the contribution of 5-HT1A, mice with a complete genetic deletion of the gene HTR1a, which encodes the 5-HT1A receptor (5-HT1A−/−), were used. The decrease of gamma oscillation power induced in WT littermates (5-HT1A+/+) by 10 μm 5-HT was similar to (56.6 ± 11.5%), if slightly smaller than that seen in rats (rats: see Fig. 1, mice: Fig. 4A, KA: 1.13 × 10−9, 6.9 × 10−10, 2.5 × 10−9 V2, +5-HT: 2.2 × 10−10, 1.8 × 10−10, 1.1 × 10−9 V2, P < 0.01, n = 14). In contrast to the WT mice, the 5-HT-induced decrease in gamma oscillation power was absent in the 5-HT1A−/– mice, with a change of only −16.1 ± 18.6% (Fig. 4A, KA: 9.5 × 10−10, 4.8 × 10−10, 1.6 × 10−9 V2, +5-HT: 3.9 × 10−10, 2.6 × 10−10, 10.0 × 10−10 V2, P = 0.21, n = 11). 5-HT1A−/– mice and their WT littermates were then used to determine the effect of 5-HT1A activation in individual cells.
Figure 4. 5-HT decreases pyramidal cell firing frequency in 5-HT1A+/+ but not 5-HT1A−/− mice.
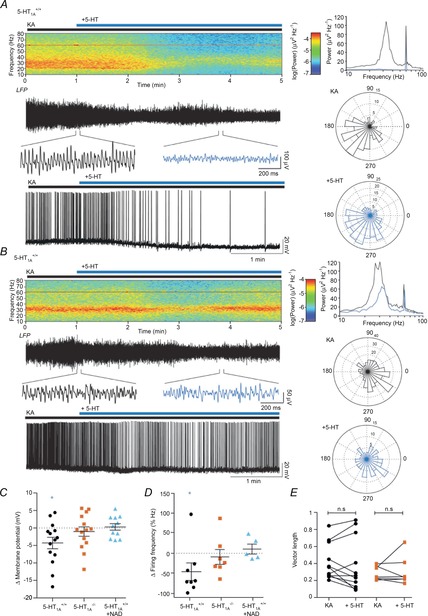
A and B, top left: averaged spectrograms of raw data. Traces: 5 min example LFP recording showing the wash-on of 5-HT (top) with insets showing gamma band-filtered oscillation in detail in control (black) and after (blue) the application of 5-HT, and whole cell traces showing example firing activity in pyramidal cells during 5-HT wash-on (bottom). Top right: power spectra showing gamma power before (black) and after (blue) application of 5-HT from shown LFP trace. Bottom right: rose plots of preferred firing phase for an example pyramidal cell control in KA (top, black) and in 5-HT (bottom, blue). A, data for 5-HT1A+/+ mice showing a decrease in gamma oscillations, and hyperpolarization of the pyramidal cell. B, data for 5-HT1A−/− mice showing a block of the decrease in gamma, and no pyramidal cell hyperpolarization. C, population data showing change in membrane potential, decreased in 5-HT1A+/+ mice, but not 5-HT1A−/− mice or 5-HT1A+/+ mice in the presence of NAD 299. D, population data showing change in firing frequency in cell-attached mode. Firing frequency is reduced in 5-HT1A+/+ mice, but not in 5-HT1A−/− mice or 5-HT1A+/+ mice in the presence of NAD 299. E, population data showing no changes in vector radius of preferred firing phase in 5-HT1A+/+ and 5-HT1A−/− mice. *P < 0.05.
Whole-cell current-clamp recordings from pyramidal cells and concomitant local field recordings were made during KA-induced gamma oscillations in both 5-HT1A−/− mice and their WT littermates. The resting membrane potential of 5-HT1A+/+ pyramidal cells hyperpolarized by 4.3 ± 1.6 mV in the presence of 5-HT (Fig. 4B, C, KA: −48.8, −49.5, −46.6 mV, +5-HT: −49.5, −55.6, −46.7 mV, P < 0.05, n = 14). This hyperpolarization was absent in 5-HT1A−/− mice (Fig. 4B, C, KA: −47.9, −50.3, −46.3 mV, +5-HT: −49.1, −53.6, −44.8 mV, P = 0.21, n = 17) and was blocked in 5-HT1A+/+ animals with the 5-HT1A antagonist NAD 299 (Fig. 4C, KA+NAD: −44.8, −47.2, −42.7 mV, +5-HT: −42.9, −49.4, −41.3 mV, P > 0.99, n = 11). Note the much smaller variance of the 5-HT effect in the presence of NAD 299 in intracellular recordings when compared to extracellular field recordings of gamma oscillations under the same conditions. This supports the assumption that when 5-HT1A receptors are blocked 5-HT-induced effects via other serotonin receptor subtypes expressed on non-pyramidal cell types participating in the network oscillation become very prominent. The selective hyperpolarization of excitatory cells but not inhibitory cells alters the equilibrium of excitation/inhibition in the neuronal network, leading to the suppression of gamma oscillations.
To determine the effect of 5-HT on suprathreshold activity, pyramidal cell firing activity was also monitored. The preferred firing phase of each pyramidal cell was analysed by comparing the peak of each action potential to the instantaneous frequency seen in the field (see Methods). For each cell that was firing sufficiently in phase with the field during the control condition and maintained firing throughout the application of 5-HT, there was no change in the vector radius measuring the preciseness of the preferred phase in either group of animals (Fig. 4A, B, E, 5-HT1A+/+ KA: 0.36, 0.26, 0.65, +5-HT: 0.25, 0.13, 0.76, P = 0.52, n = 11; 5-HT1A−/− KA: 0.35, 0.21, 0.40, +5-HT: 0.36, 0.23, 0.65, P = 0.94, n = 7). Due to non-specific run-down of firing over long drug applications, cell-attached recordings were made to assess the effects of 5-HT on firing frequency. The firing rate decreased in 5-HT1A+/+ animals in response to 5-HT application (Fig. 4A, D, KA: 5.0, 3.7, 6.3 Hz, +5-HT: 1.9, 1.0, 3.4 Hz, P < 0.05, n = 8). This decrease was absent in slices prepared from both 5-HT1A−/− (Fig. 4B, D, KA: 8.2, 4.0, 10.1 Hz, +5-HT: 6.4, 3.9, 7.8 Hz, P = 0.11, n = 7) and 5-HT1A+/+ mice in the presence of the 5-HT1A antagonist NAD 299, where neither the antagonist itself nor the 5-HT had any effect (Fig. 4D, KA: 9.3, 4.4, 17.9 Hz, +NAD: 8.0, 6.1, 13.6 Hz, +5-HT: 7.8, 6.3, 14.7 Hz, Friedman statistic = 0.0, P > 0.9, n = 6, Friedman test). The probable result of this reduction in pyramidal cell firing is insufficient excitatory drive to the network, quieting robust gamma oscillations.
Serotonin activates an inwardly rectifying outward current in pyramidal cells via 5-HT1A
To determine the ionic conductances activated in CA3 pyramidal cells, postsynaptic effects of 5-HT were recorded in the presence of synaptic blockers for AMPARs, NMDARs and GABAARs (10 μm DNQX, 50 μm AP-5 and 1 μm gabazine, respectively), metabotropic blockers for metabotropic glutamate receptors (mGluRs) and GABABRs (10 μm LY-367385 and 1 μm CGP-55845, respectively) and the Na+ channel blocker TTX (1 μm) to synaptically isolate cells. I–V curves were created from whole-cell voltage-clamp experiments where cells were subjected to negative and positive voltage steps (see Methods). Pyramidal cells from 5-HT1A+/+ mice revealed an inwardly rectifying current with a reversal potential near the calculated reversal potential for K+ (−85 mV), with the application of 5-HT, which was not seen in 5-HT1A−/− mice (Fig. 5A, B). To determine the degree of rectification, a rectification index (RI) was calculated as the ratio between the current response at −60 and −110 mV. The RI was significantly smaller in 5-HT1A−/− mice than in 5-HT1A+/+ mice (Fig. 5B–D, 5-HT1A+/+: −0.62, −0.69, −0.17; 5-HT1A−/−: 0.21, −0.53, 0.39, n1 = 9, n2 = 8, P < 0.05, Mann–Whitney U-test). Additionally, the RI was significantly different in 5-HT1A+/+ in the presence of NAD 299 as compared to the 5-HT1A+/+ mice alone (Fig. 5C, D, RI: 0.02, −0.02, 0.58, n = 5, P < 0.01, Mann–Whitney U-test). These data suggest that the hyperpolarization and subsequent reduction in firing of pyramidal cells is mediated by an inwardly rectifying current activated by 5-HT1A.
Figure 5. 5-HT activates an inwardly rectifying current in 5-HT1A+/+ but not 5-HT1A−/− mice, which is mediated via Kir3.
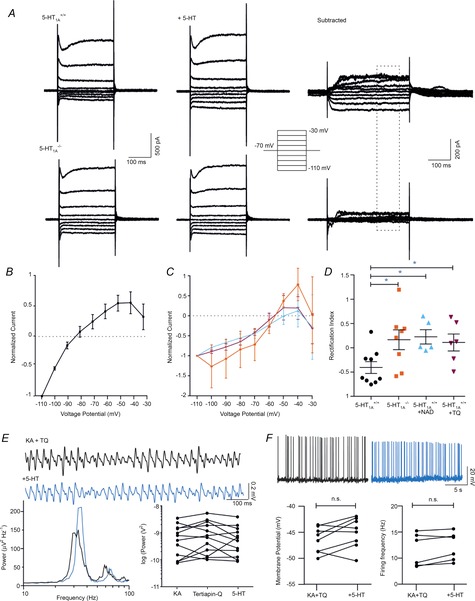
A, example current responses to voltage steps in control (left) and in the presence of 5-HT (middle). Subtraction of the two responses reveals an inwardly rectifying current that is present in 5-HT1A+/+ mice (top right) but not in the 5-HT1A−/− mice (bottom right). B, population data showing inwardly rectifying I–V curve of normalized data in 5-HT1A+/+ mice. C, population data showing linear I–V relationship in normalized data from 5-HT1A−/− mice, and in 5-HT1A+/+ mice in the presence of NAD 299 and tertiapin-Q (TQ). D, population data showing that the rectification index for the 5-HT evoked current in 5-HT1A+/+ mice is significantly smaller than that in the 5-HT1A−/− mice or in 5-HT1A+/+ mice in the presence of NAD 299 and TQ. E, example filtered LFP gamma oscillations from 5-HT1A+/+ mice in the presence of Kir channel blocker TQ (10 min, black) and after application of 5-HT (5 min, blue) and corresponding power spectra (bottom left). Population data showing a block of 5-HT elicited a decrease in gamma in the presence of TQ. F, top: example current clamp recording of firing in pyramidal cells in control KA (20 min, black) and after application of 5-HT (5 min, blue). Bottom left: population data showing no 5-HT-induced hyperpolarization, and no decrease in firing in cell-attached recordings (bottom right). *P < 0.05.
Based on the above results and the 5-HT1A-dependent hyperpolarization induced by 5-HT in pyramidal cells we hypothesize that an ionic conductance supporting an inwardly rectifying outward potassium current may be responsible for 5-HT effects observed in field and patch clamp recordings. Indeed, 5-HT1A is linked to the G-protein coupled inwardly rectifying potassium channel Kir3 (Andrade et al. 1986). To test our hypothesis we proceeded to block Kir3 channels with the bee venom toxin tertiapin-Q (TQ). In subsequent extracellular recordings the 5-HT-induced decrease in gamma oscillation power was fully blocked in WT mice (TQ: 7.21 × 10−10, 1.6 × 10−10, 1.4 × 10−9 V2, +5-HT: 5.6 × 10−10, 1.5 × 10−10, 1.3 × 10−9 V2, P = 0.11, n = 12; Fig. 5E). Additionally, the inwardly rectifying current seen in 5-HT1A+/+ animals was blocked in pyramidal cells in the presence of TQ (Fig. 5C, D, RI: 0.11, −0.29, 0.55, n = 6, P < 0.05, Mann–Whitney U-test).
On the cellular level we have shown that the resting membrane potential of 5-HT1A+/+ pyramidal cells hyperpolarizes, while this effect is absent in 5-HT1A−/− mice and can be blocked in 5-HT1A+/+ animals with the 5-HT1A antagonist NAD 299 (Fig. 4B, C). To confirm this hypothesis, 5-HT-induced hyperpolarization in the presence of TQ was assessed. Indeed, when recording pyramidal cell membrane potential in the presence of TQ no 5-HT-induced changes were observed (Fig. 5F, KA+TQ: −45.4, −48.5, −44.1 mV, +5-HT: −44.1, −46.9, −42.0 mV, P = 0.69, n = 7). Additionally, TQ blocked the 5-HT-induced reduction in firing frequency (Fig. 5F, KA: 8.4, 4.8, 12.8 Hz, +TQ: 9.9, 6.1, 12.8 Hz, +5-HT: 9.7 ± 1.5 Hz, Friedman statistic = 2.3, P = 0.43, n = 6, Friedman test).
While 5-HT clearly has multiple effects in the hippocampus, the 5-HT1A-induced activation of Kir3 and subsequent hyperpolarization of pyramidal cells appears the main mediator for the serotonergic modulation of gamma oscillations in the hippocampus.
Discussion
In this paper, we show that 5-HT1A receptor activation decreases fast, rhythmic network activity in the hippocampal CA3 region of mice and rats. This effect is strikingly different from the effect of 5-HT1A receptor activation in the prefrontal cortex where pyramidal cells are disinhibited due to a presumed 5-HT1A receptor-mediated inhibition of interneurons (Lladó-Pelfort et al. 2012). While the Lladó-Pelfort study indicates localization of 5-HT1A receptors on inhibitory rather than excitatory cell types in the prefrontal cortex, our data demonstrate a strong inhibition of pyramidal cells in the hippocampus. Release of serotonin from raphe nucleus neurons will probably have various outcomes, with variable time scales, depending on the postsynaptic receptor subtype activated. Presynaptic 5-HT1A autoreceptors on serotonergic terminals throughout the cortex regulate release of serotonin (Malagié et al. 2001). And, as shown in this study, the presence of serotonin may quickly inhibit any fast network oscillations, mediated by 5-HT1A, whereas activation of other receptors may cause longer term effects due to slower G protein-coupled mechanisms. The decrease in firing that persists in the 5-HT1A−/− mice (Fig. 5) is probably controlled by the latter mechanism.
In the hippocampus, median raphe axons target the layers lacunosum-moleculare and radiatum (Gulyás et al. 1999) and the results of this study suggest that either serotonergic fibres are forming synapses onto distal dendrites of pyramidal cells or pyramidal cells are activated via volume transmission. Histological and physiological studies suggest that calbindin-, cholecystokinin- and a subset of somatostatin-positive interneurons are sensitive to 5-HT, with evidence for 5-HT1B on cholecystokinin cells and 5-HT3 on somatostatin cells (Aznar et al. 2003; Winterer et al. 2011). The data in this study suggest that 5-HTR activation on interneurons does not contribute to the 5-HT-induced decrease in gamma oscillations, and that PV-positive interneurons are not sensitive to 5-HT.
Regarding the mechanism of action via 5-HT1A, the members of the G protein-coupled receptor inwardly rectifying potassium channel family found in CA3 are Kir3.1–3.3 (Liao et al. 1996; Kulik et al. 2006). Kir3.1 requires either Kir3.2 or Kir3.4 (found in cardiac tissue) to traffic to the membrane, and Kir3.3 is mostly found in the endoplasmic reticulum. Additionally, if Kir3.2 is knocked out, baclofen and 5-HT-induced outward currents are abolished in CA1 pyramidal cells (Lüscher et al. 1997). Thus, the current seen in CA3 pyramidal cells is probably dependent on Kir3.2.
While other studies reported a similar reduction in gamma oscillation power in response to 5-HT and 5-HT1A agonism in the carbachol gamma-induction paradigm (Krause & Jia, 2005) they also described an increase of gamma oscillation power when activating 5-HT2 receptors. The fact that we could not see any effect on gamma oscillation power when using the 5-HT2A (and 5-HT2C) agonist TCB-2 may be ascribed to the somewhat different binding profile of TCB-2 versus the agonist DOI (10.1371/journal.pone.0040906) used by Krause and Jia, resulting in a somewhat different effect overall on gamma oscillations. Also, we cannot exclude that carbachol-induced gamma oscillations are affected differently by 5-HT2 receptor agonists compared to KA-induced gamma oscillations.
Canonically, multiple oscillators are thought to drive hippocampal gamma oscillations with CA3 as the source of slow gamma (Colgin et al. 2009; Belluscio et al. 2012). Thus, a decrease in power in CA3 may result in a decrease in feed-forward slow gamma to CA1. A change in balance of these networks may affect any one of many hippocampal-dependent cognitive processes such as spatial navigation, memory consolidation or pattern separation. Our results are consistent with the strong evidence for an inhibitory role of 5-HT1A receptors in learning and memory (Ögren et al. 2008). Furthermore, the effect of 5-HT1A receptor activation involves interaction with glutamatergic or NMDA receptors in the control of hippocampal cognition (Ögren et al. 2008). Raphe signalling via 5-HT1A activation may provide an inhibitory tone, promoting quiescence in the hippocampus, which in turn may promote restfulness.
Understanding the effect of serotonin receptor activation in the network is vital for fully understanding the mechanism of atypical antipsychotic medications, as many, such as aripiprazole and clozapine, are 5-HT1A partial agonists. These drugs increase release of dopamine in the prefrontal cortex, which may explain their reduction of negative symptoms in schizophrenic patients. Additionally, atypical antipsychotics with 5-HT1A agonism reduce some side effects associated with typical antipsychotics, such as catalepsy (Prinssen et al. 2002), possibly by regulating excitation in cortical networks. Finally, it has been hypothesized that adult neurogeneration in the dentate gyrus may be stimulated with 5-HT1A agonism (Schreiber & Newman-Tancredi, 2014), suggesting possible relief of cognitive deficits in psychiatric disorders in addition to relief of negative symptoms.
In summary, our study has identified a novel cellular mechanism as a potential target for therapies that are aimed at alleviating cognitive decline by helping the brain to maintain or re-establish normal gamma oscillation levels in neuropsychiatric and neurodegenerative disorders. We further suggest that the 5-HT1A receptor and the cellular and network mechanisms modulated by its activation warrant continued investigation with the aim to consolidate and extend the identification of potential therapeutic targets.
Acknowledgments
We are grateful to Professor Hannah Monyer (University Hospital Heidelberg) for PV-GFP mice, Professor René Hen (Columbia University) for 5-HT1A−/− mice, and Professor Sven Ove-Ögren (Karolinska Institutet) for NAD 299 and comments. We also thank Drs Richard Andersson and Mick Craig for analysis scripts, analysis assistance and manuscript comments.
Glossary
- 8OH-DPAT
(±)-8-hydroxy-2-dipropylaminotetralin hydrobromide
- ACSF
artificial cerebrospinal fluid
- AP-5
d-(–)-2 amino-5-phosphonopentanoic acid
- CGP 55845
(2S)-3-[[(1S)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl] (phenylmethyl)phosphinic acid hydrochloride
- DNQX
6,7-dinitroquinoxaline-2,3-dione
- EPSC
excitatory postsynaptic current
- gabazine
SR-95531, 6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide
- IPSC
inhibitory postsynaptic current
- KA
kainic acid, (2S,3S,4S)-carboxy-4-(1-methylethenyl)-3-pyrrolidineacetic acid
- LFP
local field potential
- LY-367385
(S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid
- NAD 299
(3R)-3-(dicyclobutyl-amino)-8-fluoro-3,4-dihydro-2H-1-benzopyran-5-carboxamide hydrochloride
- PV
parvalbumin
- ritanserin
6-[2-[4-[bis(4-fluorophenyl) methylene]-1-piperidinyl]ethyl]-7-methyl-5H-thiazolo[3,2-a]pyrimidin-5-one
- RI
rectification index
- TCB-2
(4-bromo-3,6-dimethoxybenzocyclobuten-1-yl) methylamine hydrobromide
- TQ
tertiapin-Q
- tropisetron
(3-endo)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl 1H-indole-3-carboxylic acid ester monohydrochloride
Additional information
Competing interests
All authors maintain no conflict of interest.
Author contributions
A.D.J., C.J.M. and A.F. conceived and designed the experiments, A.J. conducted the experiments and analysed the data, and A.J., C.J.M. and A.F. interpreted the data and wrote the paper.
Funding
This work was supported by a Karolinska Institute-NIH PhD studentship (A.J.), the National Institutes of Health (C.J.M) and the Swedish Research Council, the Swedish Medical Association, the Swedish Brain Foundation and the Strategic Program in Neurosciences at the Karolinska Institute (A.F.).
References
- Andersson R, Johnston A, Fisahn A. Dopamine D4 receptor activation increases hippocampal gamma oscillations by enhancing synchronization of fast-spiking interneurons. PloS ONE. 2012;7:e40906. doi: 10.1371/journal.pone.0040906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson R, Lindskog M, Fisahn A. Histamine H3 receptor activation decreases kainate-induced hippocampal gamma oscillations in vitro by action potential desynchronization in pyramidal neurons. J Physiol. 2010;588:1241–1249. doi: 10.1113/jphysiol.2009.180984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade R, Malenka R, Nicoll R. A G protein couples serotonin and GABAB receptors to the same channels in hippocampus. Science. 1986;234:1261–1265. doi: 10.1126/science.2430334. [DOI] [PubMed] [Google Scholar]
- Atallah B, Scanziani M. Instantaneous modulation of gamma oscillation frequency by balancing excitation with inhibition. Neuron. 2009;62:566–577. doi: 10.1016/j.neuron.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aznar S, Qian Z, Shah R, Rahbek B, Knudsen G. The 5-HT1A serotonin receptor is located on calbindin- and parvalbumin-containing neurons in the rat brain. Brain Res. 2003;959:58–67. doi: 10.1016/s0006-8993(02)03727-7. [DOI] [PubMed] [Google Scholar]
- Belluscio M, Mizuseki K, Schmidt R, Kempter R, Buzsáki G. Cross-frequency phase-phase coupling between θ and γ oscillations in the hippocampus. J Neurosci. 2012;32:423–435. doi: 10.1523/JNEUROSCI.4122-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin J, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L-H, Moore C. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin E, Haj-Dahmane S, Torres G, Andrade R. The 5-HT4 receptor-induced depolarization in rat hippocampal neurons is mediated by cAMP but is independent of Ih. Neuroscience Lett. 2002;324:1–4. doi: 10.1016/s0304-3940(02)00113-1. [DOI] [PubMed] [Google Scholar]
- Colgin L, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser M-B, Moser E. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature. 2009;462:353–357. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Costa L, Trovato C, Musumeci S, Catania M, Ciranna L. 5-HT1A and 5-HT7 receptors differently modulate AMPA receptor-mediated hippocampal synaptic transmission. Hippocampus. 2012;22:790–801. doi: 10.1002/hipo.20940. [DOI] [PubMed] [Google Scholar]
- Csicsvari J, Jamieson B, Wise K, Buzsáki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- Day M, Olson P, Platzer J, Striessnig J, Surmeier D. Stimulation of 5-HT2 receptors in prefrontal pyramidal neurons inhibits Cav1.2 l type Ca2+ currents via a PLCβ/IP3/calcineurin signaling cascade. J Neurophysiol. 2002;87:2490–2504. doi: 10.1152/jn.00843.2001. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike F, Buhl E, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF, McBain CJ. Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci. 2004;24:9658–9668. doi: 10.1523/JNEUROSCI.2973-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyás A, Acsády L, Freund T. Structural basis of the cholinergic and serotonergic modulation of GABAergic neurons in the hippocampus. Neurochem Int. 1999;34:359–372. doi: 10.1016/s0197-0186(99)00041-8. [DOI] [PubMed] [Google Scholar]
- Hurley P, McMahon R, Fanning P, O'Boyle K, Rogers M, Martin F. Functional coupling of a recombinant human 5-HT5A receptor to G-proteins in HEK-293 cells. Br J Pharmacol. 1998;124:1238–1244. doi: 10.1038/sj.bjp.0701928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Jia Y. Serotonergic modulation of carbachol-induced rhythmic activity in hippocampal slices. Neuropharmacology. 2005;48:381–390. doi: 10.1016/j.neuropharm.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Kulik A, Vida I, Fukazawa Y, Guetg N, Kasugai Y, Marker C, Rigato F, Bettler B, Wickman K, Frotscher M, Shigemoto R. Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal cells. J Neurosci. 2006;26:4289–4297. doi: 10.1523/JNEUROSCI.4178-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Jan Y, Jan L. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci. 1996;16:7137–7150. doi: 10.1523/JNEUROSCI.16-22-07137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Setya S, Johnson-Farley N, Cowen D. Differential coupling of 5-HT1 receptors to G proteins of the Gi family. Br J Pharmacol. 2002;136:1072–1078. doi: 10.1038/sj.bjp.0704809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lladó-Pelfort L, Assié M-B, Newman-Tancredi A, Artigas F, Celada P. In vivo electrophysiological and neurochemical effects of the selective 5-HT1A receptor agonist, F13640, at pre- and postsynaptic 5-HT1A receptors in the rat. Psychopharmacology. 2012;221:261–272. doi: 10.1007/s00213-011-2569-9. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Jan L, Stoffel M, Malenka R, Nicoll R. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- Malagié I, Trillat A, Bourin M, Jacquot C, Hen R, Gardier A. 5-HT1B autoreceptors limit the effects of selective serotonin re-uptake inhibitors in mouse hippocampus and frontal cortex. J Neurochem. 2001;76:865–871. doi: 10.1046/j.1471-4159.2001.00083.x. [DOI] [PubMed] [Google Scholar]
- Ögren S, Eriksson T, Elvander-Tottie E, D'Addario C, Ekström J, Svenningsson P, Meister B, Kehr J, Stiedl O. The role of 5-HT1A receptors in learning and memory. Behav Brain Res. 2008;195:54–77. doi: 10.1016/j.bbr.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Prinssen E, Colpaert F, Koek W. 5-HT1A receptor activation and anti-cataleptic effects: high-efficacy agonists maximally inhibit haloperidol-induced catalepsy. Eur J Pharmacol. 2002;453:217–221. doi: 10.1016/s0014-2999(02)02430-5. [DOI] [PubMed] [Google Scholar]
- Ramboz S, Oosting R, Amara D, Kung H, Blier P, Mendelsohn M, Mann J, Brunner D, Hen R. Serotonin receptor 1A knockout: an animal model of anxiety-related disorder. Proc Natl Acad Sci U S A. 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber R, Newman-Tancredi A. Improving cognition in schizophrenia with antipsychotics that elicit neurogenesis through 5-HT1A receptor activation. Neurobiol Learn Mem. 2014;110:72–80. doi: 10.1016/j.nlm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- Schulz S, Heidmann K, Mike A, Klaft Z-J, Heinemann U, Gerevich Z. First and second generation antipsychotics influence hippocampal gamma oscillations by interactions with 5-HT3 and D3 receptors. Br J Pharmacol. 2012;167:1480–1491. doi: 10.1111/j.1476-5381.2012.02107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal V, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer K, Niznikiewicz M, Shenton M, McCarley R. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol Psychiatry. 2008;63:744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Samuels B, Hen R. Serotonin receptor expression along the dorsal-ventral axis of mouse hippocampus. Phil Trans R Soc Lond B Biol Sci. 2012;367:2395–2401. doi: 10.1098/rstb.2012.0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vugt M, Schulze-Bonhage A, Litt B, Brandt A, Kahana M. Hippocampal gamma oscillations increase with memory load. J Neurosci. 2010;30:2694–2699. doi: 10.1523/JNEUROSCI.0567-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Doheny HC, Traub RD, LeBeau FE, Buhl EH. Differential expression of synaptic and nonsynaptic mechanisms underlying stimulus-induced gamma oscillations in vitro. J Neurosci. 2001;21:1727–1738. doi: 10.1523/JNEUROSCI.21-05-01727.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterer J, Stempel A, Dugladze T, Földy C, Maziashvili N, Zivkovic A, Priller J, Soltesz I, Gloveli T, Schmitz D. Cell-type-specific modulation of feedback inhibition by serotonin in the hippocampus. J Neurosci. 2011;31:8464–8475. doi: 10.1523/JNEUROSCI.6382-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójtowicz A, van den Boom L, Chakrabarty A, Maggio N, Haq R, Behrens C, Heinemann U. Monoamines block kainate- and carbachol-induced gamma-oscillations but augment stimulus-induced gamma-oscillations in rat hippocampus in vitro. Hippocampus. 2009;19:273–288. doi: 10.1002/hipo.20508. [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Fries P. The role of neuronal synchronization in selective attention. Curr Opin Neurobiol. 2007;17:154–160. doi: 10.1016/j.conb.2007.02.002. [DOI] [PubMed] [Google Scholar]


