Abstract
It has been reported that the threshold to activate ‘silent’ or inactive descending facilitation of nociception is lower than that of descending inhibition. Thus, the development of pain therapy to effectively drive descending inhibition alone, without the confounding influences of facilitation is a challenge. To address this issue we investigated the effects of intramuscular stimulation with a heating-needle on spinal nociception, assessed by measuring nociceptive paw withdrawal reflex in rats. Additionally, involvement of the thalamic ‘nociceptive discriminators’ (thalamic mediodorsal (MD) and ventromedial (VM) nuclei), and opioid-mediated mechanisms were further explored. Descending facilitation and inhibition were elicited by 46°C noxious heating-needle stimulation, and were regulated by thalamic MD and VM nuclei, respectively. In contrast, innocuous heating-needle stimulation at a temperature of 43°C elicited descending inhibition modulated by the thalamic VM nucleus alone. Microinjection of μ/δ/κ-opioid receptor antagonists β-funaltrexamine hydrochloride/naltrindole/nor-binaltorphimine, into the VM nucleus attenuated the 46°C intramuscular heating-needle stimulation-evoked descending inhibition, whereas treatment of the MD nucleus with β-funaltrexamine hydrochloride significantly decreased the descending facilitation. By contrast, descending inhibition evoked by 43°C heating-needle stimulation was only depressed by naltrindole, as opposed to μ- and κ-opioid receptor antagonists, which failed to influence descending inhibition. The present study reveals distinct roles of μ-opioid receptors in the function of thalamic MD and VM nuclei, which exert facilitatory and inhibitory actions on nociception. Furthermore, innocuous, but not noxious, intramuscular heating-needle stimulation targeting δ-opioid receptors is suggested to be a promising avenue for the effective inhibition of pain.
Key points
Due to a lower triggering threshold of descending facilitation, driving of the descending inhibition alone without irritation of descending facilitation is critically challenged in the modulation of pain.
We test the hypothesis and provide the novel finding that intramuscular non-painful heat stimulation may bypass the lower triggering thresholds of descending facilitation, and initiate the descending inhibition alone, which gives us a promising avenue to treat pain.
We further reveal distinct roles of μ/δ/κ-opioid receptors in the function of the thalamic ‘nociceptive discriminators’, the thalamic mediodorsal and ventromedial nuclei, in descending facilitatory and inhibitory controls of nociception.
The present study helps us understand the mechanisms of the triggering and strengthening of endogenous descending inhibition via non-painful heat stimulation, i.e. moxibustion, in effective relief of pain.
Introduction
In the past several decades, the role and significance of descending regulation of pain and nociception has received much attention due to its implications in the development and maintenance of pathological pain that is associated with central sensitization, as evidenced by hyperalgesia and allodynia (Treede et al. 1992). From anatomical and functional perspectives, it is widely accepted that different descending pain modulatory circuits exert distinct actions, either facilitation or inhibition, on spinal nociceptive processing (Fields, 1992; Zhuo et al. 2002; for reviews see Urban & Gebhart, 1999; Millan, 2002; Heinricher et al. 2009). Enhancing descending inhibition or weakening descending facilitation by means of pharmacological and non-pharmacological interference has been suggested to have a role in the clinical control of pathological pain (Pertovaara, 2000). However, in contrast to tonic modulation of proprioceptor-mediated spinal processing, an important characteristic of endogenous descending control of nociceptor-evoked responses under physiological conditions is that it is ‘silent’ or inactive, and is triggered and initiated by sufficient peripheral C-afferent inputs (You et al. 2010). Most importantly, the threshold to trigger descending facilitation is significantly lower than that of descending inhibition (Lei et al. 2011; Lei & You, 2013; You et al. 2013). This might be a major obstacle in the initiation of descending inhibition alone to effectively relieve pain without excitation of descending facilitation. However, information transmitted by C-afferents is not restricted to nociception and includes innocuous heat signals (Besson & Chaouch, 1987). Thus, we hypothesized previously that non-painful heat stimulation, e.g. moxibustion, might be a way to trigger and strengthen descending inhibition alone (You et al. 2013), and that this approach warrants investigation.
In contrast to antinociceptive effects within the peripheral nervous system and the spinal cord, the actions of opioids in supraspinal structure, i.e. cortex and other areas, appear conflicting. Activation of opioid receptors in descending pathways, e.g. rostral ventromedial, are reported to be antinociceptive (Heinricher et al. 1992), whereas excitation of opioid receptors in other brain regions results in pronociceptive effects on nociceptive processing (Ossipov et al. 2004). Among the family of opioid receptors, effects of μ-opioid receptors on nociception have received much attention and debate (Marinelli et al. 2002). Administration of a μ-opioid receptor agonist, e.g. fentanyl, produces profound analgesia followed by long-lasting hyperalgesia, which may cause tolerance and addiction to μ-opioids with serious clinical challenge (Colpaert, 1996). To date, the interpretation of such complex and paradoxical signal transduction in nociceptive systems is still not understood.
In the present study, we investigated the potential influence of intramuscular heating-needle stimulation at different temperatures on endogenous descending modulation of pain. In addition, opioidergic mechanisms underlying the descending regulation – facilitation and inhibition – of nociception were systematically explored. For the first time we report direct, novel evidence that innocuous (43°C) heat stimulation, which targets δ-, but not μ- and κ-opioid receptors, rather than noxious (46°C) heat stimulation might be a promising approach for the effective control of pain.
Methods
Ethical approval and animals
Male Sprague–Dawley rats weighing 260–300 g (about 10 weeks old) were provided by the Animal Center of the College of Medicine, Xi'an Jiaotong University, and housed in pairs in plastic boxes under a 12 h:12 h light:dark cycle (lights on at 08:00 h) at 22–26°C with food and water available ad libitum. All experiments were approved by the Xi'an Jiaotong University Animal Care Committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23), revised in 1996, and comply with the policies and regulations of The Journal of Physiology (Drummond, 2009). The animals were acclimatized to the laboratory and habituated to the test boxes for at least 1 h for each of the days prior to testing. The rats were used only once and killed at the end of the experiment by an intraperitoneal injection of an overdose of sodium pentobarbital (200 mg kg–1). All efforts were made to minimize animal suffering and the number of animals used.
Intramuscular heating-needle stimulation
Rats were anaesthetized initially by mask inhalation of isoflurane (4% isoflurane in 96% oxygen), followed by 1% isoflurane in 99% oxygen for the maintenance of anaesthesia during the intramuscular (i.m.) heating-needle stimulation. Stimulation was induced by a concentric stainless steel heating needle (diameter, 1.05 mm; length, 30 mm) which was connected and feedback-controlled by an inner heating therapeutic device (Model: NWX-1, Acuceuticals Co., Ltd, Shanghai, China). The heating needle is filled with a heating element and temperature probe, which detects variations of temperature precisely (±0.25°C). The heating needle was inserted unilaterally into the middle part of the gastrocnemius (GS) muscle of the left hind limb, and the depth of the insertion was about 0.5 cm.
Peripheral thermal stimuli at temperatures over 45°C were perceived to be noxious (Hardy, 1953; Julius & Basbaum, 2001). It was also reported that group IV muscle fibres respond to heat stimulation at temperatures over 43°C (Kumazawa & Mizumura, 1977). In the present study, the i.m. heating-needle stimulation was tested in the experimenters’ GS muscle to mimic the situation in the rats; a period of 45 min of 46°C heat stimulation evoked a heat sensation together with a moderate and tolerable pain sensation, whereas heat stimulation at a temperature of 43°C only generated a non-painful heat sensation. Thus, the i.m. heating-needle stimulation at a temperature of 46°C in the current study was chosen as a noxious stimulus, whereas 43°C was selected for innocuous heat stimulation.
During the experiment, the durations of heating-needle stimulation were 15 min, 30 min and 45 min for each temperature to investigate the temporal characteristics of i.m. heating-needle stimulation in the modulation of spinal nociception. In addition to single heating-needle stimulation, spatial effects of double heating-needle stimulation (distance between double needles, 0.5 cm) was tested on bilateral spinally organized paw withdrawal reflexes.
Electrolytic lesion of contralateral thalamic MD and VM nuclei
Anaesthetized (sodium pentobarbital, 50 mg kg−1, i.p.) rats were mounted in a stereotaxic frame with fixation of the head by ear bars and a tooth plate (68001, RWD Co., Shenzhen, China). After local lidocaine (lignocaine) analgesia, the scalp was cut and the cranium was drilled. A thalamic nucleus located contralaterally to the i.m. heating-needle stimulation was electrolytically lesioned. To perform the electrolytic lesion of the thalamic mediodorsal (MD) and the ventromedial (VM) nuclei, an insulated stainless steel electrode (shank diameter 200 μm; tip diameter 50 μm, exposed tip 50 μm) was advanced stereotactically into the targeted thalamic nuclei areas at the following coordinates. For the MD nucleus the position of the electrode was: anteroposterior, –(2.3–2.8) mm from bregma; lateral, 0.75 mm from midline; dorsoventral, 5.2–5.4 mm from the cranium. For the VM nucleus the position of the electrode was: anteroposterior, –(2.3–2.8) mm; lateral, 1.2–1.5 mm from midline; dorsoventral, 7.1–7.2 mm from the cranium (You et al. 2013). An electrolytic lesion was made by means of an electrical stimulator (Model-2100, A-M Systems Inc., Carlsborg, WA, USA) generating a 150 μA anodal DC current for 30 s through the electrode tip. The lesion current was monitored continuously by using an oscilloscope to measure the voltage drop across a 100 Ω resistor in series with the electrode. After the electrolytic lesion, the microelectrode was slowly withdrawn, the wound was washed with sterile 0.9% saline, treated with antibiotics, and the skull was closed with dental cement. A recovery period of 7 days was allowed, during which the animals’ behaviour and motor function were monitored. Animals showing motor dysfunction assessed by means of a Rota-Rod treadmill (Model 755, IITC, Woodland Hills, CA, USA) were excluded from the remaining experiments.
Intracerebral microinjection with different opioid antagonists
Rats were anaesthetized with sodium pentobarbital (50 mg kg−1, I.P.). As described elsewhere (You et al. 2013), a craniotomy was made with a dental drill in order to perform the intracerebral (i.c.) catheterization. One guide cannula (o.d. 0.35 mm; i.d. 0.25 mm; RWD Life Science Co., Shenzhen, China) was advanced stereotactically into the different thalamic nuclei areas at the coordinates described above. After the catheterization, the wound was washed with sterile 0.9% saline, treated with antibiotics, and the skull was closed with dental cement. The animals were then put back to the box for 7 days’ recovery during which the animals’ behaviour and motor function were strictly monitored. Animals showing severe permanent neurological deficits or motor dysfunction were excluded.
In previous studies (Lund et al. 2002; Schepers et al. 2008), administration of 1 nmol of β-funaltrexamine hydrochloride (β-FNA), naltrindole or nor-binaltorphimine (nor-BNI) into the thalamus and other areas of brain has been documented to effectively affect spinally organized nociception. In the present study, all antagonists were ordered commercially (Sigma-Aldrich Chemie Gmbh, Germany), and were freshly prepared and dissolved in 0.9% saline. Thirty minutes after the i.m. heating-needle stimulation, a bolus of 0.5 μl solution containing the each of the drugs listed above was injected through the intrathalamic catheter using a 1 μl microsyringe while the rat was gently restrained by hand. All drugs were slowly infused at a constant speed over a 30 s period. The effects of each of the drugs were tested within 4 h. In some experiments, the effects of the tested drug at the higher dose of 5 nmol (0.5 μl)–1 were also tested. After the experiment, the drug injection site was marked by microinjection with Pontamine Sky Blue dye (0.5 μl; 2% in 0.5 m sodium acetate).
Experimental design
Experimental study groups were randomized and blinded. According to the different experimental purposes, rats recruited in the current study were randomly divided into several individual groups; eight rats in each group were included for the investigation. The investigator conducting the behavioural measurements was blinded to the experimental group, i.e. heating-needle stimulation vs. needle insertion without heating, and i.c. opioid receptor antagonists treatment vs. i.c. 0.9% saline treatment.
Measurement of mechanical and heat sensitivity
For the measurement of noxious mechanically evoked paw withdrawal response, rats were placed in different individual plexiglass chambers with mesh floors underneath and transparent top covers (20 cm × 20 cm × 25 cm). A hand-held electronic von Frey device (2290 Electrovonfrey, IITC) with a rigid filament was used to detect the mechanical paw withdrawal threshold. According to the mapping of the withdrawal field of the gastrocnemius muscle (You et al. 2010), the filament was applied to the heel part of the hind paw until a foot withdrawal response was elicited, indicating that the mechanical threshold (grams) and the cut-off force had been achieved.
Noxious heat-evoked paw withdrawal responses were determined using a 390G plantar stimulator Analgesia Meter (IITC). Each rat was placed individually into a Plexiglas cubicle placed onto a constant temperature controlled transparent glass used to avoid temperature sink from the tested hind paws. The radiant heat stimulus was a high-intensity beam (setting = 30–40% intensity) aimed at the heel part of the hind paw. The hind paw withdrawal latency was determined as the time from the onset of noxious heat stimulation to withdrawal of the tested hind paw. The latency of the paw withdrawal reflex was initially measured and controlled around 10–11 s, which was considered as the baseline response. The same stimulus delivered to the operator's hand evoked painful but tolerant sensations. To avoid excessive tissue injury, manual cut-off of the heat stimulus was performed if no paw withdrawal reflex could be evoked by a 20 s heat stimulus.
The paw withdrawal thresholds to noxious mechanical and heat stimuli were tested for both the ipsilateral and contralateral heel of hind paws 30 min prior to and 30 min, 1–4 h and 1–7 days after the i.m. heating-needle stimulation. At each time point, bilateral hind paws received three trials each, with at least a 30 s interval between subsequent trials, for the mean of which represented the mechanical paw withdrawal threshold (grams) and thermal paw withdrawal latency (seconds). A reduced or increased threshold for the paw withdrawal response compared with the threshold before the i.m. heating-needle stimulation was defined as hyperalgesia or hypoalgesia, respectively.
Assessment of motor function
Briefly, animals were placed on a Rota-Rod treadmill (Model 755, IITC) rotating at a gradually increasing speed from 5 to 30 r.p.m. for 30 s and maintained for another 120 s at 30 r.p.m. Rats with motor dysfunction after the implantation of an intracerebral guide cannula and 20 min after the heat stimuli were strictly excluded from the remaining experiments.
Histology for identification of the placement of the cannula
At the end of behavioural testing, the animals that received implantation of an intracerebral cannula were anaesthetized by sodium pentobarbital (50 mg kg−1, i.p.) and transcardially perfused with 10% formalin. The brains were then isolated and stored in 30% sucrose for 2 days. Frozen serial sections (50 μm) were cut in the coronal plane, treated with Nissl stain and screened under a microscope (Leica, Germany). Schematic reconstructions of the injection sites were drawn according to the stereotaxic atlas of rats (Paxinos & Watson, 1998). In brain, microinjection of 0.5 μl of solution spread from the injection site with about 0.4 mm diffusion distance. Nearby rostrocaudal and mediolateral brain structures were critically checked. The histological determination of the location of the cannula tip was performed without knowledge of the behavioural results.
Statistical analysis
All results were expressed as means ± SEM. The data were analysed using SigmaStat (Systat Software Inc., San Jose, CA, USA) and compared by either paired one-way ANOVA or two-way repeated measures ANOVA with a post hoc Bonferroni test for analysis of the differences over the entire observation time among different groups. P < 0.05 was considered statistically significant.
Results
Effects of i.m. single heating-needle stimulation on nociceptive paw withdrawal reflexes
As shown in Fig. 1, paw withdrawal reflexes to mechanical and heat stimuli were evaluated bilaterally 30 min prior to and 30 min, 1–4 h and 1–7 days after the 15–45 min i.m. single heating-needle stimulation at a temperature of either 43°C or 46°C. A period of 45 min insertion of the needle without heating served as control, which exhibited no effects on noxious mechanical- and heat-evoked paw withdrawal reflexes over time (Fig. 1).
Figure 1. Variations of bilateral noxious mechanical- and heat-evoked paw withdrawal reflexes prior to and post ipsilateral single heating-needle stimulation at a temperature of 43°C or 46°C.
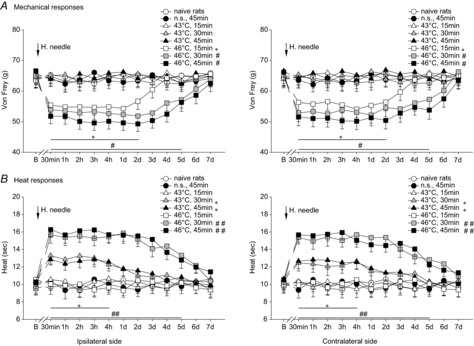
Significantly decreased mechanical paw withdrawal thresholds (mechanical hyperalgesia) were found bilaterally following the ipsilateral i.m. 15–45 min of 46°C heating-needle stimulation, whereas 15–45 min i.m. 43°C heating-needle stimulation failed to cause any effects on bilateral mechanically evoked paw withdrawal reflexes (A). Prolonged bilateral heat-evoked paw withdrawal latencies (heat hypoalgesia) were significantly observed after the 30–45 min, but not 15 min, of i.m. heating-needle stimulation at temperature of 43°C or 46°C (B). *P < 0.05, #P < 0.05 and ##P < 0.001 compared with naive rats group. Naive rats, rats without receiving any treatments; B, baseline response before the heating-needle stimulation; n.s., needle stimulation without heating. n = 8 for each group.
A period of 15–45 min ipsilateral i.m. 43°C heating-needle stimulation failed to cause any effects on bilateral mechanically evoked paw withdrawal reflexes (P > 0.05, Fig. 1A), whereas prolonged bilateral heat-evoked paw withdrawal latencies (heat hypoalgesia) were significantly observed after the 30–45 min, but not 15 min, of 43°C heating-needle intramuscular stimulation (43°C 30 min; ipsilateral: F(4,28) = 3.51, P < 0.05; contralateral: F(4,28) = 3.55, P < 0.05; Fig. 1B; 43°C 45 min; ipsilateral: F(4,28) = 3.49, P < 0.05; contralateral: F(4,28) = 3.57, P < 0.05; Fig. 1B). In contrast to no effects of 43°C heating-needle stimulation on mechanically evoked paw withdrawal reflexes, significantly enhanced mechanically evoked responses (mechanical hyperalgesia) were found following the 15–45 min of i.m. 46°C heating-needle stimulation (46°C 15 min; ipsilateral: F(6,42) = 2.74, P < 0.05; contralateral: F(6,42) = 2.81, P < 0.05; Fig. 1A; 46°C 30 min; ipsilateral: F(9,63) = 2.21, P < 0.05; contralateral: F(9,63) = 2.27, P < 0.05; Fig. 1A; 46°C 45 min; ipsilateral: F(9,63) = 2.58, P < 0.05; contralateral: F(9,63) = 2.53, P < 0.05; Fig. 1A). The onset of bilateral mechanical hyperalgesia was observed no later than 30 min after the end of 15–45 min of ipsilateral 46°C heating-needle stimulation, and the effects lasted over 2–5 days (Fig. 1A). Likewise, prolonged heat-evoked paw withdrawal latencies (heat hypoalgesia) were found bilaterally after 30–45 min, but not 15 min, of ipsilateral 46°C heating-needle stimulation. Among different treatments, the longest heat-evoked paw withdrawal latencies were found in rats that received 30–45 min of 46°C heating-needle stimulation (P < 0.001, two-way ANOVA, Fig. 1B).
Effects of i.m. double heating-needle stimulation on nociceptive paw withdrawal reflexes
In addition to single heating-needle stimulation, variations of bilateral paw withdrawal mechanical threshold and paw withdrawal thermal latency exposed to the i.m. double heating-needle stimulation at temperature of 43°C or 46°C were observed.
In the control group, the insertion of double needles without heating into the GS muscle exhibited no effects on bilateral mechanical- and heat-evoked paw withdrawal reflexes over time (P > 0.05, Fig. 2A). Ipsilateral i.m. double heating-needle stimulation at a temperature of 43°C regardless of the different durations (15–45 min) did not show any effects on bilateral mechanically evoked paw withdrawal reflexes (P > 0.05, two-way ANOVA, Fig. 2A). After 30–45 min, but not 15 min, of 43°C double heating-needle stimulation, bilateral heat-evoked paw withdrawal latencies were significantly prolonged (P < 0.05, two-way ANOVA, Fig. 2B).
Figure 2. Effects of ipsilateral double heating-needle stimulation at a temperature of either 43°C or 46°C on bilateral noxious mechanical- and heat-evoked paw withdrawal reflexes.
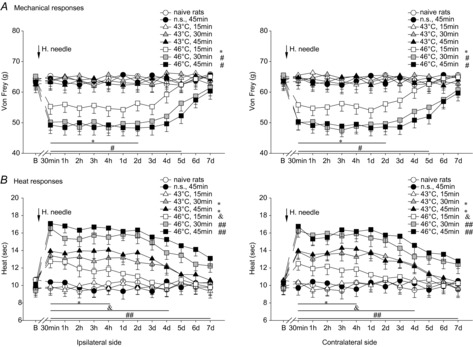
Bilateral mechanical hyperalgesia was found following a period of 15–45 min of ipsilateral i.m. double heating-needle stimulation at a temperature of 46°C, but not 43°C (A). A period of 30–45 min 43°C or 15–45 min 46°C double heating-needle stimulation caused bilateral heat hypoalgesia (B). *P < 0.05, &P < 0.05, #P < 0.05 and ##P < 0.001 compared with naive rats. Naive rats, rats without receiving any treatments; B, baseline response before the heating-needle stimulation; n.s., needle stimulation without heating. n = 8 for each group.
After a period of 15–45 min of 46°C double heating-needle stimulation, bilateral significantly enhanced mechanically evoked paw withdrawal reflexes were observed (P < 0.05, two-way ANOVA, Fig. 2A). Compared with 46°C single heating-needle stimulation, the lowest mechanical paw withdrawal threshold and the longest duration of mechanical hyperalgesia occurred following 30–45 min of 46°C double heating-needle stimulation. In contrast to no effects of 15 min 46°C single heating-needle stimulation on heat-evoked paw withdrawal latencies (Fig. 1B), ipsilateral 15 min double heating-needle stimulation at a temperature of 46°C significantly caused prolonged paw withdrawal latencies (heat hypoalgesia) bilaterally (ipsilateral: F(4,28) = 3.37, P < 0.05; contralateral: F(4,28) = 3.29, P < 0.05; Fig. 2B). The long-lasting bilateral heat hypoalgesia after the ipsilateral 30–45 min 46°C double heating-needle stimulation remained at the higher level for at least 7 days, and declined gradually within 10 days (Fig. 2B).
Influence of contralateral electrolytic lesion of the thalamic MD nucleus or VM nucleus on i.m. heating-needle stimulation-induced mechanical hyperalgesia and heat hypoalgesia
Figure 3A shows the schematic reconstructions of the electrolytic lesion sites (n = 32) of thalamic MD and VM nuclei. One week after the contralateral electrolytic lesion of the target thalamic MD or VM nucleus, variations of paw withdrawal reflexes to noxious mechanical and heat stimuli were tested. After this time, the effects of 30 min i.m. heating-needle stimulation at either 43°C or 46°C on mechanical- and heat-evoked nociceptive paw withdrawal reflexes were investigated.
Figure 3. Reconstructions of the locations of 32 electrolytic lesions of thalamic MD and VM nuclei (A), and of 128 microinjection sites within the thalamic mediodorsal (MD) and ventromedial (VM) nuclei (B).
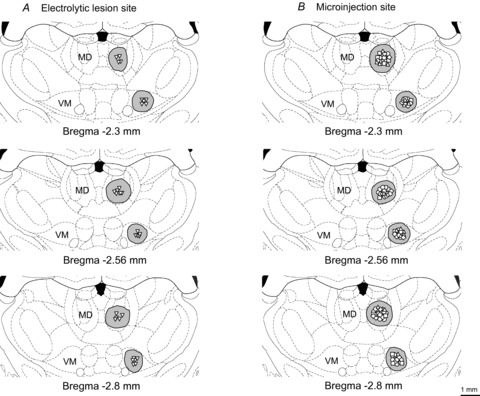
The spread regions of lesion and microinjection are marked by grey areas. Effective sites for electrolytic lesion are indicated by down-triangles in A. Microinjection sites of 0.9% saline, β-FNA, naltrindole and nor-BNI are indicated by circles, up-triangles, squares and diamonds in B, respectively. Please note that some microinjection sites overlap with others in these reconstructions.
Lesion of the contralateral thalamic MD or VM nucleus failed to affect the bilateral mechanical- and heat-evoked paw withdrawal reflexes (P > 0.05). However, after exposure to i.m. heating-needle stimulation, lesion of the contralateral thalamic MD nucleus significantly blocked the occurrence of bilateral mechanical hyperalgesia (P < 0.05, one-way ANOVA, Fig. 4A), whereas no heat hypoalgesia was observed after lesion of the contralateral thalamic VM nucleus (P < 0.05 and P < 0.001, one-way ANOVA, Fig. 4B).
Figure 4. Involvement of the contralateral thalamic MD and VM nuclei in 30 min i.m. heating-needle stimulation ipsilaterally induced effects on bilateral mechanical- and heat-evoked paw withdrawal reflexes.
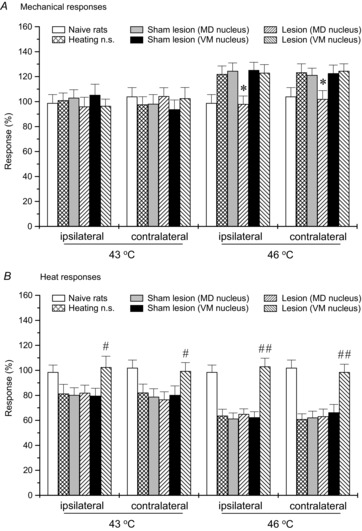
The contralateral thalamic MD and VM nuclei were electrolytically lesioned by 150 μA DC current. Lesion of the contralateral thalamic MD nucleus significantly blocked the i.m. 46°C heating-needle stimulation-induced bilateral mechanical hyperalgesia (A). Bilateral heat hypoalgesia elicited by i.m. 43°C or 46°C heating-needle stimulation was significantly prevented by lesion of the contralateral thalamic VM nucleus (B). *P < 0.05, #P < 0.05 and ##P < 0.001 compared with heating-needle stimulation group. Naive rats, rats without receiving any treatments; n.s., needle stimulation. n = 8 for each group.
Influence of i.c. microinjection of different opioid antagonists into thalamic MD or VM nucleus on i.m. 43°C heating-needle stimulation-induced effects
Figure 3B shows the schematic reconstructions of microinjection sites (n = 128) of thalamic MD and VM nuclei. Thirty minutes after the i.m. single heating-needle stimulation, a bolus of 0.5 μl solution containing one of the different opioid antagonists (β-FNA, naltrindole or nor-BNI) was injected into the target thalamic MD or VM nucleus, respectively.
In Fig. 5, i.c. administration of μ-, δ- and κ-opioid receptor antagonists into the thalamic MD nucleus did not affect mechanical- and heat-evoked paw withdrawal reflexes following the i.m. 43°C heating-needle stimulation (P > 0.05). Likewise, no influence of i.c. administration of μ-, δ- and κ-opioid receptor antagonists into the contralateral thalamic VM nucleus on noxious mechanically evoked paw withdrawal reflex was found (P > 0.05, Fig. 6A). In contrast, i.c. microinjection with δ-opioid receptor antagonist naltrindole, but not the other opioid receptor antagonists, into the contralateral thalamic VM nucleus, significantly depressed bilateral heat hypoalgesia induced by i.m. innocuous 43°C heating-needle stimulation compared with vehicle (0.9% saline) treatment (ipsilateral: F(3,21) = 3.56, P < 0.05; contralateral: F(3,21) = 3.49, P < 0.05; Fig. 6B). We further tested the effects of opioid μ- and κ-antagonists at the higher dose of 5 nmol (0.5 μl)–1. No significant effects of these higher doses of opioid receptor antagonists on 43°C heating-needle stimulation-induced descending inhibition of paw withdrawal latency were found (data not shown).
Figure 5. Effects of i.c. microinjection with β-FNA, naltrindole or nor-BNI into the contralateral thalamic MD nucleus on bilateral mechanical- and heat-evoked paw withdrawal reflexes during the condition of 30 min 43°C i.m. single heating-needle stimulation.
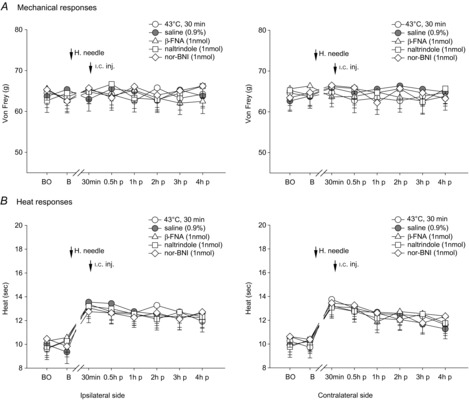
No influence of i.c. administration of μ-, δ- and κ-opioid receptor antagonists into the thalamic MD nucleus was found on mechanical- and heat-evoked paw withdrawal reflexes following the i.m. 43°C heating-needle stimulation. BO, baseline response before the operation of i.c. catheterization; B, baseline response before the i.m. heating-needle stimulation; n.s., needle stimulation; p, post i.c. injection. n = 8 for each group.
Figure 6. Effects of i.c. microinjection with β-FNA, naltrindole or nor-BNI into the contralateral thalamic VM nucleus on bilateral mechanical- and heat-evoked paw withdrawal reflexes during the condition of 30 min 43°C i.m. single heating-needle stimulation.
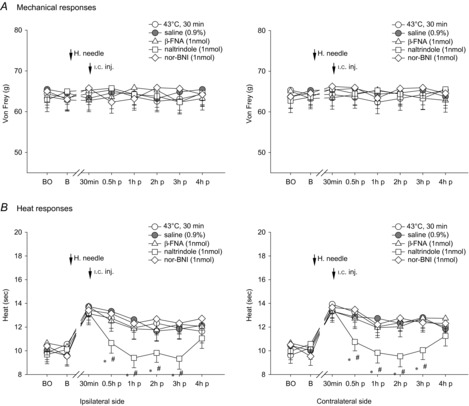
Bilateral heat hypoalgesia induced by ipsilateral i.m. 43°C heating-needle stimulation were significantly depressed by i.c. microinjection with δ-opioid antagonist naltrindole, but not the other opioid receptor antagonists, into the contralateral thalamic VM nucleus. *P < 0.05 compared with the 43°C i.m. single heating-needle stimulation group. #P < 0.05 compared with the vehicle (0.9% saline) treatment group. BO, baseline response before the i.c. catheterization; B, baseline response before the i.m. heating-needle stimulation; p, post i.c. injection. n = 8 for each group.
Influence of i.c. microinjection of different opioid antagonists into thalamic MD or VM nucleus on i.m. 46°C heating-needle stimulation-induced descending facilitation and inhibition
After the i.c. microinjection with μ-opioid receptor antagonist β-FNA into the contralateral MD nucleus, bilateral mechanical hyperalgesia elicited by the 30 min noxious 46°C heating-needle stimulation was depressed significantly (P < 0.05, two-way ANOVA), whereas δ/κ-opioid receptor antagonists naltrindole and nor-BNI had no effects (P > 0.05, two-way ANOVA; Fig. 7A). As for innocuous 43°C heating-needle stimulation, opioid antagonists administrated into the contralateral thalamic MD nucleus failed to block heat hypoalgesia during the condition of i.m. noxious 46°C heating-needle stimulation (P > 0.05, Fig. 7B). Administration of opioid antagonists into the contralateral thalamic VM nucleus did not cause any effects on i.m. 46°C heating-needle stimulation-induced mechanical hyperalgesia (P > 0.05, Fig. 8A). The bilateral heat hypoalgesia was significantly decreased by i.c. microinjection of μ-opioid receptor antagonist β-FNA, δ-opioid receptor antagonist naltrindole, or κ-opioid receptor antagonist nor-BNI into the contralateral thalamic VM nucleus (P < 0.05, two-way ANOVA; Fig. 8B).
Figure 7. Effects of i.c. microinjection with β-FNA, naltrindole or nor-BNI into the contralateral thalamic MD nucleus on bilateral noxious mechanical- and heat-evoked paw withdrawal reflexes during the exposure to the i.m. noxious 46°C single heating-needle stimulation.
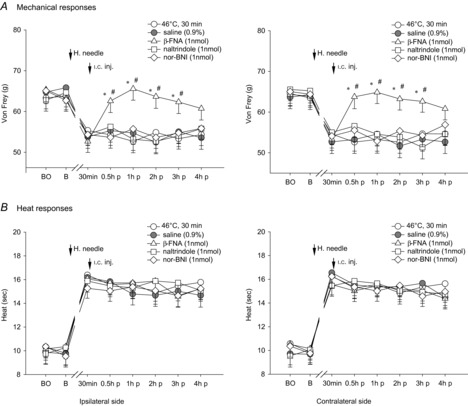
Bilateral mechanical hyperalgesia elicited by the 30 min 46°C heating-needle stimulation was depressed significantly by the i.c. microinjection with μ-opioid receptor antagonist β-FNA into the contralateral MD nucleus. It is suggested that the μ-opioid receptor is involved in thalamic MD nucleus-mediated descending facilitation of mechanically evoked nociception.*P < 0.05 compared with the 46°C i.m. single heating-needle stimulation group. #P < 0.05 compared with the vehicle (0.9% saline) treatment group. BO, baseline response before the i.c. catheterization; B, baseline response before the i.m. heating-needle stimulation; p, post i.c. injection. n = 8 for each group.
Figure 8. Effects of i.c. microinjection with β-FNA, naltrindole or nor-BNI into the contralateral thalamic VM nucleus on bilateral noxious mechanical- and heat-evoked paw withdrawal reflexes during the exposure to the i.m. noxious 46°C single heating-needle stimulation.
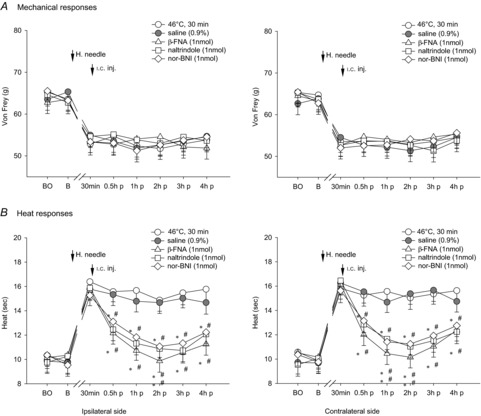
i.c. microinjection of β-FNA, naltrindole or nor-BNI into the contralateral thalamic VM nucleus markedly depressed the bilateral heat hypoalgesia. It is suggested that μ/δ/κ-opioid receptors are all involved in thalamic VM nucleus-organized descending inhibition of heat-evoked nociception. *P < 0.05 and **P < 0.001 compared with the 46°C i.m. single heating-needle stimulation group. #P < 0.05 and ##P < 0.001 compared with the vehicle (0.9% saline) treatment group. BO, baseline response before the i.c. catheterization; B, baseline response before the i.m. heating-needle stimulation; p, post i.c. injection. n = 8 for each group.
Discussion
The present study provides novel, direct evidence that innocuous, but not noxious, heating-needle stimulation may selectively initiate ‘silent’ or inactive descending inhibition alone. Central δ-opioid receptors within the thalamic VM nucleus are pivotally involved in innocuous heating-needle stimulation-induced endogenous descending inhibition. Targeting δ-, but not μ- and κ-, opioid receptors, innocuous heating stimulation may provide a promising approach in the effective control of pathological pain, and that excitation of descending facilitation associated with central sensitization can be avoided.
Heat evoked descending modulation: facilitation and inhibition, of pain
The experience and perception of pain not only depends on peripheral noxious afferents, but also relies on complex nociceptive processing and regulation within the central nervous system, in particular supraspinal regions. Over the last several decades, evidence from different laboratories has indicated that the descending controls of pain consist of two distinct modulations, facilitation and inhibition, which are mediated by multiple cortical and subcortical regions, i.e. anterior cingulate cortex, thalamus and some specific areas regarded previously to be only involved in the precise control of movement, i.e. cerebellum (Calejesan et al. 2000; Hofbauer et al. 2001; Saab & Willis, 2003; Dostrovsky & Craig, 2009). Despite much progress in the exploration of pain, basic researchers and clinicians still face serious challenges in a better understanding of pain per se and its modulation. From a functional perspective, in contrast to unconscious persistent controls of proprioception and postural movement, the question of whether descending control of pain is tonically active has been a much debated subject without a clear understanding. We recently reported that the descending modulations, facilitation and inhibition, of pain in the normal physiological state are ‘silent’ or inactive, which over time can be triggered by sufficient C-afferent input associated with central temporal and spatial summation (You et al. 2010, 2013; Lei et al. 2011). From an anatomical perspective, there is some doubt as to whether central neural circuits differ in their modulation of nociceptor-evoked mechanical and heat-evoked responses due to the fact that secondary heat hyperalgesia within the non-injury area is rarely reported (Lewis, 1936; Treede et al. 1992; Fuchs et al. 2000). In accordance with other reports conducted in humans as well as in animals (Pertovaara, 1998; Monconduit et al. 1999; Magerl et al. 2001; Shyu et al. 2004; Montes et al. 2005; Waters & Lumb, 2008; Wilson et al. 2008), it has been reported that thalamic MD and VM nuclei referred to as thalamic ‘nociceptive discriminators’ specifically participate in not only the discrimination of somatosensory afferents, but also in the precise regulation of descending controls (Lei & You, 2013; You et al. 2013). Noxious mechanically induced nociception is regulated by the activity of the thalamic MD nucleus, whereas the thalamic VM nucleus modulates heat-evoked nociceptive activities. One important finding from the current study is that electrolytic lesion of, or intracerebral administration of μ/δ/κ-opioid receptor antagonists into, the target thalamic nuclei significantly blocked and attenuated activities of descending modulations after the cessation of heating-needle stimulation, and the withdrawal of the heating-needle from the GS muscle. It could conceivably be hypothesized that, after their initiation, the activity of descending control from supraspinal regions is independent of continuous peripheral afferent input, but relies on the activity of the thalamic ‘nociceptive discriminators’. Taken together with previous studies in physiological and pathological pain states (You et al. 2010, 2013; Lei et al. 2011, Lei & You, 2013), it is further suggested that the thalamic ‘nociceptive discriminators’, thalamic MD and VM nuclei, may also function as the pivotal ‘control unit’ in maintaining the descending modulation of pain.
Based on these specific characteristics of sensory transmission within the region of the thalamus, one would expect that interference in up- and down-regulation of the activity of thalamic MD and VM nuclei may therefore have coordinated effects, resulting in a reduction of nociceptor-induced anxiety responses and abnormal activities, i.e. hyperalgesia. Unfortunately, it has been previously demonstrated that the triggering thresholds of ‘silent’ or inactive descending facilitation and inhibition are significantly different; lower thresholds for descending facilitation and higher thresholds for descending inhibition (You et al. 2010; Lei et al. 2011). These specific characteristics of lower triggering thresholds of facilitation might be a major obstacle in prompt initiation of descending inhibition alone without excitation of descending facilitation for the treatment of pain. As detailed above, ‘silent’ or inactive endogenous descending controls, facilitation and inhibition, can be triggered by peripheral C-fibre mediated afferents. In addition to nociceptors, C-fibres also convey low-threshold mechanoceptive or thermoceptive information (Besson & Chaouch, 1987). This raises the possibility of exciting descending inhibition alone by means of innocuous heat stimulation, which may bypass the lower triggering threshold of descending facilitation. The present study is the first to address this issue, and has demonstrated that innocuous 43°C heating-needle stimulation provoked descending inhibition only, whereas both facilitation and inhibition of pain were excited by i.m. heat stimulation at temperatures over 45°C.
There is considerable evidence that the initiation of endogenous descending controls, facilitation and inhibition, is dependent on the activation of peripheral C-fibre afferents associated with temporal and spatial summation (You et al. 2010, 2013; Lei et al. 2011). With respect to the characteristics of temporal summation, i.m. heating-needle stimulation with a duration longer than 30 min in the present study did not exert a more powerful effect on the enhancement of descending inhibition, and durations shorter than 15 min might not easily trigger descending inhibition (Fig. 1). In Fig. 2, double heating-needle stimulation at either 43°C or 46°C caused enhanced and longer-lasting analgesia, which is consistent with our previous finding showing characteristics of spatial summation in descending inhibition (Lei et al. 2011). Thus, 30 min of double or more heating-needle stimulation might be a good option for innocuous heating therapy in clinical usage.
Central opioid receptors and their actions on the perception of pain and its modulation
Opioids targeting several types of receptors, μ, δ, κ, exert diverse effects on pain behaviour and nociception. Experimental data showed that opioid receptors are located in areas of the brain, spinal cord and at the periphery, that are involved in pain transmission and perception (Goodman et al. 1980; Lewis et al. 1983; Gouardères et al. 1985; Stein et al. 1989). It has also been reported that all opioid receptor subtypes are located in thalamic MD and VM nuclei, and that the μ-opioid receptor has a higher density in thalamic MD and VM nuclei than δ and κ receptors (Mansour et al. 1987). Except for the antinociceptive role of opioid receptors in the spinal cord and the periphery, opioids can produce heightened pain sensations which are characterized by hyperalgesia and allodynia after short and long-term exposure (Arner et al. 1988; Celerier et al. 1999), and inconsistent reports in the literature show complex roles of opioids, in particular μ-opioids, in supraspinal regions (Nicoll et al. 1980; Gear & Levine, 2011). Analgesia followed by hyperalgesia elicited by μ-opioids has been specifically highlighted as it may induce the development of opioid tolerance and dependence (Colpaert, 1996; Vanderah et al. 2001).
Mechanisms of different actions of central opioids, in particular those that mediate hyperalgesia caused by μ-opioid receptors, in the modulation of pain within the brain are unknown, and are proposed to be dependent on the doses, receptor subtypes, and diversity of experimental pain models (Jacquet & Lajtha, 1974; Leybin et al. 1976; Childers et al. 1979; Millan, 1986; Phillips et al. 2012). From the current study, one important and unanticipated finding was that μ-opioid receptor antagonist, β-funaltrexamine hydrochloride, administered into the MD and VM nuclei exhibited significantly opposite actions upon the i.m. noxious heating-needle stimulation-induced descending facilitation and inhibition. In contrast to the antinociceptive role of μ-opioids in the thalamic VM nucleus, μ-opioid receptors in the thalamic MD nucleus showed pronociceptive effects and were involved in descending facilitation. This μ-opioid receptor-mediated pronociceptive effect in thalamic MD nucleus has been suggested to probably depend on NMDA receptor-mediated pain facilitation (Mao et al. 1994; Larcher et al. 1998; Celerier et al. 2000; Kow et al. 2002; Heinl et al. 2011). Morphological studies have shown colocalization of μ-opoid receptors and NMDA receptors in the spinal dorsal horn as well as in supraspinal sites (Gracy & Pickel, 1997; Commons et al. 1999). Further studies revealed that opiates can activate the NMDA receptor through G proteins associated with opioid receptor and/or intracellular mechanism responses (Eric & Aghajanian, 1997; Chan et al. 2002; Rang et al. 2005). Our previous studies provided indirect evidence of diverse roles of glutamate within MD and VM nuclei, exerting facilitatory and inhibitory modulation of nociception, respectively (You et al. 2010; Lei & You, 2013). These paradoxical findings suggest that the different roles of opioids in promoting descending facilitation and inhibition are closely associated with the function of specific thalamic nuclei per se in the modulation of pain. Thus, we speculate that the antinociceptive effects of systemic administration of μ-opioids, i.e. morphine, may be largely related to its peripheral antinociceptive effects (Coggeshall et al. 1997), and counteract μ-opioid-induced descending facilitation. After metabolism, the pronociceptive effects associated with tolerance of μ-opioids due to the initiation of descending facilitation emerge later.
In conclusion, descending inhibition with excitation of central δ-opioid receptors elicited by innocuous heat stimulation may open a new window, and provide a novel therapeutic target for the effective control of pathological intractable pain. More experiments related to pharmaceutical drug development, in particular the up-regulation of function of thalamic δ-opioids in the thalamic VM nucleus and down-regulation of activity of μ-opioid receptors in the thalamic MD nucleus, are required in the future. In contrast to pollution by smoke and the danger of cautery caused by moxa burning, non-painful heating-needle therapy instead of traditional moxibustion provides adjustable, steady and quantitative heat stimulation for the treatment of pain.
Glossary
- β-FNA
β-funaltrexamine hydrochloride
- GS
gastrocnemius
- i.c.
intracerebral
- i.m.
intramuscular
- MD
mediodorsal
- nor-BNI
nor-binaltorphimine
- VM
ventromedial
Additional information
Competing interests
The authors declare that there are no competing interests.
Author contributions
The experiments were performed in the Center for Biomedical Research on Pain in the College of Medicine at Xi'an Jiaotong University. H.-J.Y. contributed to the conception and design of the entire experiments, conducted the experiments and performed data analysis and interpretation, and drafted the paper. J.L. conducted the experiments and performed the data analyses, data discussion and interpretation, and assisted in the writing. G.Y., X.-L.F. and Q.L. contributed to the conduction of experiments and data analysis, and assisted in the writing. All authors approved the final version of the manuscript.
Funding
The present work was supported by grants from the National Natural Science Foundation of P.R. China (30971424, 81271228), and a grant from ShaanXi Province Science and Technology Research and Development project (2011KW-44).
References
- Arner S, Rawal N, Gustafsson LL. Clinical experience of long-term treatment with epidural and intrathecal opioids – a nationwide survey. Acta Anaesthesiol Scand. 1988;32:253–259. doi: 10.1111/j.1399-6576.1988.tb02725.x. [DOI] [PubMed] [Google Scholar]
- Besson JM, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987;67:67–186. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- Calejesan AA, Kim SJ, Zhuo M. Descending facilitatory modulation of a behavioral nociceptive response by stimulation in the adult rat anterior cingulate cortex. Eur J Pain. 2000;4:83–96. doi: 10.1053/eujp.1999.0158. [DOI] [PubMed] [Google Scholar]
- Celerier E, Laulin J, Larcher A, Le Moal M, Simonnet G. Evidence for opiate-activated NMDA processes masking opiate analgesia in rats. Brain Res. 1999;847:18–25. doi: 10.1016/s0006-8993(99)01998-8. [DOI] [PubMed] [Google Scholar]
- Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–472. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- Chan SL, Chou CC, Wong CS, Wu CC, Tao PL. Study the effect and mechanism of dextromethorphan on preventing the development of morphine tolerance and dependence in rats. J Med Sci. 2002;22:171–176. [Google Scholar]
- Childers SR, Creese I, Snownan AM, Snyder SH. Opiate receptor binding affected differentially by opiates and opioid peptides. Eur J Pharmacol. 1979;55:11–18. doi: 10.1016/0014-2999(79)90142-0. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Zhou S, Carlton SM. Opioid receptors on peripheral sensory axons. Brain Res. 1997;764:126–132. doi: 10.1016/s0006-8993(97)00446-0. [DOI] [PubMed] [Google Scholar]
- Colpaert FC. System theory of pain and of opiate analgesia: no tolerance to opiates. Pharmacol Rev. 1996;48:355–402. [PubMed] [Google Scholar]
- Commons KG, Van Bockstaele EJ, Pfaff DW. Frequent colocalization of mu opioid and NMDA-type glutamate receptors at postsynaptic sites in periaqueductal gray neurons. J Comp Neurol. 1999;408:549–559. [PubMed] [Google Scholar]
- Dostrovsky JO. The thalamus and nociceptive processing. In: Basbaum AI, Bushnell MC, Craig AD, editors. Science of Pain. San Diego: Academic Press; 2009. pp. 635–654. [Google Scholar]
- Drummond GB. Reporting ethical matters in The Journal of Physiology: standards and advice. J Physiol. 2009;587:713–719. doi: 10.1113/jphysiol.2008.167387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eric JN, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Fields HL. Is there a facilitating component to central pain modulation? APS J. 1992;1:71–78. [Google Scholar]
- Fuchs PN, Campbell JN, Meyer RA. Secondary hyperalgesia persists in capsaicin desensitized skin. Pain. 2000;84:141–149. doi: 10.1016/s0304-3959(99)00194-3. [DOI] [PubMed] [Google Scholar]
- Gear RW, Levine JD. Nucleus accumbens facilitates nociception. Exp Neurol. 2011;229:502–506. doi: 10.1016/j.expneurol.2011.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman RR, Snyder SH, Kuhar MJ, Young WS., 3rd Differentiation of delta and mu opiate receotor localizations by light microscopic autoradiography. Proc Natl Acad Sci USA. 1980;77:6239–6243. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouardères C, Cros J, Quirion R. Autoradiographic localization of mu, delta and kappa opioid receptor binding sites in rat and guinea pig spinal cord. Neuropeptides. 1985;6:331–342. doi: 10.1016/0143-4179(85)90006-x. [DOI] [PubMed] [Google Scholar]
- Gracy KN, Pickel VM. Ultrastructural localization and comparative distribution of nitric oxide synthase and N-methyl-d-aspartate receptors in the shell of the rat nucleus accumbens. Brain Res. 1997;747:259–272. doi: 10.1016/s0006-8993(96)01249-8. [DOI] [PubMed] [Google Scholar]
- Hardy JD. Thresholds of pain and reflex contraction as related to noxious stimulation. J Appl Physiol. 1953;5:725–739. [Google Scholar]
- Heinl C, Drdla-Schutting R, Xanthos DN, Sandkühler J. Distinct mechanisms underlying pronociceptive effects of opioids. J Neurosci. 2011;31:16748–16756. doi: 10.1523/JNEUROSCI.3491-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinricher MM, Morgan MM, Fields HL. Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience. 1992;48:533–543. doi: 10.1016/0306-4522(92)90400-v. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J Neurophysiol. 2001;86:402–411. doi: 10.1152/jn.2001.86.1.402. [DOI] [PubMed] [Google Scholar]
- Jacquet YF, Lajtha A. Paradoxical effects after microinjection of morphine in the pariaqueductal grey matter in the rat. Science. 1974;185:1055–1057. doi: 10.1126/science.185.4156.1055. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kow LM, Commons KG, Ogawa S, Pfaff DW. Potentiation of the excitatory action of NMDA in ventrolateral periaqueductal gray by the μ-opioid receptor agonist, DAMGO. Brain Res. 2002;935:87–102. doi: 10.1016/s0006-8993(02)02532-5. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Mizumura K. Thin-fibre receptors responding to mechanical, chemical and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977;273:179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher A, Laulin JP, Celerier E, Le Moal M, Simonnet G. Acute tolerance associated with a single opiate administration: involvement of N-methyl-d-aspartate-dependent pain facilitatory systems. Neuroscience. 1998;84:583–589. doi: 10.1016/s0306-4522(97)00556-3. [DOI] [PubMed] [Google Scholar]
- Lei J, Jin L, Zhao Y, Sui MY, Huang L, Tan YX, Chen YK, You HJ. Sex-related differences in descending norepinephrine and serotonin controls of spinal withdrawal reflex during intramuscular saline induced muscle nociception in rats. Exp Neurol. 2011;228:206–214. doi: 10.1016/j.expneurol.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Lei J, You HJ. Endogenous descending facilitation and inhibition differ in control of formalin intramuscularly induced persistent muscle nociception. Exp Neurol. 2013;248:100–111. doi: 10.1016/j.expneurol.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Lewis ME, Pert A, Pert CB, Herkenham M. Opiate receptor localization in rat cerebral cortex. J Comp Neurol. 1983;216:339–353. doi: 10.1002/cne.902160310. [DOI] [PubMed] [Google Scholar]
- Lewis T. Experiments relating to cutaneous hyperalgesia and its spread through somatic nerves. Clin Sci. 1936;2:373–423. [Google Scholar]
- Leybin L, Pinsky C, LaBella FS, Havlicek V, Rezek M. Intraventricular Met5-enkephalin causes unexpected lowering of pain threshold and narcotic withdrawal signs in rats. Nature. 1976;264:458–459. doi: 10.1038/264458a0. [DOI] [PubMed] [Google Scholar]
- Lund I, Ge Y, Yu LC, Uvnas-Moberg K, Wang J, Yu C, Kurosawa M, Agren G, Rosén A, Lekman M, Lundeberg T. Repeated massage-like stimulation induces long-term effects on nociception: contribution of oxytocinergic mechanisms. Eur J Neurosci. 2002;16:330–338. doi: 10.1046/j.1460-9568.2002.02087.x. [DOI] [PubMed] [Google Scholar]
- Magerl W, Fuchs PN, Meyer RA, Treede RD. Roles of capsaicin-insensitive nociceptors in cutaneous pain and secondary hyperalgesia. Brain. 2001;124:1754–1764. doi: 10.1093/brain/124.9.1754. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli S, Vaughan CW, Schnell SA, Wessendorf MW, Christie MJ. Rostral ventromedial medulla neurons that project to the spinal cord express multiple opioid receptor phenotypes. J Neurosci. 2002;22:10847–10855. doi: 10.1523/JNEUROSCI.22-24-10847.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. Multiple opioid systems and pain. Pain. 1986;27:303–347. doi: 10.1016/0304-3959(86)90158-2. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Monconduit L, Bourgeais L, Bernard JF, Le Bars D, Villanueva L. Ventromedial thalamic neurons convey nociceptive signals from the whole body surface to the dorsolateral neocortex. J Neurosci. 1999;19:9063–9072. doi: 10.1523/JNEUROSCI.19-20-09063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montes C, Magnin M, Maarrawi J, Frot M, Convers P, Mauguière F, Garcia-Larrea L. Thalamic thermo-algesic transmission: ventral posterior (VP) complex versus VMpo in the light of a thalamic infarct with central pain. Pain. 2005;113:223–232. doi: 10.1016/j.pain.2004.09.044. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Alger BE, Jahr CE. Enkephalin blocks inhibitory pathways in the vertebrate CNS. Nature. 1980;287:22–25. doi: 10.1038/287022a0. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lai J, King T, Vanderah TW, Malan, Hruby VJ, Porreca F. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:126–148. doi: 10.1002/neu.20091. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th edn. San Diego: Elsevier Academic Press; 1998. [Google Scholar]
- Pertovaara A. A neuronal correlate of secondary hyperalgesia in the rat spinal dorsal horn is submodality selective and facilitated by supraspinal influence. Exp Neurol. 1998;149:193–202. doi: 10.1006/exnr.1997.6688. [DOI] [PubMed] [Google Scholar]
- Pertovaara A. Plasticity in descending pain modulatory systems. Prog Brain Res. 2000;129:231–242. doi: 10.1016/S0079-6123(00)29017-1. [DOI] [PubMed] [Google Scholar]
- Phillips RS, Cleary DR, Nalwalk JW, Arttamangkul S, Hough LB, Heinricher MM. Pain-facilitating medullary neurons contribute to opioid-induced respiratory depression. J Neurophysiol. 2012;108:2393–2404. doi: 10.1152/jn.00563.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rang HP, Dale MM, Ritter JM. Pharmacology. 1st edn. London: Churchill Livingstone; 2005. [Google Scholar]
- Saab CY, Willis WD. The cerebellum: organization, functions and its role in nociception. Brain Res Rev. 2003;42:85–95. doi: 10.1016/s0165-0173(03)00151-6. [DOI] [PubMed] [Google Scholar]
- Schepers RJ, Mahoney JL, Gehrke BJ, Shippenberg TS. Endogenous kappa-opioid receptor systems inhibit hyperalgesia associated with localized peripheral inflammation. Pain. 2008;138:423–439. doi: 10.1016/j.pain.2008.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu BC, Lin CY, Sun JJ, Chen SL, Chang C. BOLD response to direct thalamic stimulation reveals a functional connection between the medial thalamus and the anterior cingulate cortex in the rat. Magn Reson Med. 2004;52:47–55. doi: 10.1002/mrm.20111. [DOI] [PubMed] [Google Scholar]
- Stein C, Millan MJ, Shippenberg TS, Herz A. Peripheral opioid receptors mediating antinociception in inflammation. Evidence for involvement of mu, delta and kappa receptors. J Pharmacol Exp Ther. 1989;248:1269–1275. [PubMed] [Google Scholar]
- Treede RF, Meyer RA, Raja SN, Campbell JN. Peripheral and central mechanisms of cutaneous hyperalgesia. Prog Neurobiol. 1992;38:397–421. doi: 10.1016/0301-0082(92)90027-c. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci USA. 1999;96:7687–7692. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Mechanisms of opioid-induced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001;92:5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Lumb BM. Descending control of spinal nociception from the periaqueductal grey distinguishes between neurons with and without C-fibre inputs. Pain. 2008;134:32–40. doi: 10.1016/j.pain.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Wilson HD, Uhelski ML, Fuchs PN. Examining the role of the medial thalamus in modulating the affective dimension of pain. Brain Res. 2008;1229:90–99. doi: 10.1016/j.brainres.2008.06.009. [DOI] [PubMed] [Google Scholar]
- You HJ, Lei J, Niu N, Yang L, Fan XL, Tjølsen A, Li Q. Specific thalamic nuclei function as novel ‘nociceptive discriminators’ in the endogenous control of nociception in rats. Neuroscience. 2013;232:53–63. doi: 10.1016/j.neuroscience.2012.12.021. [DOI] [PubMed] [Google Scholar]
- You HJ, Lei J, Sui MY, Huang L, Tan YX, Tjølsen A, Arendt-Nielsen L. Endogenous descending modulation: spatiotemporal effect of dynamic imbalance between descending facilitation and inhibition of nociception. J Physiol. 2010;588:4177–4188. doi: 10.1113/jphysiol.2010.196923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Sengupta JN, Gebhart GF. Biphasic modulation of spinal visceral nociceptive transmission from the rostroventral medial medulla in the rat. J Neurophysiol. 2002;87:2225–2236. doi: 10.1152/jn.2002.87.5.2225. [DOI] [PubMed] [Google Scholar]


