Abstract
Innovative, culturally tailored strategies are needed to extend diabetes education and support efforts in low-resourced primary care practices serving racial/ethnic minority groups. A randomized controlled trial examined the effect of a diabetes self-care coaching intervention delivered by medical assistants and the joint effect of intervention and ethnicity over time. The randomized repeated-measures design included 270 low-income African American and Hispanic/Latino patients with type 2 diabetes. The one-year clinic- and telephone-based medical assistant coaching intervention was culturally tailored and guided by theoretical frameworks. A1C was obtained, and a self-care measure was completed at baseline, 6 months, and 12 months. Data were analyzed using mixed-effects models with and without adjustment for covariates. There was significant overall improvement in mean self-care scores across time, but no intervention effect. Results revealed differences in self-care patterns across racial/ethnic subgroups. No differences were found for A1C levels across time or group.
Keywords: Diabetes Mellitus, self-management, minority, health behavior
Approximately 25.8 million children and adults in the United States (8.3% of the population) have diabetes (Centers for Disease Control and Prevention [CDC], 2013). Direct and indirect U.S. expenditures attributed to diabetes in 2012 were estimated at $245 billion, including $176 and $69 billion in direct and indirect (disability, work loss, premature mortality) costs, respectively (American Diabetes Association [ADA], 2013a). The burden of diabetes, including prevalence and risk of complications, is greater for economically disadvantaged individuals and minority groups, especially Latinos and African Americans in the United States (Smedley, Stith, & Nelson, 2003). After adjusting for population age differences, 7.1% of non-Hispanic Whites, 11.8% of Hispanics, and 12.6% of non-Hispanic Blacks have diagnosed diabetes (CDC, 2013).
Diabetes Self-Management
Diabetes self-management is an important component of overall diabetes care. Research on self-care patterns has shown that it is complex and challenging in general (Delameter, 2006; Ruggiero et al., 1997). In addition, a recent 5-year longitudinal study found that after controlling for covariates, Hispanic and African American participants spent fewer days engaging in self-care activities compared with White participants (Trief et al., 2013). Research, including a systematic review (Peek, Cargill, & Huang, 2007), has examined the effectiveness of patient-level self-management education interventions in improving diabetes outcomes among racial/ethnic minorities receiving care at primary care clinics and support through community-based health workers and other resources. In general, self-management interventions, especially culturally tailored approaches, showed promise in improving diabetes outcomes. Continued research is needed to help understand diabetes self-care patterns in racial/ethnic minority groups to further support cultural tailoring.
There is strong evidence regarding the value of diabetes education, self-management training, and support delivered by a professional diabetes educator: that is, a health care professional (e.g., nurse, dietitian, pharmacist) who has specialized training in diabetes care (ADA, 2013b; Duncan et al., 2011; Haas et al., 2012). Some primary care clinics may have limited or no access to formal diabetes education, training, and support programs (Emerson, 2006). Therefore, in clinical settings with limited resources, alternative or supplemental models, such as peer (e.g., Lorig, Ritter, Villa, & Armas, 2009) or technology-based (e.g., Dick et al., 2011; Khan et al., 2011; Walker et al., 2011) approaches, are needed to help provide culturally tailored diabetes education, training, and support to all patients.
The American Association of Diabetes Educators (AADE) recognized the value of the use of multi-level teams, including para-professionals (e.g., certified medical assistants) and community health workers (CHWs), as important potential resources for providing diabetes education, self-management training, and support (AADE, 2011). There is growing evidence supporting the impact of CHWs in diabetes prevention and care (Norris et al., 2006; Ruggiero, Castillo, Quinn, & Hochwert, 2012). In addition, inclusion of certified medical assistants in diabetes health care teams has shown promise. (Ruggiero et al., 2010; Langford, Sawyer, Gioimo, Brownson, & O'Toole, 2007). Medical assistants are commonly available in primary care clinics and may serve as integral members of health care teams. They have the potential to expand educational efforts and outreach to underserved populations, especially in clinical practices with limited resources. Like CHWs, they may also help connect with patients through shared racial/ethnic backgrounds or communities.
Purpose
The overall purpose of this study was to develop, implement, and evaluate the effectiveness of a culturally tailored medical assistant self-care coaching (MAC) intervention in low-income racial/ethnic minority populations with type 2 diabetes. The MAC intervention is innovative in the following ways: (a) delivered by medical assistants integrated into the clinical environment and specially trained and supervised in diabetes self-care and behavioral self-management coaching; (b) guided by theoretical frameworks applying well-known behavioral and counseling strategies; and (c) tailored for both the African American and Hispanic/Latino groups. The MAC intervention was designed to extend our reach and efforts in low-resourced primary care practices to support racial/ethnic minority groups. This paper describes the joint effect of the intervention and ethnicity (Hispanic/Latino, African American) over time on self-care and A1C.
Methods
Design
The overall study was a randomized controlled trial (RCT) that included a 2 intervention group (Treatment as Usual [TAU] vs. MAC) X 3 time-point (baseline, 6 months, 12 months) repeated-measures design with three fixed factors: (a) race/ethnicity (African American, Hispanic/Latino); (b) clinic site (Clinics 1 to 4); and (c) diabetes medication regimen (non-insulin, insulin). This created homogeneous blocks of patients where patients within each block were randomly assigned with equal probability to intervention condition (TAU, MAC), thereby creating a randomized complete block design. For the current analyses, each intervention group is divided by ethnicity forming 4 groups: MAC-African American, MAC-Hispanic/Latino, TAU-African American, and TAU-Hispanic/Latino.
Setting, Sample, and Recruitment
This study was conducted at four primary care clinics that are part of a large ambulatory care network of federally qualified health centers (FQHCs) that serves predominately uninsured and Medicaid patients in Chicago and its surrounding suburbs. Specifically, participants were enrolled in this study based on the following inclusion criteria: (1) diagnosis of type 2 diabetes for at least 6 months; (2) receiving medication therapy for diabetes; (3) Hispanic/Latino or African American; (4) age equal or greater than 18 years; (5) fluent in English or Spanish; (6) recent A1C value ≥ 6.5%; and (7) able to provide informed consent. Participants were not eligible if they met any of the following criteria: (1) pregnant, or planning a pregnancy during the study period (this would necessitate significant intervention for intensive therapy); (2) serious comorbid medical (e.g., cancer; recent cardiac-related event) or mental health conditions (e.g., schizophrenia; dementia) or serious complications of diabetes (e.g., dialysis; limb amputation) that might impact participation; (3) not available by phone; and (4) currently enrolled in another diabetes-related research study or had a household member enrolled in this study.
Initial contact occurred at the clinic through referral by health care providers or other clinic staff, especially medical assistants, or through self-referral based on recruitment materials in clinics or “word of mouth.” The overall recruitment strategy in all four clinics included informational sessions for clinic staff and the display and availability of informational materials, such as brochures and posters, in the patient waiting areas. Patients who were identified as potentially eligible and expressed interest in the study met with the project research specialist (RS) to confirm eligibility. A full-time bachelor-level RS was placed at each clinic to conduct the eligibility screenings and all study self-report assessments with patients. If a patient was determined to be eligible by the RS, a detailed description of the study, including randomization, study protocol, and incentives (i.e., $20 cash for baseline assessment, and $25 cash follow-up assessments), was provided in lay language in either English or Spanish according to the patient’s preference. If the patient agreed to participate, the RS obtained the informed consent.
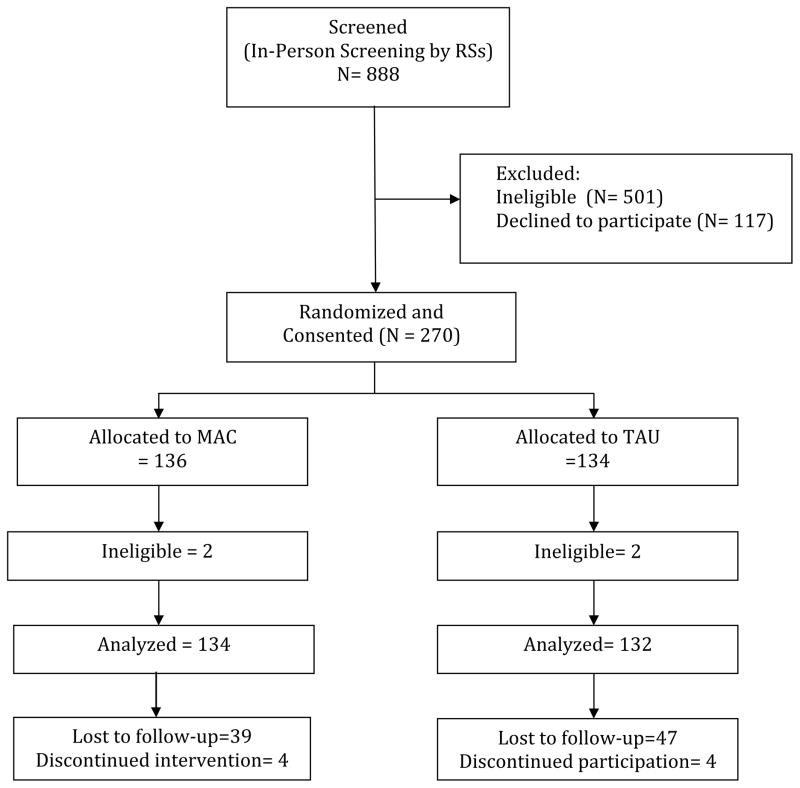
A total of 1,294 patients from 4 clinics were identified by staff as possibly eligible (See Figure 1). Of these, 888 were screened by RSs and 501 were found to be ineligible based on inclusion-exclusion criteria. Of the 387 eligible patients, 117 declined to participate and 270 were consented and randomized. Four were removed from the sample by researchers due to the presence of major comorbidities that were identified after baseline. A total of 266 are included in the final sample for data analyses on self-care. Of the total analyzed sample, 4 from each group actively withdrew from participation and 39 and 47 were lost to follow-up for the MAC and TAU groups, respectively. All recruitment and consent materials were reviewed and approved by the Institutional Review Board of the University of Illinois at Chicago.
Figure 1.
Consort Figure
Staff Training
All staff were trained by the PI and/or project manager on the study protocol relevant to their role. All staff also participated in Human Subjects, HIPAA, and cultural sensitivity training. Research specialists were responsible for the consent process, all self-report assessments, maintaining project materials and supplies at the clinic, and tracking related activities. They were trained by the Project Manager in the use of a Tablet PC for conducting the computer-based self-report assessments; appropriate assessment techniques and data collection methods; and completion of the study activities tracking protocol.
Certified medical assistants served as our Medical Assistant Coaches (MACs). In addition to the standard medical assistant training received during their education and through the health system, they received greater than 40 hours of initial project training, ongoing booster trainings as needed, and ongoing supervision by members of the multidisciplinary project team (i.e., physician, nurse CDE, psychologist, pharmacist) and/or relevant multidisciplinary experts. Trainings included: basics of diabetes self-management; behavioral counseling strategies guided by the Transtheoretical Model (Ruggiero, 2000); Empowerment Theory (Funnell, Nwankwo, Gillard, Anderson, & Tang, 2005); Motivational Interviewing (Miller & Rollnick, 2002); 5 A’s of Counseling (Goldstein, Whitlock, & DePue, 2004); and the structured coaching protocol. Training on diabetes self-management was based on the Diabetes Empowerment Education Program (DEEP; Castillo et al., 2010). A master trainer conducted the 20-hour training covering basic information on diabetes epidemiology, its risk factors, clinical characteristics, complications, medication, self-management, nutrition, physical activity, and psychosocial factors.
Intervention Conditions
Primary care-enhanced treatment as usual (TAU)
Both groups received TAU at the participating clinics, which included regular visits with a primary health care provider, referrals for specialty care such as foot and eye exams, as well as visits to the endocrinologist when necessary, and basic education provided by a physician and/or other provider. Although there was one CDE available for referral at another site within the health center network, there were no CDEs on site at any of the study clinics. To ensure that all patients in this trial had received basic diabetes education, all participants were given the “Diabetes: You’re in Control” educational booklet at the baseline assessment contact. This booklet was developed for low literacy and is available in Spanish. Additional activities related to the study for both groups included participation in repeated study assessments involving in-person contact, completion of the battery of behavioral and psychosocial measures, and a telephone prompt from the RSs to complete follow-up assessments. In addition, if the patient’s score on the depression measure (described in Measures) indicated possible depression and/or suicidal ideation (i.e., above cut-off of 10; suicidal ideation item endorsed), a form summarizing the score was given to the patient’s provider during the visit to facilitate any necessary follow-up.
MAC intervention
The MAC intervention was designed to be delivered across a 12-month period with in-person contacts at regular clinic visits and follow-up phone calls between the clinic-based contacts. The MAC intervention was tailored using the following strategies: (a) MACs were of the same ethnicity as the predominant group served in a particular clinic (i.e., African American or Hispanic/Latino); (b) written educational materials were chosen to minimize literacy barriers (e.g., written at or below 5th grade level; available in English and Spanish); (c) educational materials were culturally appropriate to either racial/ethnic subgroup; and (d) bilingual (Spanish/English) MACs were based in clinics serving Hispanic/Latino individuals. Self-care coaching was person-centered, individualized, and guided by theory (e.g., Transtheoretical Model, empowerment framework) and best practice counseling approaches (e.g., 5 A's of counseling; motivational interviewing). The focus was on helping the person learn the necessary information and skills to make informed self-care choices and changes.
Content tailoring was based on the patient’s choice from: healthy eating, physical activity, blood glucose self-monitoring, medication adherence, foot care, smoking cessation, and healthy coping. MAC interactions were protocol-driven and supported by a computer program that followed the 5 A’s of counseling framework (i.e., Assess, Agree, Advise, Assist, Arrange). This program provided specific questions to assess (e.g., self-care patterns, stage of change) for each self-care area addressed, agreed upon the agenda for the interaction based on patient preference, and offered suggestions for individualized stage-matched coaching feedback and educational resources to provide (i.e., advise, assist). The MAC also supported the patient in arranging appointments and setting personal self-care goals. Written educational materials from available resources (e.g., National Diabetes Education Program’s “4 Steps to Control Your Diabetes For Life”) were provided and, where possible, were chosen to be culturally tailored, in the appropriate language, and matched to stage of change. Two very brief videos tailored to ethnic group and available in Spanish were available to describe proper foot care and meal portion size, where appropriate.
The MAC Intervention used two delivery approaches: face-to-face coaching during diabetes-related routine clinic visits and brief monthly telephone follow-up. MAC clinic-based (face-to-face) coaching sessions were designed to be brief (< 30 minutes) and be delivered quarterly during routine diabetes care visits. After the initial clinic-based coaching session, the MACs initiated monthly follow-up calls during the one-year intervention period, except for months when in-person routine clinic visits occurred. The objectives of these calls were to: (a) provide follow-up self-care coaching; (b) answer patient questions; and (c) remind patients of medical visits and tests and help arrange necessary appointments. The telephone MAC interactions were designed to be brief (<15 minutes). If a patient missed or was not scheduled for a quarterly clinic visit, a telephone MAC session was attempted as a substitute intervention contact. When a patient needed or requested guidance or information regarding their medical care, the MAC referred the patient to the primary care provider. The MAC was considered a member of the health clinic team but dedicated to the research study and supervised by a health system physician on clinical matters and by the multidisciplinary research team (i.e., physician, nurse/CDE, psychologist) for research activities.
Managing Threats to Internal Validity
A number of strategies were used to minimize threats to internal validity in this study, including: (a) a research specialist conducted all self-report assessments; (b) the standardized questionnaires were conducted using interactive computer-delivered approaches in either English or Spanish; and (c) once randomized to condition, the research protocol for the TAU group did not include research contact with the study medical assistant. After randomization, the only other face-to-face research interactions with the TAU group were with the RS at the 6-month and 12-month clinic visits to conduct the self-report assessment. Fidelity of the MAC intervention delivered by the medical assistants was also monitored in several ways, including: (a) review of tracking and intervention reports, notes, and charts conducted by the project coordinator and PI; (b) occasional direct observation by a trained research assistant; and (c) periodic PI observation of intervention role-plays during ongoing training and supervision sessions. Deviations from the intervention protocol were discussed to correct any drift from the protocol and to ensure delivery of the intervention as intended.
Measures and Assessment Procedure
Summary of Diabetes Self-Care Activities Questionnaire (SDSCA)
Self-care outcomes were measured using the SDSCA. The SDSCA is a 13-item self-report measure of the frequency for completion of regimen activities over the past 7 days. The instrument assesses the subscales of general diet, specific diet, exercise/physical activity, blood-glucose testing, foot care, medication adherence, and smoking using the average score (range = 0–7 days). Reliability, validity, and normative data on the SDSCA from 7 different studies (N = 1,988) indicated that this measure has adequate internal and test-retest reliability, and evidence of validity and sensitivity to change (Toobert, Glasgow, & Hampson, 2000). The average inter-item correlations within scales were high (mean = 0.47), test-retest correlations were moderate (mean = 0.40), and correlations with multiple validated measures of diet and exercise supported the validity of the SDSCA subscales (mean = 0.23; Toobert et al., 2000). A Spanish-version SDSCA with previous psychometric testing is available. This measure demonstrated adequate internal consistency with a Cronbach's alpha level of 0.73 in the current sample. Consistent with previous studies, the SDSCA items capturing medication use displayed a ceiling effect (i.e., greater than 6 days/week) (Toobert et al., 2000). For this reason, medication adherence was excluded as a self-care outcome. The current paper examines the following subscales: general diet, specific diet, exercise, self-testing, and foot care.
A1C
Long-term glycemic control was examined using A1C values collected at each time point. When available, laboratory values for A1C were recorded from electronic or paper health records. When an A1C value was not available via the patient health record at the assessment time-point, it was obtained by the MAC using the DCA 2000+ Analyzer from Bayer (Mishawaka, IN).
Covariates
Covariates included information from baseline on socio-demographic characteristics (i.e., age and gender), length of diabetes duration (in years), insulin use (Yes/No), body mass index (BMI), depressive symptoms, and self-care self-confidence. Change in insulin use across time was also considered. This was a categorical variable that indicated if the participant used insulin at any point in the study after baseline (i.e., continued use or newly prescribed). Self-report measurements of height and weight were used to calculate baseline BMI.
The previously validated Patient Health Questionnaire (PHQ-9) (Kroeke, Spitzer, & Williams, 2001) was used to evaluate depressive symptomatology. The 9-item PHQ-9 is considered an effective screening tool for depression and has adequate validity and test-retest reliability reported across multiple community samples. The PHQ-9 uses a 4-point Likert scale (0 = not at all to 3 = nearly every day) to probe the extent to which individuals have been troubled with depressive symptoms within the last two weeks; possible scores range 0–27. It has been found valid in African American and Hispanic/Latino populations (Huang, 2006), is available in Spanish, and has demonstrated applicability to Hispanic/Latino patients with diabetes (Gilmer, 2008). This measure demonstrated adequate internal consistency within the current sample with a Cronbach's alpha of 0.88.
A multi-item index of self-confidence was used to assess targeted diabetes care behaviors (e.g., self-testing, exercise). This index is based on previous work of an author (Jones et al., 2003) and was also translated into Spanish and used in our previously published pilot study (Ruggiero et al., 2010). In our pilot sample, the translated version was found to be understandable, acceptable, and it demonstrated adequate reliability (Cronbach's alpha; α = 0.77) in the current sample.
Assessment Procedure
All self-report information and measures were collected at each time point by the RSs. Baseline assessment was conducted during a regularly scheduled diabetes clinic visit and prior to randomization to intervention condition. Following the consent process, the baseline assessment was conducted. The behavioral and psychosocial measures were delivered through an interactive (touchscreen) computerized assessment, including audio and Spanish translation (where desired). At baseline, RSs obtained date of birth (month/year) and gender. Length of diabetes (duration in years), baseline insulin use, and change in insulin use (continued use or newly prescribed) were based on self-report information captured using tablet PCs during scheduled assessments.
Randomization
Each participant was randomly assigned to one of the study groups (i.e., TAU or MAC) following completion of the baseline self-report assessment. Within each participating clinic, randomization to experimental condition (MAC or TAU) was stratified by gender, race/ethnicity (African American or Hispanic/Latino) and by diabetes medication type (oral and/or insulin). A participant’s race/ethnicity, gender, and medication type were entered into a computer randomization program that carried out the random assignment to condition on an equiprobability basis.
Statistical Analyses
Baseline descriptive statistics were computed by both intervention assignment and ethnicity. Analyses of variance (ANOVA) and chi-square tests were used as appropriate to perform baseline group comparisons. In order to assess the joint effect of intervention and ethnicity over time on diabetes self-care behavior, a series of 4 (Group: MAC-African American, MAC-Hispanic, TAU-African American, TAU-Hispanic) by 3 (Time-point: Baseline, 6-month, and 12-month follow-up) mixed-effects models were constructed for each self-care subscale. Model 1 was unadjusted and Model 2 was adjusted for age, gender, duration of diabetes, baseline insulin use, change in insulin use (i.e., sustained use vs. newly prescribed), recruitment site (i.e., 4 clinics), and baseline BMI, baseline outcome measure, depressive symptoms, and self-care self-confidence. A random intercept was specified in the model for each recruitment site. All covariates were treated as fixed effects. After entering Model 2 covariates, non-significant covariates were subsequently removed. Model covariance structure goodness-of-fit was assessed by the Bayesian Information Criterion (BIC; Schwarz, 1978). The BIC is a consistent model selection criterion that includes a penalty term for model complexity. The covariance structure (i.e., compound symmetric, first-order autoregressive, Toeplitz, unstructured) resulting in the lowest value of BIC was employed in the final model for the given outcome variable. A significant Group x Time interaction effect was followed by analysis of simple effects for each intervention group. Significant simple effects or main effects for time were followed by post-hoc pairwise comparisons using the Sidak adjustment. All data analyses were performed using SAS statistical software version 9.3.
Results
Baseline Characteristics and Comparisons
A total of 266 participants, including 134 randomized to the MAC condition and 132 to the TAU condition, were included in the analyses. Table 1 presents the sample characteristics. No significant intervention group differences were observed on baseline sociodemographic variables or insulin use.
Table 1.
Baseline Socio-demographic Characteristics and Insulin Use of the Study Sample by Intervention Status
| Variable | Total (N=266) | MAC (n=134) | TAU (n=132) | p |
|---|---|---|---|---|
| Age, M (SD) | 53.15 (12.36) | 53.24 (11.72) | 53.06 (13.01) | 0.90 |
| Female, n (%) | 183 (68.8) | 91 (67.9) | 92 (69.7) | 0.75 |
| Race/Ethnicity, n (%) | 0.71 | |||
| African American | 140 (52.6) | 69 (51.5) | 71 (53.8) | |
| Hispanic/Latino | 126 (47.4) | 65 (46.5) | 61 (46.2) | |
| Education, n (%) | 0.86 | |||
| < High School | 148 (59.9) | 76 (61.3) | 72 (58.6) | |
| High School/GED | 47 (19.0) | 22 (17.7) | 25 (20.3) | |
| > High School | 52 (21.1) | 26 (21.0) | 26 (21.1) | |
| Annual household income, n (%) | 0.82 | |||
| Less than $20,000 | 142 (73.6) | 75 (74.3) | 67 (72.8) | |
| More than $20,000 | 51 (26.4) | 26 (25.7) | 25 (27.2) | |
| Employment Status, n (%) | 0.95 | |||
| Currently Employed | 96 (39.8) | 49 (39.5) | 47 (40.2) | |
| Unemployed | 53 (22.0) | 27 (21.8) | 26 (22.2) | |
| Other | 92 (38.2) | 48 (38.7) | 44 (37.6) | |
| Health Insurance, n (%) | 0.71 | |||
| Yes | 144 (60.0) | 70 (58.8) | 74 (61.2) | |
| No | 96 (40.0) | 49 (41.2) | 47 (38.8) | |
| Marital Status, n (%) | ||||
| Married | 96 (38.9) | 49 (39.5) | 47 (38.2) | 0.53 |
| Never Married | 59 (23.9) | 26 (21.0) | 33 (26.8) | |
| Other | 92 (37.2) | 49 (39.5) | 43 (35.0) | |
| Others w. Diabetes in Household, n (%) | 0.36 | |||
| None | 44 (18.3) | 25 (21.0) | 19 (15.6) | |
| One | 145 (60.2) | 72 (60.5) | 73 (59.8) | |
| More than one | 52 (21.6) | 22 (18.5) | 30 (24.6) |
Data are n (%) or means (± SD). Employment status category of “other” includes homeworkers, students, and those retired and/or unable to work. Marital status category of “other” includes separated, divorced, and widowed. Participants who responded “Don’t know, not sure/ Prefer not to answer” were excluded from these analyses.
Table 2 shows baseline descriptive statistics on biopsychosocial variables by intervention group. No significant baseline intervention group differences were found for BMI, A1C, smoking, depression, confidence, and all self-care behaviors, with the exception of General Diet [F(1,248) = 3.94, p < .05], with MAC participants having significantly higher scores than their TAU counterparts (MAC: M = 3.95; TAU: M = 3.38). For all participants at baseline, mean A1C was 8.63 and mean BMI was 33.18. Mean baseline self-care varied across behaviors, with the greatest reported level for medication use and the lowest reported level for physical activity.
Table 2.
Baseline Bio-psychosocial Factors of the Study Sample by Intervention Status
| Variable | Total (N=266) | MAC (n=134) | TAU (n=132) | p |
|---|---|---|---|---|
| Baseline Biomedical Variables | ||||
| BMI, M (SD) | 33.18 (7.10) | 32.99 (6.40) | 33.38 (7.76) | 0.67 |
| Glycemic Control (A1C), M (SD) | 8.63 (2.37) | 8.72 (2.40) | 8.53 (2.34) | 0.54 |
| Diabetes Self-Care Behavior | ||||
| General Diet, M (SD) | 3.66 (2.31) | 3.95 (2.33) | 3.38 (2.26) | 0.048 |
| Specific Diet, M (SD) | 3.95 (1.65) | 4.14 (1.69) | 3.77 (1.59) | 0.08 |
| Physical Activity, M (SD) | 2.48 (2.11) | 2.65 (2.16) | 2.31 (2.05) | 0.20 |
| Glucose Testing, M (SD) | 3.61 (2.68) | 3.65 (2.73) | 3.56 (2.63) | 0.79 |
| Foot Care, M (SD) | 4.53 (2.47) | 4.66 (2.45) | 4.41 (2.49) | 0.44 |
| Medication Adherence | ||||
| Insulin | 6.45 (2.58) | 6.76 (2.35) | 6.14 (2.77) | 0.06 |
| Oral Medication/Pills | 6.30 (1.97) | 6.50 (1.73) | 6.10 (2.17) | 0.15 |
| Smoking, n (%) | 0.22 | |||
| Yes | 196 (78.4) | 102 (81.6) | 94 (75.2) | |
| No | 54 (21.6) | 23 (18.4) | 21 (24.8) | |
| Depressive Symptomatology, M (SD) | 6.04 (5.71) | 5.60 (5.68) | 6.49 (5.71) | 0.22 |
| Self-Confidence | 3.73 (0.75) | 3.75 (0.76) | 3.70 (0.74) | 0.60 |
Baseline Characteristics of the Sample by Intervention and Ethnicity
The two ethnic groups were compared within each intervention condition. African Americans were more educated than their Hispanic/Latino counterparts in both treatment conditions (p's < 0.001), and Hispanics were more likely to be married (MAC: p < 0.002; TAU: p < 0.001). In the MAC group, Hispanic/Latino participants were more likely to report one or more members in their household having diabetes (80.3%) compared to African Americans (71.4%; p < .05).
Missing Data and Intervention Contacts
Of the 266 participants, 170 (63.9%) provided responses on the SDSCA measure at baseline and 12-month follow-up. A total of 16 participants had missing data at baseline, and 80 participants had missing data at 12 months. Comparison of participants who provided data at both baseline and 12 months versus participants with missing data at 12 months revealed no significant differences with respect to age, gender, education, or baseline level of self-care behavior, insulin use, BMI, A1C, or depressive symptoms (all p’s > 0.15). Study completion rates based on self-care data for the four intervention/ethnicity groups ranged from 59.0% (MAC-Hispanic) to 79.4% (TAU-African Americans). An average of 4 MAC contacts were completed per participant across the one-year period, and the majority of the intervention contacts were clinic-based.
Comparisons of Intervention/Ethnicity Groups over Time
Table 3 presents descriptive statistics (means and standard errors) on the SDSCA scales as a function of intervention condition, ethnicity, and time. Table 4 presents the F and p values for the main and interaction effects for Models 1 and 2. Across all of the SDSCA scales, there was a significant overall effect for time (both unadjusted and adjusted models; all p's < .01). The pattern of results was similar for the unadjusted and adjusted models (Models 1 and 2, respectively). In the unadjusted model, post-hoc analyses revealed significant improvements on all SDSCA scales from baseline to six months (all p’s < .05). There were also significant post-hoc comparisons between baseline and 12 months on General Diet, Specific Diet and Foot Care outcomes (all p’s < .01). There was also a significant improvement in Foot Care from 6 to 12 months (p < .01). In the adjusted models, significant improvements were observed from baseline to six months on all SDCSA scales (p’s < .01) and from baseline to 12 months (General Diet and Specific Diet: p < .01; Physical Activity and Glucose Testing: p < .05). Only with respect to Foot Care was there a significant change from 6 to 12 months (p < .01).
Table 3.
Means (SEs) of Diabetes Self-Care and A1C by Treatment Group, Ethnicity, and Time
| MAC | TAU | |||
|---|---|---|---|---|
|
| ||||
| African American | Hispanic/Latino | African American | Hispanic/Latino | |
| A1C | ||||
| Baseline | 9.09 (1.03) | 7.76 (1.03) | 8.31 (1.03) | 8.12 (1.03) |
| 6 months | 8.95 (1.03) | 7.88 (1.03) | 8.17 (1.03) | 7.63 (1.03) |
| 12 months | 9.01 (1.04) | 7.86 (1.04) | 8.06 (1.04) | 7.69 (1.04) |
| SDSCA General Diet | ||||
| Baseline | 4.08 (0.28) | 3.80 (0.29) | 3.26 (0.27) | 3.55 (0.30) |
| 6 months | 4.50 (0.34) | 3.92 (0.35) | 4.40 (0.32) | 4.30 (0.33) |
| 12 months | 4.54 (0.37) | 4.06 (0.43) | 4.52 (0.34) | 4.45 (0.40) |
| SDSCA Specific Diet | ||||
| Baseline | 3.81 (0.20) | 4.49 (0.20) | 3.52 (0.19) | 4.07 (0.21) |
| 6 months | 4.21 (0.24) | 4.76 (0.25) | 3.89 (0.22) | 4.40 (0.23) |
| 12 months | 4.44 (0.25) | 4.59 (0.30) | 4.26 (0.24) | 4.52 (0.28) |
| SDSCA Physical Activity | ||||
| Baseline | 2.81 (0.28) | 2.43 (0.29) | 2.45 (0.27) | 2.10 (0.30) |
| 6 months | 3.43 (0.34) | 3.67 (0.35) | 3.27 (0.31) | 2.75 (0.33) |
| 12 months | 3.27 (0.35) | 2.76 (0.40) | 2.94 (0.32) | 2.49 (0.38) |
| SDSCA Blood Glucose Testing | ||||
| Baseline | 4.14 (0.33) | 3.08 (0.34) | 4.00 (0.32) | 3.09 (0.35) |
| 6 months | 4.91 (0.39) | 3.43 (0.40) | 4.60 (0.36) | 3.74 (0.38) |
| 12 months | 5.32 (0.42) | 3.45 (0.48) | 4.26 (0.39) | 3.46 (0.45) |
| SDSCA Foot Care | ||||
| Baseline | 4.78 (0.29) | 4.56 (0.29) | 4.37 (0.38) | 4.51 (0.30) |
| 6 months | 5.14 (0.35) | 5.42 (0.36) | 4.21 (0.42) | 5.28 (0.34) |
| 12 months | 5.43 (0.36) | 6.54 (0.41) | 4.93 (0.34) | 5.77 (0.39) |
Table 4.
Mixed Effects Analyses for Unadjusted and Adjusted Effects of the Intervention over Time by Ethnicity for A1C and Each Self-Care Outcome
| A1C and Self-Care Outcomes | Model 1 (unadjusted) F-Statistic, (p-value) | Model 2 (adjusted) F-Statistic, (p-value) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Trt/Ethnicity | Time Trt/Ethnic. X Time | Trt/Ethnicity | Time | Trt/Ethnic. X Time | ||
| A1C | 5.77 (0.001) | 1.19 (0.31) | 0.79 (0.58) | 1.89 (0.13) | 1.51 (0.22) | 1.67 (0.13) |
| General Diet | 0.60 (0.62) | 7.13 (<0.01) | 0.86 (0.52) | 2.58 (0.05) | 9.75 (<0.01) | 1.78 (0.10) |
| Specific Diet | 3.23 (0.02) | 6.65 (<0.01) | 0.45 (0.85) | 0.98 (0.40) | 9.32 (<0.01) | 0.37 (0.89) |
| Physical Activity | 1.44 (0.23) | 12.50 (<0.01) | 0.45 (0.84) | 0.41 (0.75) | 16.32 (<0.01) | 0.83 (0.55) |
| Glucose Testing | 5.68 (<0.01) | 5.21 (<0.01) | 0.52 (0.79) | 4.50 (<0.01) | 7.03 (<0.01) | 1.15 (0.34) |
| Foot Care | 2.75 (0.04) | 17.10 (<0.01) | 1.87 (0.09) | 7.78 (<0.01) | 23.28 (<0.01) | 2.82 (0.01) |
Unadjusted group main effects were observed on A1C (p < 0.001), Specific Diet (p < .02), Glucose Testing (p < .01), and Foot Care (p < .04). Significant adjusted group differences were observed on the Glucose Testing and Foot Care measures. In the adjusted model, Hispanics in both conditions evidenced significantly higher levels of foot care compared to their African American counterparts. In contrast, MAC-African Americans had significantly higher levels of glucose testing than Hispanics in both conditions (p’s < .01).
Finally, whereas no significant Group x Time interaction was observed in the unadjusted analyses, a significant interaction effect was found for Foot Care (p < .01) after adjusting for covariates. Post-hoc analyses revealed significant change in Foot Care behavior over time in every group except MAC-African American. Pairwise comparisons between all waves were statistically significant among MAC-Hispanics, with greatest change occurring between baseline and 12 months (t = 5.80, p < .01) and between 6 and 12 months (t = 3.36, p < .01). The TAU-African American group only evidenced a significant change from 6 to 12 months (t = 2.46, p < .05). In contrast, TAU-Hispanics evidenced significant change from baseline to 12 months (t = 3.91, p < .01). Over the course of the study, MAC-Hispanic participants evidenced the greatest improvement in Foot Care behavior, followed by TAU-Hispanics.
Discussion
The overall findings of this study demonstrate that all groups reported improvements in self-care across time, especially from baseline to six months, but no intervention effect was found and no differences were found for A1C. In addition, differences were found in the patterns of self-care changes for the two ethnic groups. In particular, Hispanic/Latino participants in both conditions evidenced both significantly higher levels and greater rate of change in foot care compared to their African American counterparts. In particular, Hispanic/Latino participants in the MAC condition reported the greatest improvement in foot care behavior at the end of the intervention (i.e., reported checking their feet nearly daily on average). In addition, African Americans in the MAC condition reported significantly higher levels of glucose testing than Hispanics in both intervention conditions.
The pre-intervention patterns across self-care behaviors are consistent with those found in other studies indicating the highest levels of reported adherence to the medication regimen and the greatest challenges in getting regular physical activity (Delameter, 2006; Ruggiero et al., 1997). In African Americans, the patterns found for self-care in this study are consistent with those found in a similar sample (Tang, Brown, Funnell, & Anderson, 2008). The pre-intervention levels of self-care from highest to lowest (excluding medication) for African American participants were: foot care, glucose self-testing, healthy eating, and physical activity and for Hispanic/Latino participants were: foot care, healthy eating, glucose self-testing, and physical activity.
Studies focused on examining innovative approaches to enhancing diabetes self-management in low-income racial/ethnic populations have had mixed results. Although some controlled studies found significant differences between intervention groups on diabetes self-care measures, other studies did not (e.g., Anderson, Christison-Lagay, Villagra, Liu, & Dziura, 2010; Frosch, Uy, Ochoa, & Mangione, 2011; Khan et al., 2011). In particular, one study (Anderson et al., 2010) with an intensive one-year telephone-based intervention delivered by nurses in an ethnically diverse population and setting similar to that of the current study did not find differences in self-care or glycemic control. The results across these studies indicate the need for more controlled studies to help identify effective culturally tailored diabetes self-management interventions for low-income racial/ethnic minority populations.
The overall goal was to examine the real world effectiveness of a culturally tailored medical assistant coaching intervention designed to improve diabetes self-care in low-income minority adults with type 2 diabetes. The limitations, challenges, and lessons learned regarding study methodology, population, and setting will be described.
This study examined self-care outcomes using one self-report measure; therefore, measurement issues could have affected the results. Although efforts were made to maximize validity (e.g., measure validated in English and Spanish) and accuracy in the assessment process (e.g., interactive computer assessment with audio in English and Spanish), this study may still have suffered from common challenges related to self-report data, such as social desirability bias. Future studies should ideally use multiple reliable and valid measurement approaches. In particular, since a ceiling effect was experienced in the measurement of medication adherence, multiple methods (e.g., pill counts, electronic monitoring, pharmacy refill data) and new measures (Morisky, Ang, Krousel-Wood, & Ward, 2008) should be considered in assessing medication use/adherence since it may directly influence A1C.
Although a variety of strategies were used to minimize threats to internal validity, challenges were encountered in delivering the research intervention protocol within the real-world clinic setting. For example, contamination across interventions was possible because blinding of MACs and clinicians was not feasible; and patient contact by MACs may have occurred in the course of their general clinic duties, but was considered consistent with “treatment as usual.” Also, clinic staff-initiated co-interventions occurred (e.g., support group started in one clinic). Setting-related barriers to delivering the planned quarterly clinic contacts were experienced, including variability in spacing/scheduling of routine diabetes visits and attrition related to an increase in the clinic fee structure.
Although the MAC intervention was designed to be intensive and the researchers monitored MAC procedures for fidelity, the majority of participants did not receive the intended dose of the intervention. Two potential reasons for the low dose included patients frequent “no showing” for scheduled routine clinic appointments and challenges in reaching participants by phone for follow-up contacts between clinic visits despite multiple call attempts. In general, participants had multiple real-world barriers (e.g., scheduling conflicts, transportation problems, frequent disconnected phones) to accessing this intensive intervention.
Many lessons were learned from this study. The results underscore the importance of identifying and understanding differences in self-care patterns between racial/ethnic groups and unique barriers and facilitators to self-care. For example, the improvement found in our study for the Hispanic/Latino group on foot care may be related to the culturally and language-tailored video on foot care shown by the MAC. Future research is needed with large and diverse samples with respect to race/ethnicity and socioeconomic status to better understand diabetes self-care patterns and correlates and help identify effective culturally tailored self-care interventions. Another study from our work (Hernandez et al., 2013) examined potential correlates of self-care at baseline and found that socio-demographic and psychosocial factors help explain the different patterns found across ethnocultural groups.
It is notable that each of the four groups evidenced improvements in self-care across time. One hypothesis that may help explain our results is the provision of a standard diabetes education booklet (i.e., enhanced TAU) and increased attention related to diabetes self-management may have been a useful intervention. Therefore, the model of extending educational opportunities in low-resourced settings with trained and supervised medical assistants deserves further consideration. A large randomized controlled study, ideally randomizing at the clinic level and blinding clinicians to intervention, could further examine the effectiveness of a MAC model in other groups/settings. If effective, examination of cost-effectiveness would be beneficial and should consider the resources needed to provide the MAC intervention, such as initial training and ongoing supervision. In addition, innovative methods are needed to minimize attrition, such as maintaining contact through social networking.
Innovative approaches are needed to overcome the barriers experienced, both at the population and setting levels. To overcome access barriers at the patient level, alternative approaches are needed to provide culturally tailored educational activities that are convenient (e.g., evenings, weekends, on-demand) and delivered where people work, play, pray, and spend the day. For example, technology-based interventions (Haas et al., 2012) and community- or peer-based approaches (Norris et al., 2002; Lorig et al., 2009) may help remove access barriers, extend the reach, and improve effectiveness of clinic-based interventions. To address other barriers experienced, an integrative comprehensive approach (e.g., Peek et al., 2012) that intervenes at multiple levels (patients, practice teams, communities, health systems) based on the chronic care model (Wagner et al., 2001) should be considered.
Acknowledgments
Research reported in this publication was supported by National Institute of Nursing Research of the National Institutes of Health under grant number R01 NR010313. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the position or policy of the Department of Veterans Affairs or the United States government. R.H. was supported by the National Institute of Health (NIH) under Award Number 5T32 HL069771-10 (M. Daviglus, PI).
The authors would like to acknowledge the support of the following individuals and groups: Roxana Barron, Mike Berbaum, Ph.D., Desiree Bond, Young-Ku Choi, Ph.D., Daisy Cintron, M.A., Nilsa Dominguez, Mickey Eder, Ph.D., Berenice Hernandez, Jairo Mejia, M.D., Sandra Franklin, Sonya Hopkins, Teresa Mantiñan, Ada Moadsiri, Dr.PH., Erica Seltzer, Dr.PH., Yashika Watkins, PhD, Steve Whitman, Ph.D., and Shikhi Bhansari, along with the entire Diabetes Self-Care research team and the Access Community Health Network Clinic Staff at study sites. The authors wish to thank Kevin Grandfield for editorial assistance.
Abbreviations
- MAC
Medical Assistant Coach or Coaching
- RCT
Randomized Clinical Trial
- TAU
Treatment as Usual
References
- American Association of Diabetes Educators. AADE guidelines for the practice of diabetes self-management education and training (DSME/T) Chicago, IL: Author; 2011. [Google Scholar]
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013a;36(4):1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2013b;36(Supplement 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DR, Christison-Lagay J, Villagra V, Liu H, Dziura J. Managing the space between visits: A randomized trial of disease management for diabetes in a community health center. Journal of General Internal Medicine. 2010;25(10):1116–1122. doi: 10.1007/s11606-010-1419-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. National diabetes fact sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: US Department of Health and Human Services; 2013. [Google Scholar]
- Castillo A, Giachello A, Bates R, Concha J, Ramirez V, Sanchez C, Arrom J. Community-based diabetes education for Latinos: The Diabetes Empowerment Education Program. Diabetes Educator. 2010;36(4):586–94. doi: 10.1177/0145721710371524. [DOI] [PubMed] [Google Scholar]
- Delameter AM. Improving patient adherence. Clinical Diabetes. 2006;24:71–77. [Google Scholar]
- Dick JJ, Nundy S, Solomon MC, Bishop KN, Chin MH, Peek ME. Feasibility and usability of a text message-based program for diabetes self-management in an urban African American population. Journal of Diabetes Science and Technology. 2011;5(5):1246–1254. doi: 10.1177/193229681100500534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan I, Ahmed T, Li QE, Stetson B, Ruggiero L, Burton K, Fitzner K. Assessing the value of the diabetes educator. The Diabetes Educator. 2011;37(5):638–57. doi: 10.1177/0145721711416256. [DOI] [PubMed] [Google Scholar]
- Emerson S. Implementing diabetes self-management education in primary care. Diabetes Spectrum. 2006;19(20):79–83. [Google Scholar]
- Frosch DL, Uy V, Ochoa S, Mangione CM. Evaluation of a behavior support intervention for patients with poorly controlled diabetes. Archives of Internal Medicine. 2011;171(22):2011–2017. doi: 10.1001/archinternmed.2011.497. [DOI] [PubMed] [Google Scholar]
- Funnell MM, Nwankwo R, Gillard ML, Anderson RM, Tang TS. Implementing an empowerment-based diabetes self-management education program. The Diabetes Educator. 2005;31(53):55–6. 61. doi: 10.1177/0145721704273166. [DOI] [PubMed] [Google Scholar]
- Gilmer TP. Improving treatment of depression among Latinos with diabetes using Project Dulce and IMPACT. Diabetes Care. 2008;31(7):1324–1326. doi: 10.2337/dc08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein MG, Whitlock EP, DePue J. Multiple behavioral risk factor. Medicine. 2004;27(Suppl 2):61–79. doi: 10.1016/j.amepre.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Haas L, Maryniuk M, Beck J, Cox CE, Duker P, Edwards L, Youssef G. National standards for diabetes self-management education and support. Diabetes Care. 2012;35:2393–2401. doi: 10.2337/dc12-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez R, Ruggiero L, Riley BB, Wang Y, Chavez N, Quinn LT, Choi YK. Correlates of self-care in low-income African American and Latino patients with diabetes. Health Psychology. 2013 doi: 10.1037/hea0000043. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang FY. Using the Patient Health Questionnaire-9 to measure depression among racially and ethnically diverse primary care patients. Journal of General Internal Medicine. 2006;21(6):547–552. doi: 10.1111/j.1525-1497.2006.00409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H, Edwards L, Vallis M, Ruggiero L, Rossi SR, Rossi JS, Zinman B. Changes in diabetes self-care behaviors make a difference in glycemic control: The Diabetes Stages of Change (DiSC) study. Diabetes Care. 2003;26:732–7. doi: 10.2337/diacare.26.3.732. [DOI] [PubMed] [Google Scholar]
- Khan MA, Shah S, Grudzien ·A, Onyejekwe ·N, Banskota ·P, Karim S, Gerber BS. A diabetes education multimedia program in the waiting room setting. Diabetes Therapy. 2011;2(3):178–188. doi: 10.1007/s13300-011-0007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeke K, Spitzer RL, Williams JBW. The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford AT, Sawyer DR, Gioimo S, Brownson CA, O'Toole ML. Patient-centered goal setting as a tool to improve diabetes self-management. The Diabetes Educator. 2007;33(Suppl 6):139S–144S. doi: 10.1177/0145721707304475. [DOI] [PubMed] [Google Scholar]
- Lorig K, Ritter PL, Villa FJ, Armas J. Community-based peer-led diabetes self-management: A randomized trial. The Diabetes Educator. 2009;35(4):641–51. doi: 10.1177/0145721709335006. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. New York, NY: Guilford Press; 2002. [Google Scholar]
- Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. Journal of Clinical Hypertension. 2008;210:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Norris SL, Chowdhury FM, Van Le K, Horsley T, Brownstein JN, Zhang X, Satterfield DW. Effectiveness of community health workers in the care of persons with diabetes. Diabetic Medicine. 2006;23(5):544–556. doi: 10.1111/j.1464-5491.2006.01845.x. [DOI] [PubMed] [Google Scholar]
- Norris SL, Nichols PJ, Caspersen CJ, Glasgow RE, Engelgau MM, Jack L Task Force on Community Preventive Services. Increasing diabetes self-management education in community settings: A systematic review. American Journal of Preventive Medicine. 2002;222:39–66. doi: 10.1016/s0749-3797(02)00424-5. [DOI] [PubMed] [Google Scholar]
- Peek ME, Cargill A, Huang ES. Diabetes health disparities: A systematic review of health care interventions. Medicine Care Research Review. 2007;64:101S–158S. doi: 10.1177/1077558707305409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peek ME, Wilkes AE, Roberson TS, Goddu AP, Nocon RS, Tang H, Chin MH. Early lessons from an initiative on Chicago's south side to reduce disparities in diabetes care and outcomes. Health Affairs. 2012;31(1):177–186. doi: 10.1377/hlthaff.2011.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero L. Helping people with diabetes change behavior: From theory to practice. Diabetes Spectrum. 2000;13(3):125–132. [Google Scholar]
- Ruggiero L, Glasgow R, Dryfoos JM, Rossi JS, Prochaska JO, Orleans CT, Johnson S. Diabetes self-management. Self-reported recommendations and patterns in a large population. Diabetes Care. 1997;20(4):568–76. doi: 10.2337/diacare.20.4.568. [DOI] [PubMed] [Google Scholar]
- Ruggiero L, Moadsiri A, Butler P, Oros SM, Berbaum ML, Whitman S, Cintron D. Supporting diabetes self-care in underserved populations: A randomized pilot study using medical assistant coaches. The Diabetes Educator. 2010;36(1):127–31. doi: 10.1177/0145721709355487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero L, Castillo A, Quinn L, Hochwert M. Translation of the diabetes prevention program’s lifestyle intervention: Role of community health workers. Current Diabetes Research. 2012;12(2):127–137. doi: 10.1007/s11892-012-0254-y. [DOI] [PubMed] [Google Scholar]
- Schwarz GE. Estimating the dimension of a model. Annals of Statistics. 1978;6(2):461–464. doi: 10.1214/aos/1176344136. [DOI] [Google Scholar]
- Smedley B, Stith A, Nelson A. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: National Academy Press; 2003. [PubMed] [Google Scholar]
- Tang TS, Brown MB, Funnell MM, Anderson RM. Social support, quality of life, and self-care behaviors among African Americans with type 2 diabetes. The Diabetes Educator. 2008;34(2):266–76. doi: 10.1177/0145721708315680. [DOI] [PubMed] [Google Scholar]
- Toobert DJ, Glasgow RE, Hampson S. The summary of diabetes self-care activities measure: Results from seven studies and a revised scale. Diabetes Care. 2000;23:943–950. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]
- Trief PM, Izquierdo R, Eimicke JP, Teresi JA, Goland R, Palmas W, Weinstock RS. Adherence to diabetes self-care for white, African American and Hispanic/Latino American telemedicine participants: 5 year results from the IDEATel project. Ethnicity & Health. 2013;18(1):83–96. doi: 10.1080/13557858.2012.700915. [DOI] [PubMed] [Google Scholar]
- Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Affairs (Millwood) 2001;20:64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- Walker EA, Shmukler C, Ullman R, Blanco E, Scollan-Koliopoulus M, Cohen HW. Results of a successful telephonic intervention to improve diabetes control in urban adults: A randomized trial. Diabetes Care. 2011;34:2–7. doi: 10.2337/dc10-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]