Abstract
Inhibitors of glycosylation provide a tool for studying the biology of glycoconjugates. One class of inhibitors consists of glycosides that block glycoconjugate synthesis by acting as primers of free oligosaccharide chains. A typical primer contains one sugar linked to a hydrophobic aglycone. In this report, we describe a way to use disaccharides as primers. Chinese hamster ovary cells readily take up glycosides containing a pentose linked to naphthol, but they take up hexosides less efficiently and disaccharides not at all. Linking phenanthrol to a hexose improves its uptake dramatically but has no effect on disaccharides. To circumvent this problem, analogs of Xyl beta 1-->6Gal beta-O-2-naphthol were tested as primers of glycosaminoglycan chains. The unmodified disaccharide did not prime, but methylated derivatives had activity in the order Xyl beta 1-->6Gal(Me)3-beta-O-2-naphthol > Xyl beta 1-->6Gal (Me)2 beta-O-2-naphthol >> Xyl beta 1-->6Gal(Me)beta-O-2-naphthol. Acetylated Xyl beta 1-->6Gal beta-O-2-naphthol also primed glycosaminoglycans efficiently, suggesting that the terminal xylose residue was exposed by removing the acetyl groups. The general utility of using acetyl groups to create disaccharide primers was shown by the priming of oligosaccharides on peracetylated Gal beta 1-->4GlcNAc beta-O-naphthalenemethanol. This disaccharide inhibited sialyl Lewis X expression on HL-60 cells.
Full text
PDF
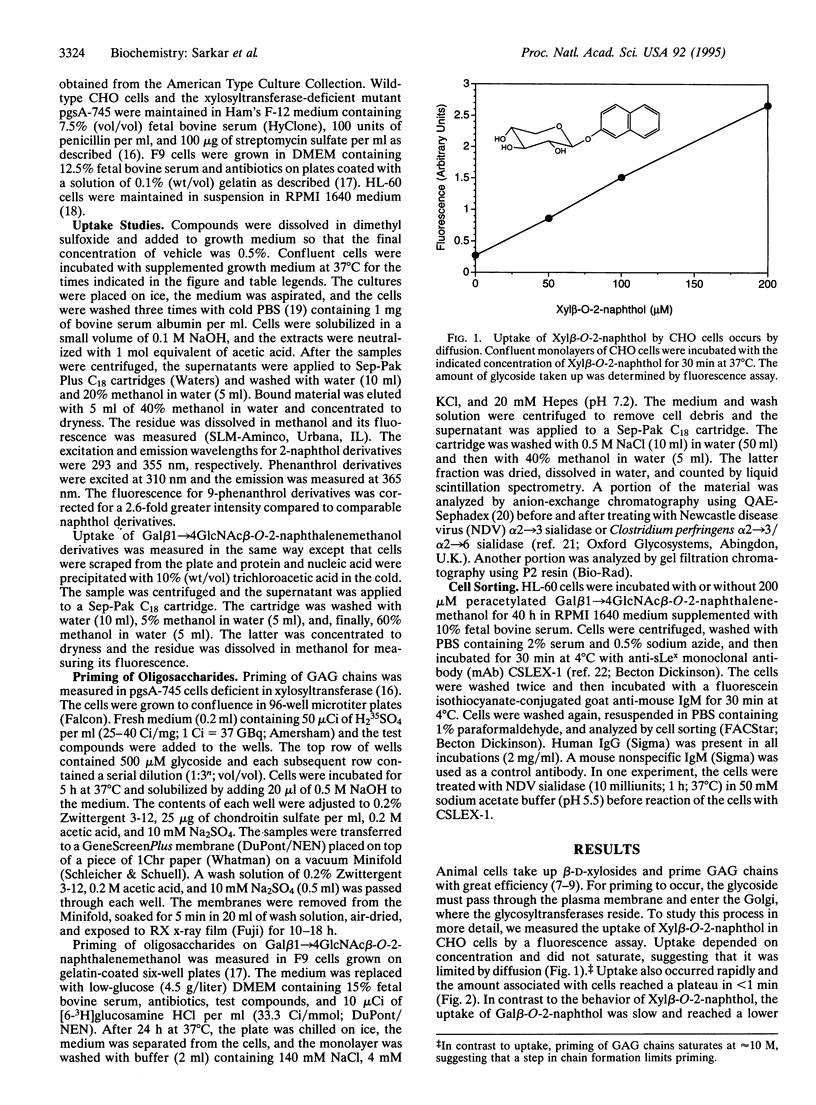
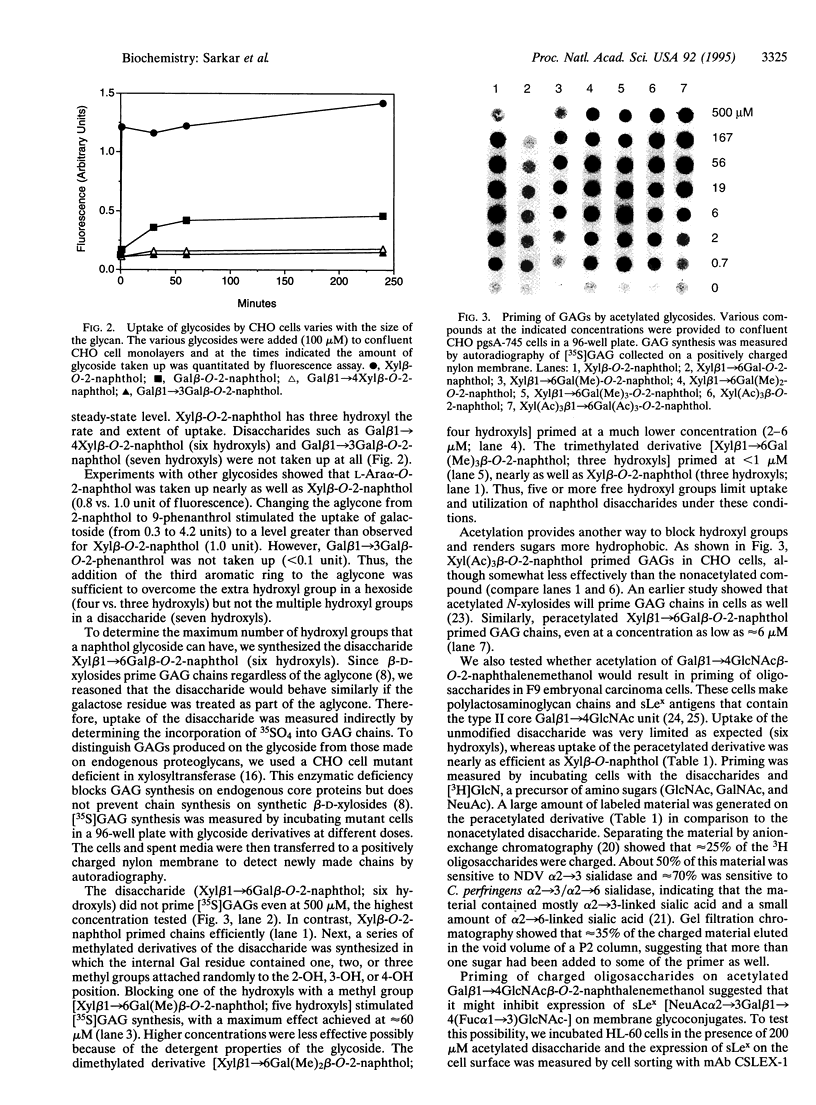
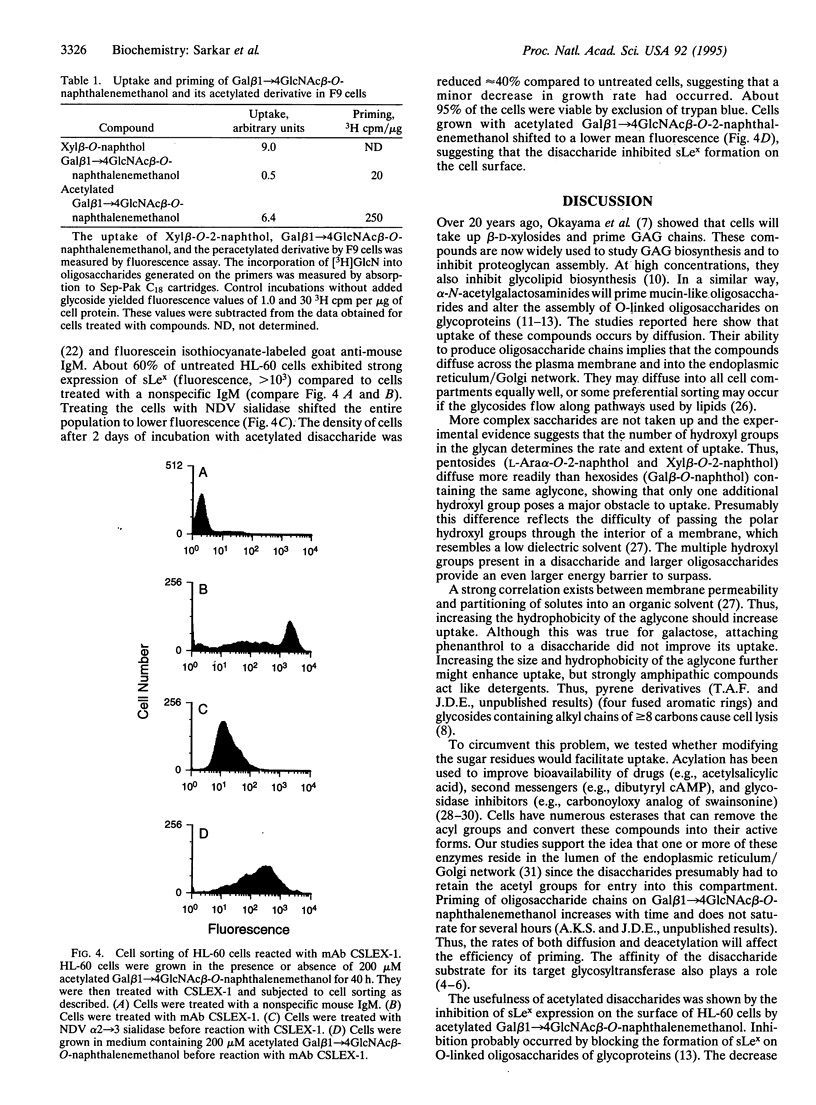

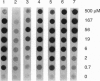
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elbein A. D. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB J. 1991 Dec;5(15):3055–3063. doi: 10.1096/fasebj.5.15.1743438. [DOI] [PubMed] [Google Scholar]
- Fritz T. A., Lugemwa F. N., Sarkar A. K., Esko J. D. Biosynthesis of heparan sulfate on beta-D-xylosides depends on aglycone structure. J Biol Chem. 1994 Jan 7;269(1):300–307. [PubMed] [Google Scholar]
- Hindsgaul O., Kaur K. J., Srivastava G., Blaszczyk-Thurin M., Crawley S. C., Heerze L. D., Palcic M. M. Evaluation of deoxygenated oligosaccharide acceptor analogs as specific inhibitors of glycosyltransferases. J Biol Chem. 1991 Sep 25;266(27):17858–17862. [PubMed] [Google Scholar]
- Khan S. H., Crawley S. C., Kanie O., Hindsgaul O. A trisaccharide acceptor analog for N-acetylglucosaminyltransferase V which binds to the enzyme but sterically precludes the transfer reaction. J Biol Chem. 1993 Feb 5;268(4):2468–2473. [PubMed] [Google Scholar]
- Lowary T. L., Hindsgaul O. Recognition of synthetic O-methyl, epimeric, and amino analogues of the acceptor alpha-L-Fuc p-(1-->2)-beta-D-Gal p-OR by the blood-group A and B gene-specified glycosyltransferases. Carbohydr Res. 1994 Jan 3;251:33–67. doi: 10.1016/0008-6215(94)84275-2. [DOI] [PubMed] [Google Scholar]
- Miao H. Q., Fritz T. A., Esko J. D., Zimmermann J., Yayon A., Vlodavsky I. Heparan sulfate primed on beta-D-xylosides restores binding of basic fibroblast growth factor. J Cell Biochem. 1995 Feb;57(2):173–184. doi: 10.1002/jcb.240570202. [DOI] [PubMed] [Google Scholar]
- Okayama M., Kimata K., Suzuki S. The influence of p-nitrophenyl beta-d-xyloside on the synthesis of proteochondroitin sulfate by slices of embryonic chick cartilage. J Biochem. 1973 Nov;74(5):1069–1073. [PubMed] [Google Scholar]
- Winchester B., Fleet G. W. Amino-sugar glycosidase inhibitors: versatile tools for glycobiologists. Glycobiology. 1992 Jun;2(3):199–210. doi: 10.1093/glycob/2.3.199. [DOI] [PubMed] [Google Scholar]
- Zacharias C., van Echten-Deckert G., Plewe M., Schmidt R. R., Sandhoff K. A truncated epoxy-glucosylceramide uncouples glycosphingolipid biosynthesis by decreasing lactosylceramide synthase activity. J Biol Chem. 1994 May 6;269(18):13313–13317. [PubMed] [Google Scholar]