Abstract
Purpose of review
To highlight the importance of immune reconstitution inflammatory syndrome (IRIS) affecting the brain in HIV-infected individuals in the absence of opportunistic infections. To describe the varied clinical manifestations, unifying pathophysiological features and discuss the principles of management of this syndrome.
Recent Findings
IRIS within the brain is commonly seen in patients with HIV infection upon initiation of antiretroviral drugs. The fulminant forms occur in the face of opportunistic infections or uncontrolled viral replication within the brain. In this case the enhanced immune response is targeted against the microbial agent, and the brain suffers bystander damage. Treatment requires the combination of the antimicrobial agent, continued antiretrovirals and in some cases corticosteroids. It is increasingly being recognized that despite adequate control of viral replication in the brain some patients develop a chronic form of T cell encephalitis which appears to be driven by continued production of HIV-Tat protein. In others the immune response may be targeted against the host antigens in the brain.
Summary
In patients with CNS-IRIS the use of corticosteroids and strategies that prevent T cell migration into the brain may be needed. Extreme caution is necessary if viral eradication strategies are to employed that involve activation of viral reservoirs, since these patients may be at risk for developing CNS-IRIS.
Keywords: Brain, antiretrovirals, HIV associated neurocognitive disorders, Tat, encephalitis
Introduction
The availability of antiretroviral therapy (ART) has dramatically changed the face of the Human Immunodeficiency Virus (HIV) pandemic with a major improvement of life expectancy. However at the same time, it was realized that some patients with HIV infection develop a paradoxical worsening of clinical status after the initiation of ART. This has been termed Immune Reconstitution Inflammatory Syndrome (IRIS) (1-3). As the viral load diminishes and the immune system recovers, patients may develop immune pathology either in the periphery or in the central nervous system (CNS). While the term IRIS has been used to describe the more severe form of inflammation, and is thereby recognizable from clinical signs, it is becoming increasingly apparent that IRIS is a spectrum disorder and may range in severity and chronicity. All patients with HIV infection managed with ART undergo acute immune dysregulation and chronic inflammation and immune dysfunction (4, 5) that may contribute to HIV associated pathologies such as stroke (6), autoimmunity (7, 8), HIV-associated neurological disorders (HAND) (9, 10), cancer (11, 12) and premature aging (13-15).
Understanding the consequences of immune reactivation in the context of HIV infection has taken up new importance since current strategies for viral eradication (16, 17) include several drugs that would activate viral reservoirs with the hope that cytotoxic immune responses would then eradicate the reservoirs (18, 19). Driving further immune activation in patients with chronic aberrant immune function may actually trigger IRIS or IRIS-like clinical conditions which may be particularly devastating given the extent of CNS HIV infection (20). Any eradication strategy should prepare for this potential complication.
Incidence of IRIS and CNS-IRIS
A meta-analysis of 17 cohorts not restricted in the type of IRIS, estimated an IRIS incidence ranging from 3-39% (21). Retrospective studies from Mexico (22) and Mozambique (23) of 390 and 136 patients respectively initiating ART indicate that the incidence of IRIS is approximately 27%. The vast majority of IRIS cases are associated with opportunistic infections (OI) either recognized prior to initiation of ART but worsening after therapy (paradoxical IRIS) or recognized after therapy initiation due to the restoration of the immune system (unmasking IRIS) (Figure 1). The overall incidence of CNS-IRIS inclusive of all patients initiating ART is estimated to be approximately 1% (24). However, similar to IRIS, CNS-IRIS often occurs within the context of an OI and therefore the incidence of CNS-IRIS is frequently assessed in populations with a particular OI. Thus the incidence of CNS-IRIS is highly variable, averaging 15-25% and ranging upwards of 40% in high-risk individuals (25-29**). A study of 110 consecutive patients with HIV and a diagnosed CNS OI demonstrated the occurrence of CNS-IRIS to be 16.4% (29**). Similarly, a study of 620 patients admitted to hospital with new or worsening neurological disease and HIV demonstrated an overall CNS-IRIS incidence of 11.8% (26); however 40% of patients with CNS tuberculosis in this cohort developed CNS-IRIS. The wide range in incidence of CNS-IRIS reflects diagnostic challenges and regional resource availability, but also may represent biological consequences related to specific pathogen associations and disease mechanisms.
Figure 1.

Paradoxical IRIS is the worsening of a recognized OI after ART initiation whereas Unmasking IRIS is the revealing of an underlying OI after the initiation of ART.
IRIS: Mortality and Risk Factors
IRIS is now recognized as a significant cause of morbidity and mortality in the HIV-managed population. IRIS is associated with an increased risk of mortality (mortality ratio of 2.3) and hospitalization (three fold risk) after adjusting for confounding factors (22). Similar trends in mortality were found in another adult (23) and a pediatric cohort (30*). While CNS-IRIS is a smaller proportion of all IRIS cases, it represents the most debilitating form of IRIS and is associated with death or permanent neurological deficit in an estimated 16-50% of cases (27, 29**, 31, 32). The CD4 nadir, an indicator of the degree of immune suppression, and the presence of an OI at the time of initiation of ART are the greatest risk factors for the development of IRIS (3, 22, 33). A reduced body mass index was also independently associated with the development of IRIS in two separate cohorts; however occurrence of CNS-IRIS was not included in the analysis (22, 23).
CNS-IRIS in association with Opportunistic Infection
A hallmark of CNS-IRIS is the infiltration of the CNS with activated T-cells. Many factors may drive the immune response during IRIS; however the majority of CNS-IRIS cases are associated with an OI. Although ART has contributed to a reduced incidence of CNS OI (29**), OI within the CNS are potent immune stimulators for the restoring immune system and the limited physical space of the brain predisposes it to damage from edema during inflammatory events. Several pathogens are associated with the development of CNS-IRIS but Mycobacterium tuberculosis (25, 34**, 35), Cryptococcus neoformans (34**, 36, 37) and JC virus (38-40) account for the majority of CNS-IRIS incidents. The clinical features, estimated rates of IRIS occurrence and risk factors associated with these infections were recently reviewed by Bahr and colleagues (34**). Other opportunistic infections, such as Varicella Zoster Virus (41-43), Cytomegolovirus (44, 45), Candida species (46) and Toxoplasma gondii (47-49) are associated with CNS-IRIS, but contribute to the frequency of disease at much reduced rates as compared to cryptococcal meningitis (CM) and tuberculosis (TB) infections. Studies on the prevention of CNS-IRIS suggest that a one-size-fits-all approach is not appropriate once a patient is immune compromised. Ideally, early detection of HIV infection through routine screening would enable initiation of therapy before CD4+ T cell counts are below 500 (50), or if resources are available, immediately after diagnosis of HIV infection. By preventing the immune system from becoming compromised some of the risk of immune dysregulation and IRIS are avoided. If HIV infection is diagnosed in patients who are already immune compromised, careful screenings for CNS-OI prior to ART initiation should be completed. Reducing the antigenic burden within the CNS may help prevent adverse neurologic inflammation. Delaying ART for five weeks to first treat CM has been shown to improve survival although there was no statistically significant difference in occurrence of CNS-IRIS (early ART = 20% IRIS, delayed ART = 13% IRIS) (51**). In contrast, it is essential to begin ART immediately if JCV infection is detected within the CNS regardless of the risk of IRIS as the immune system is the only current defense against this pathogen and corticosteroids have shown some benefit if CNS-IRIS does occur (39, 40). In a study of immediate versus delayed ART in 806 patients with TB, 61 patients developed IRIS of which four developed CNS-IRIS. Of these four patients, 75% received early ART and 25% received delayed ART (52*). In the case of tuberculosis meningitis (TBM) delaying ART reduced the most severe adverse clinical events but did not impact mortality (53). Therefore, it is currently recommended that in the case of TBM ART be delayed four weeks (34**).
CNS-IRIS and HIV encephalitis
Although not considered an OI, residual or untreated HIV replication within the CNS may lead to HIV encephalitis (HIVE) and may also contribute to the development of CNS-IRIS and chronic inflammation. IRIS is distinct from neurological complications associated with viral replication in the CNS and therapy failure, although clinically the presentation may mimic IRIS and may involve inflammation (54, 55). Elevated CNS viral loads, even in the presence of reduced viral loads in the periphery, may result not only in CNS inflammation but also in neurotoxicity and neurodegeneration from viral products (56-59). Reduction of CNS viral loads through increasing ART CNS penetration can lead to improved neurological status (9, 60*, 61) therefore it is important to assess if the CNS is not virally suppressed. Other reports suggest that ART and long-term higher CNS penetrating ART regimes may be associated with neurologic damage and increased risk of HAND (62-64*), however during frank CNS viral replication controlling the virus and thereby reducing neurotoxicity and inflammation within the CNS, is imperative.
IRIS in the absence of Opportunistic Infections
CNS-IRIS can occur in the absence of OI and these cases may be extremely informative on the mechanism of IRIS and HIV-associated immune pathologies. The clinical manifestations of CNS-IRIS without OI are highly diverse and include encephalitis (65**, 66**,67), demyelinating lesions (65**, 67, 68), and may present as headaches (69, 70), nausea (69), hearing impairment (69), weakness (70, 71), impaired speech (71), disorientation and ataxia (72**) or ischemic events (6). Although the exact forces driving the immune system are not well defined, the immune response may be directed at residual virus in the CNS, persistent release of HIV-Tat protein from HIV infected cells despite control of viral replication or to self-antigens (Figure 2). In one well studied cohort of 10 patients treated with ART that developed subacute encephalopathy, biopsy pathology demonstrated robust CD8+ lymphocyte infiltration both in the parenchyma and perivascular regions (65**). Furthermore, reactive astrocytosis and microglial activation were ubiquitous. Axonal damage and loss of myelin were observed in a subset of patients. When corticosteroids were initiated in these patients 60% showed improvement neurologically and had decreased presence of CNS lymphocytes. The other patients continued to deteriorate with 40% fatality in this cohort (65**). Within this cohort, six patients had increasing viral loads which may indicate that the immune response was targeted at HIV. However, three of the patients developed neurological symptoms after the initiation of ART without an increase in viral load; one at three months, one at nine months and one at two years post-ART-initiation, all coinciding with increasing CD4+ cell counts (65**, 66**). All of these findings are highly suggestive of IRIS. Importantly, of these patients 66% (2/3) improved with corticosteroids and 33% (1/3) died.
Figure 2.
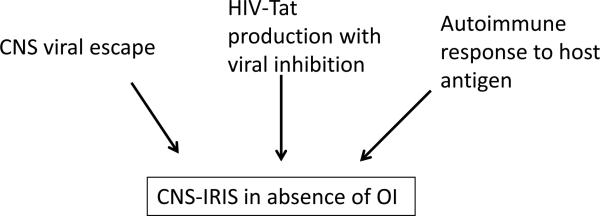
In the absence of an OI, CNS-IRIS may be driven by multiple antigenic stimuli.
Acute versus Chronic IRIS
In addition to suggesting that IRIS may mimic HIV-mediated encephalitis this cohort also supports the concept of acute and chronic IRIS (Figure 3). Two of the three patients developed immune pathology within a year of initiating ART, whereas one patient developed immune pathology two years after initiation of ART (65**, 66**). Case reports of CNS-IRIS in the absence of OI suggest that there can be varying degrees of onset and severity. A case report describes a patient with rapid immune recovery (CD4+ count 133/ mm3 to 1,251/mm3 in three months) and severe neurological impairment including deafness that improved with corticosteroid treatment (69). Biopsy showed infiltrating lymphocytes, primarily CD8+ cells, neuronal loss and fibrous astrocytosis. This patient showed substantial neurological improvement and returned to work. In this case, the presence of HIV in the CNS was not examined. In a separate report, a patient developed neurological complications 10 years after initiation of ART. Initially treated with corticosteroids the patient improved and two years later relapsed with ongoing neurological complications. Corticosteroids were again employed with slight improvement. After an additional two year interval modification to ART regime to improving CNS penetration was included as well as an additional corticosteroid intervention. Brain biopsy showed robust CD8+ lymphocytic infiltrates, some CD4+ cells and occasional IL-17+ cells. There were no HIV p24 in the CNS but robust levels of HIV Tat (72**). This patient has a permanent cognitive impairment. Similar cases have been reported. In a cohort of six patients examined for suspected IRIS, one developed neurological complications 10 years after ART initiation with CD8+ parenchymal and perivascular infiltrates and lasting sequelae whereas one patient developed neurological signs five months after ART initiation. Immune restoration was rapid and dramatic and biopsy showed CD8+ infiltrates. The patient improved with CNS penetrating ART (70). Other reports indicate IRIS may occur two years after initiation of ART, with recurrent bouts of resolving neurological complications (68) or as severe neurological manifestations three weeks after ART initiation that improved with corticosteroid use but worsened and caused fatality during corticosteroid taper (71). Together these cases, in conjunction with the previously described cohort, suggest that IRIS may develop in an acute or chronic form (summarized in Table 1). Both forms of IRIS are associated with increasing CD4+ cell counts, and therefore are a reflection of ongoing immune restoration. Immune response in these patients may be targeted against replicating virus in the brain or HIV-Tat protein if viral replication is controlled by ART.
Figure 3.
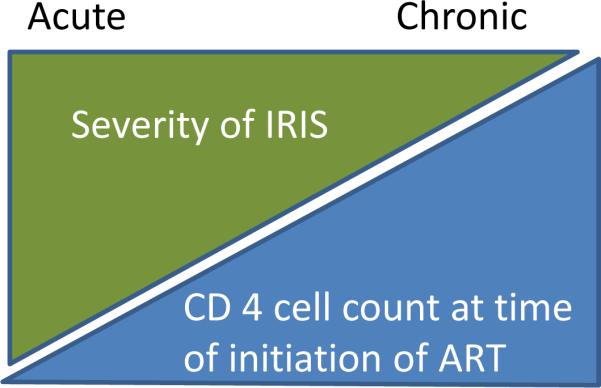
There is an inverse relationship between the severity of CNS-IRIS and the degree of immune suppression at the time when ART is initiated.
Table 1.
Comparison of acute and chronic CNS-IRIS.
| Acute CNS-IRIS | Chronic CNS-IRIS | |
|---|---|---|
| Timing | <1 year after initiation of ART | >1 year – 10 years after initiation of ART |
| Presentation | Severe clinical signs, eg. Encephalitis with seizures, altered consciousness and focal signs | Moderate to subtle clinical signs that may spontaneously remit; clinical signs may worsen over time; eg. headache, nausea, impaired hearing and vision, weakness, neurocognitive impairment |
| MRI | May have enhancing lesions or increased water signal | Generalized or subcortical atrophy |
| Prognosis | Potential for good recovery if intervention is rapid | Permanent impairment from accumulating CNS damage |
Chronic IRIS and Neurocognitive Impairment
Although ART has reduced the occurrence of HIV-associated dementia (HAD) dramatically (73), HAND persists and even asymptomatic cognitive impairment heralds the risk of further cognitive decline (74*). Interestingly, similar to IRIS, the risk of neurocognitive impairment is also tightly correlated with a lower CD4 nadir (75) suggesting that immune depletion then restoration plays an important role in the development of HAND. It is likely that viral entry into the brain occurs during the time of immune depletion and immune restoration targets the virus or viral products in the brain. Preliminary data from our laboratory suggests that nearly 30-40% of patients with undetectable HIV in the CSF still have detectable HIV-Tat protein, suggesting that it may drive the chronic IRIS and associated neurocognitive impairment. Furthermore, the chronic IRIS form is often associated with recurrent bouts of neurological manifestations that can improve under corticosteroid therapy (68, 72**). This is similar to some autoimmune disorders, such as multiple sclerosis in its relapsing-remitting form (76). The pathobiological mechanisms driving chronic IRIS may be informative for autoimmune diseases in which an underlying pathogen is suspected but not detected.
Approaches to treatment
As discussed above corticosteroids have been often used to treat CNS-IRIS. While short term use of corticosteroids seems safe, long term use has the potential for development to OI and other know sequelae such as bone mineral loss and adrenal suppression. Hence other approaches need to be considered. In patients with viral escape, i.e., who have higher viral loads in the CSF compared to the blood, antiretroviral regimen should be altered to include those that have a higher CNS penetration. In patients who develop autoimmune responses to CNS antigens, therapies that block lymphocyte trafficking into the CNS such as natalizumab may be considered. This is preferable to other drugs that target activated lymphocytes, since natalizumab leaves the peripheral immune system intact. However due to the risk of PML associated with this drug only patients known to be negative for antibodies to JC virus should be treated. Patients with detectable Tat protein in CSF in the absence of detectable HIV should ideally be treated with a Tat antagonist. However in the absence of such a compound, T cell trafficking to the brain should be controlled as mentioned above.
Conclusion
CNS-IRIS and chronic CNS inflammation in HIV infected populations is a serious complication associated with ART. The syndrome has a varied clinical presentation with acute to chronic forms and thus remains under recognized. Treatment strategies may vary depending on the underlying pathophysiological mechanism but patients should be continued on a CNS penetrating ART regimen with aggressive treatment of an underlying opportunistic infection and liberal use of corticosteroids as necessary. Acute forms of CNS-IRIS may be prevented by starting ART early in the course of infection, delaying ART in patients with OI being treated with antimicrobials and avoiding strategies that lead to activation of viral reservoirs.
Key Points.
CNS-IRIS in the absence of opportunistic infections may be much more common than originally found and may range from acute fulminant forms to chronic forms that lead to progressive T cell mediated neurodegeneration.
In the subacute or chronic forms of CNS-IRIS despite adequate control of HIV replication, there is continued released of HIV-Tat protein which is a potent activator of T cells and results in a T cell mediated encephalitis.
In patients with opportunistic infections and CNS-IRIS, antiretrovirals should be delayed by a few weeks if antimicrobials are available to treat the opportunistic infection. However in patients with PML, antiretrovirals should not be delayed even at the risk of developing IRIS.
Extreme caution is necessary in the use of therapeutic strategies that are based on reactivation of viral reservoirs, since they may result in IRIS within the brain.
Acknowledgements
Supported by intramural funds from National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Funding: Financial support for this work was provided by the National Institutes of Health.
References
- 1.French MA. Disorders of immune reconstitution in patients with HIV infection responding to antiretroviral therapy. Curr HIV/AIDS Rep. 2007 Feb;4(1):16–21. doi: 10.1007/s11904-007-0003-z. [DOI] [PubMed] [Google Scholar]
- 2.Shelburne SA, 3rd, Hamill RJ. The immune reconstitution inflammatory syndrome. AIDS Rev. 2003 Apr-Jun;5(2):67–79. [PubMed] [Google Scholar]
- 3.Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA, Giordano TP, White AC, Jr., et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005 Mar 4;19(4):399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- 4.Ndumbi P, Gillis J, Raboud J, Cooper C, Hogg RS, Montaner JS, et al. Characteristics and determinants of T-cell phenotype normalization in HIV-1-infected individuals receiving long-term antiretroviral therapy. HIV Med. 2014 Mar;15(3):153–64. doi: 10.1111/hiv.12096. [DOI] [PubMed] [Google Scholar]
- 5.Sinclair E, Ronquillo R, Lollo N, Deeks SG, Hunt P, Yiannoutsos CT, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immune Defic Syndr. 2008 Apr 15;47(5):544–52. doi: 10.1097/QAI.0b013e318162754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer EJ, Valdes-Sueiras M, Commins DL, Yong W, Carlson M. HIV stroke risk: evidence and implications. Ther Adv Chronic Dis. 2013 Mar;4(2):61–70. doi: 10.1177/2040622312471840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carugati M, Franzetti M, Torre A, Giorgi R, Genderini A, Strambio de Castilla F, et al. Systemic lupus erythematosus and HIV infection: a whimsical relationship. Reports of two cases and review of the literature. Clin Rheumatol. 2013 Sep;32(9):1399–405. doi: 10.1007/s10067-013-2271-x. [DOI] [PubMed] [Google Scholar]
- 8.Iordache L, Launay O, Bouchaud O, Jeantils V, Goujard C, Boue F, et al. Autoimmune diseases in HIV-infected patients: 52 cases and literature review. Autoimmun Rev. 2014 Apr 18; doi: 10.1016/j.autrev.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011 Feb;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao VR, Ruiz AP, Prasad VR. Viral and cellular factors underlying neuropathogenesis in HIV associated neurocognitive disorders (HAND). AIDS Res Ther. 2014;11:13. doi: 10.1186/1742-6405-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helleberg M, Gerstoft J, Afzal S, Kronborg G, Larsen CS, Pedersen C, et al. Risk of cancer among HIV-infected individuals compared to the background population: impact of smoking and HIV. AIDS. 2014 Jun 19;28(10):1499–508. doi: 10.1097/QAD.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 12.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992-2003. Ann Intern Med. 2008 May 20;148(10):728–36. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 13.Deeks SG, Phillips AN. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338:a3172. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 14.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011 Dec;53(11):1120–6. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 15.Tsoukas C. Immunosenescence and aging in HIV. Curr Opin HIV AIDS. 2014 Jul;9(4):398–404. doi: 10.1097/COH.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson M. CROI 2014: basic science review. Top Antivir Med. 2014 May;22(2):574–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor BS, Shalev N, Wilkin TJ. CROI 2014: advances in antiretroviral therapy. Top Antivir Med. 2014 May;22(2):616–31. [PMC free article] [PubMed] [Google Scholar]
- 18.Novis CL, Archin NM, Buzon MJ, Verdin E, Round JL, Lichterfeld M, et al. Reactivation of latent HIV-1 in central memory CD4(+) T cells through TLR-1/2 stimulation. Retrovirology. 2013;10:119. doi: 10.1186/1742-4690-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sgarbanti M, Battistini A. Therapeutics for HIV-1 reactivation from latency. Curr Opin Virol. 2013 Aug;3(4):394–401. doi: 10.1016/j.coviro.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Nath A, Clements JE. Eradication of HIV from the brain: reasons for pause. AIDS. 2011 Mar 13;25(5):577–80. doi: 10.1097/QAD.0b013e3283437d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010 Apr;10(4):251–61. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoyo-Ulloa I, Belaunzaran-Zamudio PF, Crabtree-Ramirez B, Galindo-Fraga A, Perez-Aguinaga ME, Sierra-Madero JG. Impact of the immune reconstitution inflammatory syndrome (IRIS) on mortality and morbidity in HIV-infected patients in Mexico. Int J Infect Dis. 2011 Jun;15(6):e408–14. doi: 10.1016/j.ijid.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Letang E, Miro JM, Nhampossa T, Ayala E, Gascon J, Menendez C, et al. Incidence and predictors of immune reconstitution inflammatory syndrome in a rural area of Mozambique. PLoS One. 2011;6(2):e16946. doi: 10.1371/journal.pone.0016946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCombe JA, Auer RN, Maingat FG, Houston S, Gill MJ, Power C. Neurologic immune reconstitution inflammatory syndrome in HIV/AIDS: outcome and epidemiology. Neurology. 2009 Mar 3;72(9):835–41. doi: 10.1212/01.wnl.0000343854.80344.69. [DOI] [PubMed] [Google Scholar]
- 25.Asselman V, Thienemann F, Pepper DJ, Boulle A, Wilkinson RJ, Meintjes G, et al. Central nervous system disorders after starting antiretroviral therapy in South Africa. AIDS. 2010 Nov 27;24(18):2871–6. doi: 10.1097/QAD.0b013e328340fe76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai L, Mahajan SD, Guo C, Zhang T, Wang W, Li T, et al. Spectrum of central nervous system disorders in hospitalized HIV/AIDS patients (2009-2011) at a major HIV/AIDS referral center in Beijing, China. J Neurol Sci. 2014 Jul 15;342(1-2):88–92. doi: 10.1016/j.jns.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Narendran G, Andrade BB, Porter BO, Chandrasekhar C, Venkatesan P, Menon PA, et al. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One. 2013;8(5):e63541. doi: 10.1371/journal.pone.0063541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepper DJ, Marais S, Maartens G, Rebe K, Morroni C, Rangaka MX, et al. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis. 2009 Jun 1;48(11):e96–107. doi: 10.1086/598988. [DOI] [PubMed] [Google Scholar]
- 29**.Riveiro-Barciela M, Falco V, Burgos J, Curran A, Van den Eynde E, Navarro J, et al. Neurological opportunistic infections and neurological immune reconstitution syndrome: impact of one decade of highly active antiretroviral treatment in a tertiary hospital. HIV Med. 2013 Jan;14(1):21–30. doi: 10.1111/j.1468-1293.2012.01033.x. [Prospective study evaluating 110 consecutive patients with CNS OI and examined clinical manifestations and rates of IRIS with associated fungal, bacterial, viral and parasitic OI.] [DOI] [PubMed] [Google Scholar]
- 30*.Gkentzi D, Tebruegge M, Tudor-Williams G, Walters S, Lyall H, Sharland M, et al. Incidence, Spectrum and Outcome of Immune Reconstitution Syndrome in HIV-Infected Children Following Initiation of Antiretroviral Therapy. Pediatr Infect Dis J. 2014 Mar 10; doi: 10.1097/INF.0000000000000331. [One of the few studies investigating IRIS in pediatric patients.] [DOI] [PubMed] [Google Scholar]
- 31.Lortholary O, Fontanet A, Memain N, Martin A, Sitbon K, Dromer F. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS. 2005 Jul 1;19(10):1043–9. doi: 10.1097/01.aids.0000174450.70874.30. [DOI] [PubMed] [Google Scholar]
- 32.Rupali P, Mannam S, Bella A, John L, Rajkumar S, Clarence P, et al. Risk factors for mortality in a south Indian population on generic antiretroviral therapy. J Assoc Physicians India. 2012 Dec;60:11–4. [PubMed] [Google Scholar]
- 33.Johnson T, Nath A. Immune reconstitution inflammatory syndrome and the central nervous system. Curr Opin Neurol. 2011 Jun;24(3):284–90. doi: 10.1097/WCO.0b013e328346be57. [DOI] [PubMed] [Google Scholar]
- 34**.Bahr N, Boulware DR, Marais S, Scriven J, Wilkinson RJ, Meintjes G. Central nervous system immune reconstitution inflammatory syndrome. Curr Infect Dis Rep. 2013 Dec;15(6):583–93. doi: 10.1007/s11908-013-0378-5. [Excellent current reviewof CNS-IRIS, associated opportunistic infections, biomarkers and treatment strategies. Highlights the differences between IRIS occuring with differing OI and common immune mechanisms.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008 Aug;8(8):516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boulware DR, Meya DB, Bergemann TL, Wiesner DL, Rhein J, Musubire A, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7(12):e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang CC, Omarjee S, Lim A, Spelman T, Gosnell BI, Carr WH, et al. Chemokine levels and chemokine receptor expression in the blood and the cerebrospinal fluid of HIV-infected patients with cryptococcal meningitis and cryptococcosis-associated immune reconstitution inflammatory syndrome. J Infect Dis. 2013 Nov 15;208(10):1604–12. doi: 10.1093/infdis/jit388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corti M, Villafane M, Trione N, Yampolsky C, Sevlever G. Progressive multifocal leukoencephalopathy presenting as IRIS in an AIDS patient. A case report and literature review. Neuroradiol J. 2013 Apr;26(2):151–4. doi: 10.1177/197140091302600203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirsch HH, Kardas P, Kranz D, Leboeuf C. The human JC polyomavirus (JCPyV): virological background and clinical implications. APMIS. 2013 Aug;121(8):685–727. doi: 10.1111/apm.12128. [DOI] [PubMed] [Google Scholar]
- 40.Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology. 2009 Apr 28;72(17):1458–64. doi: 10.1212/01.wnl.0000343510.08643.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iro MA, Kirkham FJ, Macdonald JH, Tebruegge M, Faust SN, Patel SV. Varicella zoster virus central nervous system immune reconstitution inflammatory syndrome presenting in a child. Pediatr Infect Dis J. 2013 Nov;32(11):1283–4. doi: 10.1097/INF.0b013e31829aa4fc. [DOI] [PubMed] [Google Scholar]
- 42.Newsome SD, Nath A. Varicella-zoster virus vasculopathy and central nervous system immune reconstitution inflammatory syndrome with human immunodeficiency virus infection treated with steroids. J Neurovirol. 2009 May;15(3):288–91. doi: 10.1080/13550280902913610. [DOI] [PubMed] [Google Scholar]
- 43.Teo S, Raha D, Warren D, Hassan A, Monteiro E. Central nervous system-immune reconstitution inflammatory syndrome presenting as varicella zoster virus-mediated vasculitis causing stroke. Int J STD AIDS. 2014 Jan 9; doi: 10.1177/0956462413518501. [DOI] [PubMed] [Google Scholar]
- 44.Karavellas MP, Lowder CY, Macdonald C, Avila CP, Jr., Freeman WR. Immune recovery vitritis associated with inactive cytomegalovirus retinitis: a new syndrome. Arch Ophthalmol. 1998 Feb;116(2):169–75. doi: 10.1001/archopht.116.2.169. [DOI] [PubMed] [Google Scholar]
- 45.Sugar EA, Jabs DA, Ahuja A, Thorne JE, Danis RP, Meinert CL. Incidence of cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2012 Jun;153(6):1016–24. e5. doi: 10.1016/j.ajo.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berkeley JL, Nath A, Pardo CA. Fatal immune reconstitution inflammatory syndrome with human immunodeficiency virus infection and Candida meningitis: case report and review of the literature. J Neurovirol. 2008 May;14(3):267–76. doi: 10.1080/13550280801993622. [DOI] [PubMed] [Google Scholar]
- 47.Dinardo AR, Lewis DS, Koo HL, Goodman JC, Chiao E, Andrade R. Paradoxical immune reconstitution inflammatory syndrome due to toxoplasmic encephalitis: two cases and review of initiation of antiretroviral timing in toxoplasmic encephalitis IRIS. F1000Res. 2013;2:133. doi: 10.12688/f1000research.2-133.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfeffer G, Prout A, Hooge J, Maguire J. Biopsy-proven immune reconstitution syndrome in a patient with AIDS and cerebral toxoplasmosis. Neurology. 2009 Jul 28;73(4):321–2. doi: 10.1212/WNL.0b013e3181af788e. [DOI] [PubMed] [Google Scholar]
- 49.Tsambiras PE, Larkin JA, Houston SH. Case report. Toxoplasma encephalitis after initiation of HAART. AIDS Read. 2001 Dec;11(12):608–10. 15–6. [PubMed] [Google Scholar]
- 50.Hirnschall G, Harries AD, Easterbrook PJ, Doherty MC, Ball A. The next generation of the World Health Organization's global antiretroviral guidance. J Int AIDS Soc. 2013;16:18757. doi: 10.7448/IAS.16.1.18757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014 Jun 26;370(26):2487–98. doi: 10.1056/NEJMoa1312884. [Prospective, multi-center, randomized trial which conclusively demonstrates that deferring ART improves survival in patients with cryptococcal meningitis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Luetkemeyer AF, Kendall MA, Nyirenda M, Wu X, Ive P, Benson CA, et al. Tuberculosis immune reconstitution inflammatory syndrome in A5221 STRIDE: timing, severity, and implications for HIV-TB programs. J Acquir Immune Defic Syndr. 2014 Apr 1;65(4):423–8. doi: 10.1097/QAI.0000000000000030. [Prospective, randomized study investigating the consequence of delayed versus early ART on the incidence of TB-IRIS. Found that TB IRIS occurred more often with early ART then deferred ART. While this study did not exclusively examine CNS-IRIS, there were CNS-IRIS events in this cohort.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Torok ME, Yen NT, Chau TT, Mai NT, Phu NH, Mai PP, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)--associated tuberculous meningitis. Clin Infect Dis. 2011 Jun;52(11):1374–83. doi: 10.1093/cid/cir230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hornik A, Rodriguez-Porcel F, Wallery S, Flaster M, Lee JM, Biller J. Late onset CNS immune reconstitution inflammatory syndrome in an immunocompetent patient. Front Neurol. 2013;4:12. doi: 10.3389/fneur.2013.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langford TD, Letendre SL, Marcotte TD, Ellis RJ, McCutchan JA, Grant I, et al. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS. 2002 May 3;16(7):1019–29. doi: 10.1097/00002030-200205030-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang W, Chen L, Zhang B, Park M, Toborek M. PPAR agonist-mediated protection against HIV Tat-induced cerebrovascular toxicity is enhanced in MMP-9-deficient mice. J Cereb Blood Flow Metab. 2014 Apr;34(4):646–53. doi: 10.1038/jcbfm.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, Xu C, Chen L, Xu P, Xiong H. Involvement of Kv1.3 and p38 MAPK signaling in HIV-1 glycoprotein 120-induced microglia neurotoxicity. Cell Death Dis. 2012;3:e254. doi: 10.1038/cddis.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin AH, Thayer SA. Human immunodeficiency virus-1 protein Tat induces excitotoxic loss of presynaptic terminals in hippocampal cultures. Mol Cell Neurosci. 2013 May;54:22–9. doi: 10.1016/j.mcn.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spudich SS. CROI 2014: neurologic complications of HIV infection. Top Antivir Med. 2014 May;22(2):594–601. [PMC free article] [PubMed] [Google Scholar]
- 60*.Chan P, Brew BJ. HIV Associated Neurocognitive Disorders in the Modern Antiviral Treatment Era: Prevalence, Characteristics, Biomarkers, and Effects of Treatment. Curr HIV/AIDS Rep. 2014 Jun 27; doi: 10.1007/s11904-014-0221-0. [Comprehensive review on HIV Associated Neurocognitive Disorder.] [DOI] [PubMed] [Google Scholar]
- 61.McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005 Sep;4(9):543–55. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- 62.Abers MS, Shandera WX, Kass JS. Neurological and psychiatric adverse effects of antiretroviral drugs. CNS Drugs. 2014 Feb;28(2):131–45. doi: 10.1007/s40263-013-0132-4. [DOI] [PubMed] [Google Scholar]
- 63.Akay C, Cooper M, Odeleye A, Jensen BK, White MG, Vassoler F, et al. Antiretroviral drugs induce oxidative stress and neuronal damage in the central nervous system. J Neurovirol. 2014 Feb;20(1):39–53. doi: 10.1007/s13365-013-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64*.Caniglia EC, Cain LE, Justice A, Tate J, Logan R, Sabin C, et al. Antiretroviral penetration into the CNS and incidence of AIDS-defining neurologic conditions. Neurology. 2014 Jun 6; doi: 10.1212/WNL.0000000000000564. [Large, multi-center, prospective cohort compairing neurological outcomes between patients on low and high central nervous system penetrating ART regimes. This study found highly penetrating regimes were associated with increased risk of dementia but not of OI. Did not discuss milder forms of neurocognitive impairments among the different regimes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65**.Gray F, Lescure FX, Adle-Biassette H, Polivka M, Gallien S, Pialoux G, et al. Encephalitis with infiltration by CD8+ lymphocytes in HIV patients receiving combination antiretroviral treatment. Brain Pathol. 2013 Sep;23(5):525–33. doi: 10.1111/bpa.12038. [Reports neuropathological findings of 10 patients with CD8+ T cell encephalitis and HIV. Detailed clinical findings and case histories are suggestive of IRIS occurence in three of these patients, two with an acute form of IRIS and one with a delayed form of IRIS.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66**.Lescure FX, Moulignier A, Savatovsky J, Amiel C, Carcelain G, Molina JM, et al. CD8 encephalitis in HIV-infected patients receiving cART: a treatable entity. Clin Infect Dis. 2013 Jul;57(1):101–8. doi: 10.1093/cid/cit175. [Retrospective study of HIV encephalitis patients reporting MRI, CSF and neuropathology findings including patients with and without IRIS.] [DOI] [PubMed] [Google Scholar]
- 67.Miller RF, Isaacson PG, Hall-Craggs M, Lucas S, Gray F, Scaravilli F, et al. Cerebral CD8+ lymphocytosis in HIV-1 infected patients with immune restoration induced by HAART. Acta Neuropathol. 2004 Jul;108(1):17–23. doi: 10.1007/s00401-004-0852-0. [DOI] [PubMed] [Google Scholar]
- 68.Lindzen E, Jewells V, Bouldin T, Speer D, Royal W, 3rd, Markovic-Plese S. Progressive tumefactive inflammatory central nervous system demyelinating disease in an acquired immunodeficiency syndrome patient treated with highly active antiretroviral therapy. J Neurovirol. 2008 Nov;14(6):569–73. doi: 10.1080/13550280802304753. [DOI] [PubMed] [Google Scholar]
- 69.Roh D, Glenn MD, Petito CK, Post MJ, Verma A. Reversible severe encephalitis and word deafness following rapid immune reconstitution in AIDS: a case report. J Neurovirol. 2013 Apr;19(2):190–3. doi: 10.1007/s13365-013-0161-2. [DOI] [PubMed] [Google Scholar]
- 70.Rushing EJ, Liappis A, Smirniotopoulos JD, Smith AB, Henry JM, Man YG, et al. Immune reconstitution inflammatory syndrome of the brain: case illustrations of a challenging entity. J Neuropathol Exp Neurol. 2008 Aug;67(8):819–27. doi: 10.1097/NEN.0b013e318181b4da. [DOI] [PubMed] [Google Scholar]
- 71.Zaffiri L, Verma R, Struzzieri K, Monterroso J, Batts DH, Loehrke ME. Immune reconstitution inflammatory syndrome involving the central nervous system in a patient with HIV infection: a case report and review of literature. New Microbiol. 2013 Jan;36(1):89–92. [PubMed] [Google Scholar]
- 72**.Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A. 2013 Aug 13;110(33):13588–93. doi: 10.1073/pnas.1308673110. [Describes the robust expression of HIV-Tat protein in brain and CSF in chronic CNS-IRIS despite control of viral replication.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mocroft A, Katlama C, Johnson AM, Pradier C, Antunes F, Mulcahy F, et al. AIDS across Europe, 1994-98: the EuroSIDA study. Lancet. 2000 Jul 22;356(9226):291–6. doi: 10.1016/s0140-6736(00)02504-6. [DOI] [PubMed] [Google Scholar]
- 74*.Grant I, Franklin DR, Jr., Deutsch R, Woods SP, Vaida F, Ellis RJ, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014 Jun 10;82(23):2055–62. doi: 10.1212/WNL.0000000000000492. [Prospective study demonstrating that asymptomatic neurocognitive impairment was associated with increased risk of further neurological decline despite adequate viral supression and independent of antiretroviral regime.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, et al. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011 Sep 10;25(14):1747–51. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology. 2014 May 28; doi: 10.1212/WNL.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]


