Abstract
Induction of mixed allogeneic chimerism is a promising approach for achieving donor-specific tolerance, thereby obviating the need for life-long immunosuppression for solid organ allograft acceptance. In mice receiving a low dose (3Gy) of total body irradiation, allogeneic bone marrow transplantation combined with anti-CD154 tolerizes peripheral CD4 and CD8 T cells, allowing achievement of mixed chimerism with specific tolerance to donor. With this approach, peripheral CD8 T-cell tolerance requires recipient MHC class II, CD4 T cells, B cells and DCs. Recipient-type B cells from chimeras that were tolerant to donor still promoted CD8 T-cell tolerance, but their role could not be replaced by donor-type B cells. Using recipients whose B cells or DCs specifically lack MHC class I and/or class II or lack CD80 and CD86, we demonstrate that dendritic cells (DCs) must express CD80/86 and either MHC class I or class II to promote CD8 tolerance. In contrast, B cells, though required, did not need to express MHC class I or class II or CD80/86 to promote CD8 tolerance. Moreover, recipient IDO and IL-10 were not required. Thus, antigen presentation by recipient DCs and not by B cells is critical for peripheral alloreactive CD8 T cell tolerance.
Keywords: Allograft tolerance, anti-CD154 monoclonal antibody, B cell, bone marrow transplantation, CD8, dendritic cell
Introduction
Allograft tolerance could prevent the deleterious effects of immunosuppression and chronic rejection. Mixed chimerism promotes durable allograft acceptance without maintenance immunosuppression in humans (1–3). However, host conditioning in these studies has involved depletion of preexisting T cells. T-cell recovery is slow in adults, leaving the recipient immunocompromized for a period of time. Therefore, it would be desirable to develop a conditioning regimen that avoids host T-cell depletion and graft-versus-host disease while achieving durable mixed chimerism.
A mouse model of bone marrow transplantation (BMT) with minimal conditioning involves low-dose (3Gy) total body irradiation (TBI) and costimulatory blockade with anti-CD154 mAb. This regimen overcomes peripheral CD4 and CD8 T-cell-mediated resistance to fully MHC-mismatched donor marrow engraftment, achieving durable mixed chimerism with skin graft tolerance (4). Because anti-CD154 is not appropriate for clinical use due to its thromboembolic complications (5), we aim to understand its mechanism of action in order to identify clinically useful replacements.
Prior work with this model has shown that peripheral alloreactive CD8 T-cell tolerance requires host expression of MHC class II, recipient B cells, recipient dendritic cells (DCs) and recipient CD4 T cells (6,7). Based on these observations, we hypothesize that antigen presentation to recipient CD4 T cells via the indirect pathway conditions CD4 T cells and/or antigen-presenting cells (APCs) in a manner that promotes tolerance of alloreactive CD8 T cells. We now report that DCs, and not B cells, appear to be the required APC for peripheral alloreactive CD8 T-cell tolerance.
Methods
Mice
Female C57BL/6 (B6: H-2b, CD45.2), CD45.1 congenic B6, B10.A (H-2a), CIITA−/− B6, CD80/86−/− B6, indoleamine 2,3-dioxygenase (IDO) knockout B6, IL-10 knockout B6, B-cell-deficient B6 μMT and CD11c promoter-driven diphtheria toxin receptor (DTR)-transgenic B6 mice were purchased from Frederick Cancer Research Center or Jackson Laboratory. KbDb−/− B6 mice were purchased from Taconic Farms. CIITA−/−KbDb−/− B6 mice were bred in our facility. All mice were housed in a specific pathogen-free microisolator environment.
Nonmyeloablative conditioning
Age-matched mice received 3Gy TBI from a 137Cesium irradiator on Day −1. Mice received 2 mg antimouse CD154 mAb i.p. (MR1; 8); produced at the National Cell Culture Center [NCCC], Minneapolis, MN) on Day 0 prior to i.v. injection of 15 × 106 to 25 × 106 fully MHC-mismatched B10.A BMCs. Donor BM was T-cell depleted using magnetic cell sorting (Miltenyi Biotec, Bergisch Gladbach, Germany).
Lethal irradiation and BM reconstitution
Eleven- to twelve-week-old B6 mice received 10.25Gy TBI from a 137Cesium irradiator followed 6–8 h later by i.v. injection with a mixture of 5 × 106 T-cell-depleted syngeneic BM cells from CD11c-DTR transgenic mice plus a similar inoculum from either B6, CD80/86−/−, CIITA−/−, KbDb−/− or CI-ITA−/−KbDb−/− mice. Control mice received CD11c-DTR BMCs alone. Mice that were reconstituted with syngeneic MHC class I-deficient marrow or class I- and class II-deficient marrow were also depleted of NK cells by injection of anti-NK1.1 mAb PK136 (9) (0.15 mg; i.p.) on Day −1.
In vivo cell depletion
CD8 T cells were depleted using anti-CD8 mAb (2.43; 1.44 mg/mouse; produced at NCCC) injected i.p. on Day −1. CD11c+ DCs were depleted from reconstituted CD11c-DTR transgenic mice with DT (Sigma-Aldrich Corp., St. Louis, MO) injected (i.p.; 100 ng) on Day -1 and three times per week through Day 17. Efficiency of DC depletion was 90–95% in mice reconstituted with DTR-transgenic marrow, as shown by analysis of splenocytes from a nonchimeric mouse from each group on day 15 (Figure 3B). B cells were depleted (Figure 1) using 250 μlg anti-CD20 (clone 18B12; kindly provided by Robert Dunn, Biogen-Idec) given i.v. on Days −10 and 7 relative to BMT. At 2 weeks post-BMT, approximately 3.5% of recipient peripheral blood lymphocytes were B220+ in animals receiving anti-CD20 and anti-CD154, compared to 69% in animals receiving only anti-CD154.
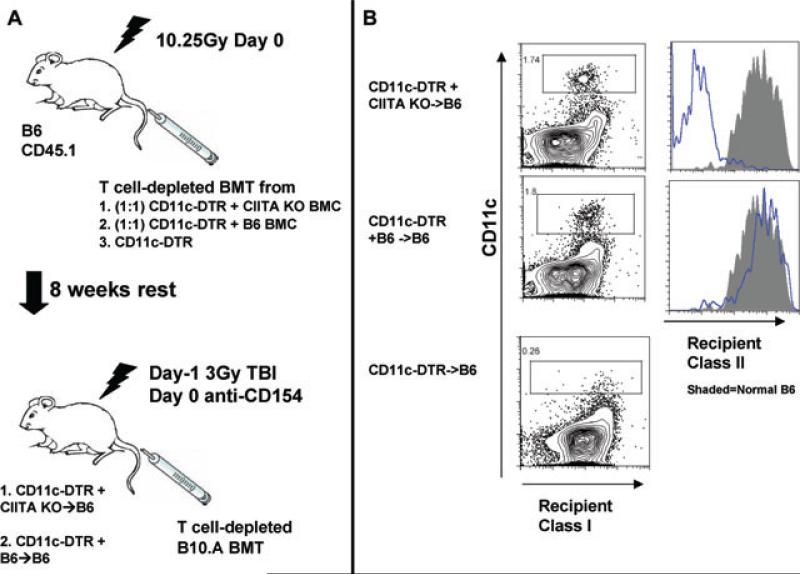
Figure 3. Generation of mice with selective absence of MHC class II expression on dendritic cells.
(A) Lethally irradiated B6 mice were reconstituted with an equal mixture of TCD BMC from CD11c-DTR transgenic (DTR) and MHC class II-deficient mice, from DTR and B6 mice or from only DTR mice. Eight weeks later, mice received T-cell-depleted B10.A BMT and DT treatment with 3Gy TBI/anti-CD154. DT treatment selectively depleted CD11c-DTR transgenic DCs, leaving only DCs that are MHC class II-deficient in CD11c-DTR + CIITA−/− mice. (B) Two weeks following conditioning (no allo-BMT), one mouse from each group was euthanized and spleens were digested with collagenase. A third DT-treated animal that had been reconstituted with DTR marrow alone (DTR→B6) is shown in the bottom panel. Histograms show MHC class II-expression for gated CD11c+ DCs of treated (CIITA−/− + DTR → B6) (B6 + DTR B6) chimeras. The shaded histograms data naive B6 mouse.
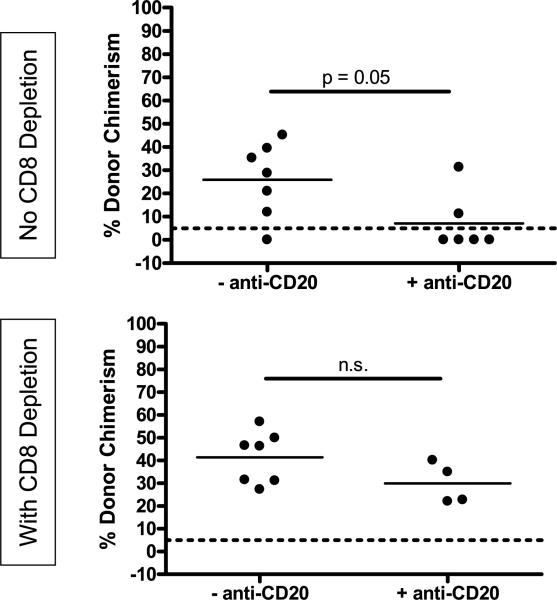
Figure 1. Depletion of recipient B cells confirms their requirement for peripheral CD8 T-cell tolerance.
WT B6 animals received B10.A BMC and anti-CD154 on Day 0 following 3Gy TBI on Day –1 and anti-CD20 and anti-CD8 where indicated. Percent donor chimerism in the Mac-1+ lineage of the peripheral blood at 2 weeks post-BMT is depicted. The dotted line at 5% donor chimerism depicts the cutoff for referring to an animal as a chimera.
Flow cytometric analysis of multilineage chimerism among WBCs
Chimerism was assessed in peripheral blood by three-color flow cyto-metric analysis using FITC-conjugated anti-Dd mAb 34-2-12 with PE- or allophycocyanin-conjugated anti-CD4, anti-CD8, anti-B220 (BD Biosciences, San Jose, CA, San Diego, CA) and anti-Mac1 (Caltag/Invitrogen, Carlsbad, CA) mAbs. HOPC1-FITC (prepared in our laboratory) and rat antimouse IgG2a-PE or allophycocyanin (BD Pharmingen) mAbs were used as negative controls. Propidium iodide staining was used to exclude dead cells. A lineage was defined as chimeric when ≥5% cells were donor MHC class I positive. Comparison of WBC chimerism at 16 weeks and spleen, lymph node and bone marrow chimerism at 18 weeks in seven long-term multilineage chimeras and five failed chimeras (prepared in μMT mice with and without B-cell transfer) showed a close correlation between WBC chimerism and chimerism of the same lineages in the lymphoid tissues and marrow (data not shown). Four of four mice lacking peripheral blood myeloid chimerism had undetectable myeloid chimerism in the marrow; five of five animals lacking peripheral blood T-cell chimerism had undetectable T-cell chimerism in the lymph nodes or spleen. Thus, chimerism in the peripheral blood is indicative of an overall chimerism.
B-cell infusions
Splenic B cells from 8–12-week-old WT B6, KbDb−/−, CIITA−/−, CIITA−/−KbDb−/− or CD80/86−/− mice were purified using magnetic cell sorting with a nontouch B-cell isolation kit (Miltenyi Biotec). The 15 × 106 to 25 × 106 B cells (purity >97%) were injected i.v. into B-cell-deficient (μlMT) mice on Day 0. For the experiment shown in Figure 7A, B6 mice were lethally irradiated with 10.25 Gy and reconstituted with either T- and B-cell-depleted (Miltenyi MACS system) B6 marrow or with a combination of T-and B-cell-depleted B6 plus B10.A marrow. Four weeks later, splenic B cells (depleted of B10.A B cells by adding anti-Dd mAb 34.2.12 to the nontouch B-cell-depletion cocktail) from these mice were adoptively transferred into μMT recipient mice receiving B10.A BMT with the anti-CD154/3 Gy TBI protocol. In Figure 7B, 43 × 106 purified B10.A B cells and 25 × 106 purified WT B6 B cells were transferred.
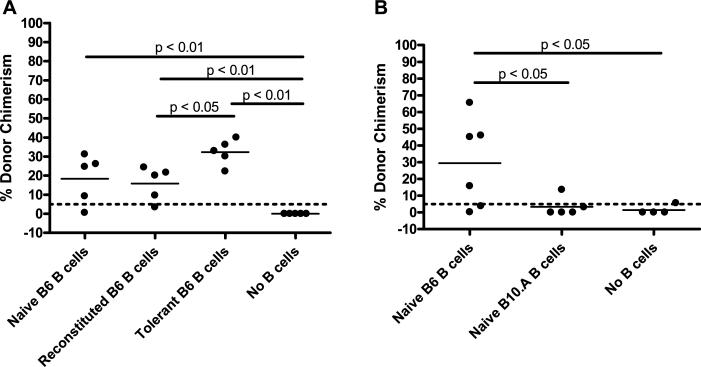
Figure 7. Transfer of recipient-type B cells tolerized to donor BM, but not donor B cells, rescues chimerism development in B-cell-deficient mice.
B-cell-deficient (μlMT) mice received B10.A BMC and anti-CD154 on Day 0 following 3Gy TBI on Day –1. The 15 × 106 to 25 × 106 recipient-type B cells were adoptively transferred on Day 0. Tolerant B6 B cells were taken from B6 + B10.A → B6 mixed chimeras, whereas reconstituted B6 B cells were taken from B6 → B6 controls (A). The dose of adoptively transferred donor-type B10.A B cells was increased to 43 × 106, whereas 25 ×6 recipient-type B6 B cells were transferred (B). Mean percentage of donor Mac-1+ WBC chimerism in the peripheral blood at 4 weeks post-BMT is indicated by the solid line. The dotted line at 5% donor chimerism depicts our cutoff for referring to an animal as a chimera.
Analysis of CD80/86 expression
B6 animals were given 3 Gy TBI on Day −1 and T-cell-depleted B10.A BM cells i.v. on Day 0 with or without anti-CD154 i.p. On Day 4 post-BMT, animals were sacrificed and their spleens digested with collagenase. Cells were stained with FITC-conjugated anti-Dd mAb 34-2-12, PE-conjugated anti-CD80 or anti-CD86 and APC-conjugated anti-CD19 or anti-CD11c and analyzed on a FACS Calibur.
Statistical analysis
The Mann–Whitney test was used to compare levels of chimerism between groups. For Figures 2 and 4, the ‘WT cells’ group and the ‘No cells’ group were each compared with all other groups. p-Values are indicated only when p < 0.05. n.s. where n.s. not significant (p > 0.05).
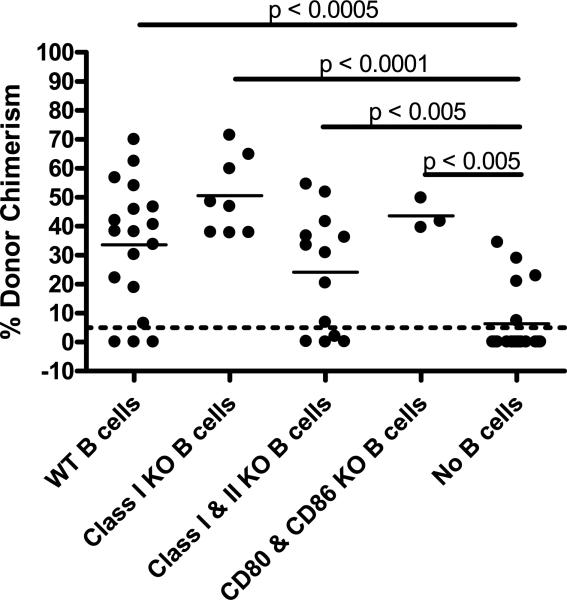
Figure 2. B-cell expression of MHC class I, MHC class II or CD80/86 is not required to promote CD8 T-cell tolerance.
B-cell-deficient (μMT) mice received B10.A BMC and anti-CD154 on Day 0 following 3Gy TBI on Day –1. As indicated, 15 × 106 to 25 × 106 WT or mutant recipient-type B cells were adoptively transferred on Day 0. Mean percentage of donor Mac-1+ WBC chimerism in the peripheral blood at 4 weeks post-BMT is indicated by the solid lines. For groups with WT, Class I KO, Class I and II KO, CD80 and CD86 KO and No B cells, combined experiments yielded sample sizes of n = 18, 8, 13, 3 and 18, respectively. The dotted line at 5% donor chimerism depicts the level at which our cutoff for calling an animal is referred to as a chimera.
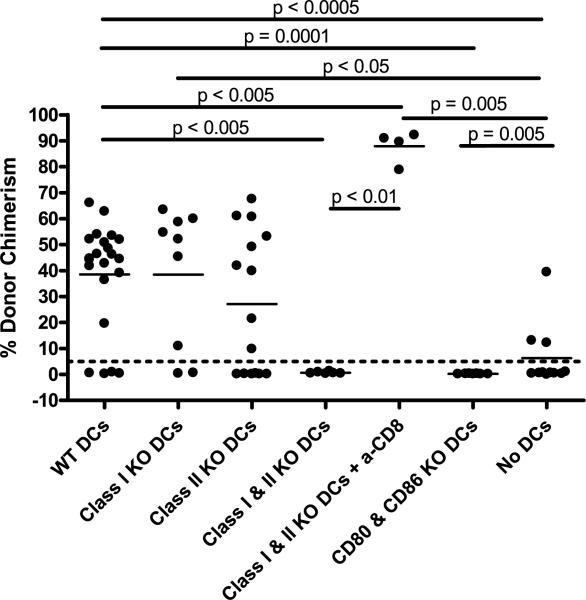
Figure 4. Expression of either MHC class I or II, as well as CD80/86, is required on host dendritic cells to promote CD8 T-cell tolerance.
Radiation chimeras prepared as described in Figure 2 received conditioning with Day-1 3Gy TBI and Day 0 anti-CD154 and B10.A BMT in addition to DT treatment. Some animals in each experimental group received anti-CD8 mAb to deplete host CD8 T cells. The percentage of donor chimerism in the Mac-1+ lineage of the peripheral blood at 4 weeks post-BMT for all mice in four separate experiments is shown. Recipient groups included: WT DC (B6 + DTR → B6) n = 22, MHC class I KO DC (KbDb−/− + DTR → B6) n = 9, MHC class II KO DC (CIITA−/− + DTR → B6) n = 15, MHC class I & II KO DC (KbDb−/−CIITA−/− + DTR → B6) n = 6, MHC class I and II KO DC + anti-CD8 n = 4, CD80/86 KO DC (CD80/86−/− + DTR → B6) n = 7 and No DC (DTR → B6) n = 11. The dotted line at 5% donor chimerism depicts our cutoff for referring to an animal as a chimera.
Results
Though required for CD8+ T-cell tolerance, recipient B cells need not express either MHC or costimulatory CD80/86 molecules
Previous studies have shown that μMT animals, which lack B cells due to deletion of the transmembrane domain of the μ heavy chain (10), reject allogeneic BM when conditioned with 3Gy TBI/anti-CD154 unless recipient CD8 T cells are depleted (6). However, this defect in CD8 T-cell tolerance is reversed by adoptive transfer of wild-type (WT), recipient-type B cells at the time of BMT. MHC class II-deficient CIITA−/− (class II transactivator−/−) B cells of recipient origin also promote CD8 T-cell tolerance in B-cell-deficient μMT mice conditioned with anti-CD154 and 3Gy TBI (6), indicating that MHC class II need not be expressed on the B cells that promote CD8 T-cell tolerance.
To confirm that these results reflected a true requirement for recipient B cells for CD8 T-cell tolerance induction rather than an abnormality in the immune environment of μMT mice, we evaluated the effect of depleting B cells with anti-CD20 mAb on the achievement of chimerism in WT mice that did or did not receive depleting anti-CD8 mAb. As expected, most animals depleted of recipient B cells rejected the donor BM graft unless they were also CD8 depleted (Figure 1). These data confirm the requirement for B cells for the tolerization of CD8 cells but not for that of CD4 cells in this model.
To further investigate the role for recipient B cells in promoting CD8 T-cell tolerance, we evaluated the requirement for MHC class I. Other studies in our laboratory have suggested the existence of an indirectly alloreactive CD8 T-cell-mediated pathway of marrow rejection (Fehr et al., unpublished data), so it was conceivable that B cells are required to present antigen to and tolerize indirectly allore-active CD8 cells. We therefore adoptively transferred B cells from MHC class I heavy chain-deficient (KbDb−/−) B6 mice (11) into B6 μMT recipients conditioned with 3Gy TBI on Day −1 and anti-CD154 before receiving T-cell-depleted B10.A BMC. Other groups (positive and negative controls, respectively) received B cells isolated from WT B6 splenocytes or no adoptive transfer. Mac-1+ white blood cell (WBC) chimerism in peripheral blood is generally representative of all lineages in this model and is the best lineage for evaluation in these experiments because chimeric recipients genetically deficient in B cells show 100% donor chimerism in the B-cell lineage and T-cell chimerism does not appear until much later (6). Thus, for simplicity, peripheral blood Mac-1+ WBC chimerism is presented here. At 4 weeks post-BMT, there was no significant difference in the level of donor chimerism between the groups that received WT or MHC class I-deficient B cells (Figure 2). In contrast, there was a statistically significant increase in the level of donor chimerism in groups receiving WT or MHC class I-deficient B cells versus no B cells. Thus, B cells deficient in MHC class I are able to promote CD8 T-cell tolerance in recipients of allogeneic BMT with anti-CD154/3Gy TBI.
The lack of requirement for MHC class I or class II on recipient B cells prompted evaluation of adoptive transfer of B cells lacking both MHC class I and class II, generated by crossing B6 KbDb−/− double-deficient mice with B6 CIITA−/− (12). We further addressed the role of antigen presentation on B cells by including a group that received B cells deficient in the costimulatory molecules CD80 and CD86. After conditioning with 3Gy TBI and anti-CD154, B6 μMT mice received T- cell-depleted B10.A BMC and recipient-type B cells from WT, CIITA−/−KbDb−−/−, or CD80/86−/− mice. At 4 weeks post-BMT, there was no difference in the level of donor chimerism between recipients of WT versus MHC class I- and class II-deficient B cells. There was also no difference between WT and CD80/86 KO B cells (Figure 2). However, there was a statistically significant difference in the level of donor chimerism between the group with no B cells and the groups receiving either MHC class I/II KO B cells or CD80/86 KO B cells. In summary, recipient B cells lacking classical MHC molecules as well as those lacking the costimulatory CD80/86 molecules are able to promote CD8 T-cell tolerance, suggesting that the B-cell contribution to CD8 T-cell tolerance does not involve antigen presentation.
Requirement for expression of antigen presentation molecules on recipient DCs for CD8 T-cell tolerance
Based on our prior observation that both recipient DCs and recipient MHC class II are required for CD8 T-cell tolerance (6), we asked whether the recipient DCs are needed to express MHC class II to promote CD8 T-cell tolerance. To address this question, we used CD11c-DTR-transgenic mice, which express the primate diphtheria toxin receptor (DTR) as a transgene under control of the murine CD11c promoter. We lethally irradiated B6 mice and reconstituted them with a 1:1 mixture of BMC from CD11c-DTR and CIITA−/− mice. These reconstituted mice lack all MHC class II+ DCs after diphtheria toxin (DT) treatment but have WT cells of all other lineages. One control group received a mixture of CD11c-DTR and B6 BMC (WT DC control) and another control group received only CD11c-DTR BM (no DC control) (Figure 3). After 8 weeks of rest, these mice were conditioned as earlier with 3Gy TBI and anti-CD154 and treated with DT. Appropriate DC depletion was confirmed by sacrificing one control animal in each conditioned group (no allo-BMT), digesting the spleen with collagenase and examining MHC class II expression on CD11chigh DCs by flow cytometry (Figure 3B). At 4 weeks post-BMT, there was no difference in the level of donor chimerism in mice lacking expression of MHC class II on recipient DC compared to the WT controls (Figure 4). However, due to the high variability in chimerism levels in animals with MHC class II-deficient DCs, a significant difference in the level of donor chimerism was not demonstrated between mice with MHC class II KO DCs versus mice lacking DCs. A similar experiment was performed to investigate the requirement for MHC class I expression on recipient DC. There was no difference in the level of chimerism between recipients with KbDb−/− DCs versus those with WT DC. There was, however, a significant difference between animals with no DCs and those with MHC class I KO DCs (Figure 4). Taken together, these data indicate that DCs deficient in either MHC class I or class II are still able to promote CD8 T-cell tolerance, although MHC class II-deficient DCs may be less effective.
Next, we asked whether DCs deficient in both MHC class I and class II and whether DCs deficient in CD80/86 could still promote CD8 T-cell tolerance. Using the same system described earlier, we found that CIITA−/−KbDb−/− DCs failed to promote chimerism unless CD8 T cells were depleted (Figure 4). Moreover, none of the mice with CD80/86-deficient DCs accepted the donor marrow (Figure 4). Together, these data indicate that expression of either MHC class I or class II, as well as expression of costimulatory CD80/86, by recipient DCs is necessary to promote CD8 T-cell tolerance. Thus, while both B cells and DCs are independently required for CD8 T-cell tolerance, only the DCs appear to be acting in an antigen-presenting capacity.
Preservation of CD80/CD86 expression on recipient DCs of tolerant compared to rejecting mice
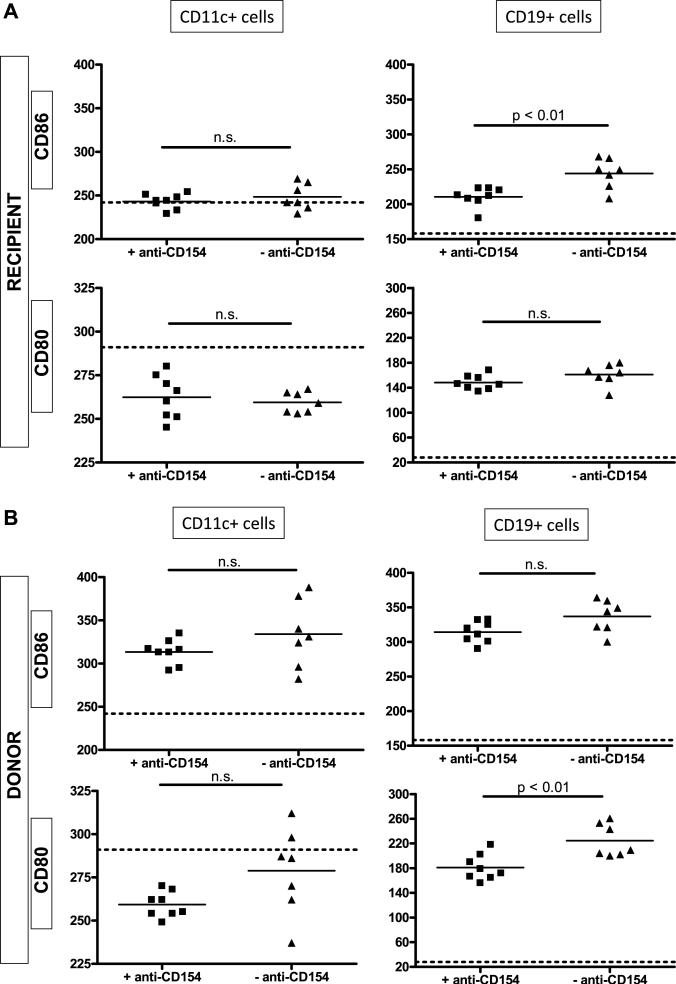
Blockade of CD40–CD154 interactions inhibits the upregulation of CD80 and CD86 on APCs (13). We compared the expression of these molecules on donor and recipient B cells and DCs in 3Gy-irradiated B6 recipients of B10.A BMT with or without anti-CD154. As shown in Figure 5, BMT under nontolerizing conditions (i.e. without anti-CD154) was associated with upregulation of CD80 and CD86 on donor (Figure 5B) and recipient (Figure 5A) B cells and with upregulation of CD86 on donor DCs. The upregulation of CD86 on recipient B cells and of CD80 on donor B cells was significantly reduced and expression of both molecules trended downward on donor DCs in recipients of BMT with anti-CD154 (tolerizing conditions) compared to those without anti-CD154. In contrast to the cell types mentioned earlier, there was no reduction in the levels of either costimulatory molecule on recipient DCs from animals given allogeneic BMT under tolerizing compared to nontolerizing conditions. The relative preservation of these molecules on recipient DCs in tolerant compared to rejecting mice is consistent with the antigen-presenting role of recipient DCs in tolerance induction.
Figure 5. Preservation of CD80/ CD86 expression on recipient DCs of tolerant compared to rejecting mice.
B6 animals were given 3Gy TBI on Day –1 followed by B10.A BM with or without anti-CD154 on Day 0. On Day 4 post-BMT, the animals were sacrificed and their splenocytes analyzed for expression of CD80 and CD86 on B cells and DCs of recipient and donor origin (MFI, median fluorescence intensity). Each symbol represents an individual animal. Dashed lines indicate median fluorescence intensity for an untreated 2C.B6 synchimera.
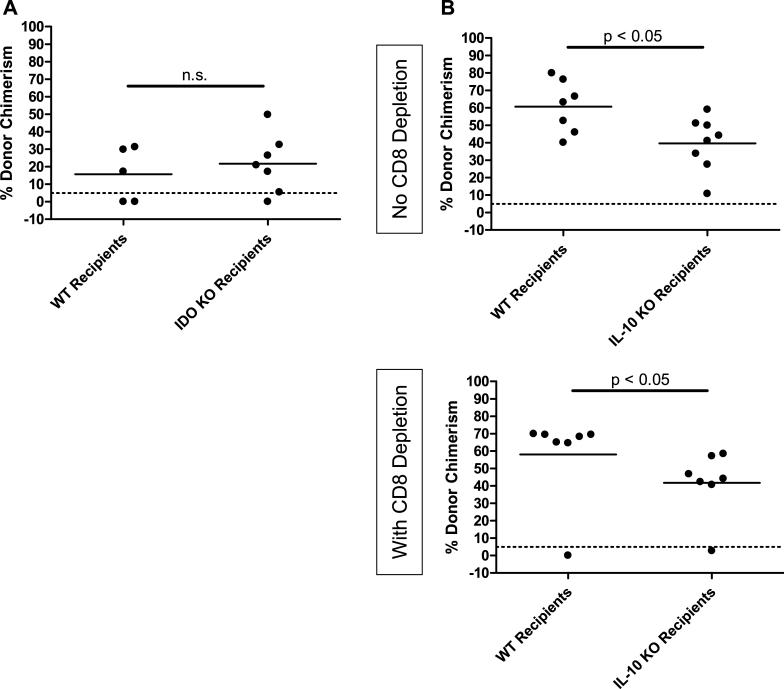
Recipient IDO and IL-10 are dispensable for CD8 T-cell tolerance
IDO produced by DCs can suppress T-cell reactivity by inducing tryptophan catabolism (14). To determine whether or not recipient DCs or B cells might promote tolerance via production of IDO, we utilized IDO KO B6 mice as recipients of allogeneic BMT with anti-CD154. As shown in Figure 6A, no difference in the level of chimerism was observed in IDO KO compared to WT recipients. To address the possibility that recipient-derived IL-10 might participate in tolerance induction, we compared the chimerism achieved when BALB/c marrow was administered to IL-10-deficient or WT B6 mice, with and without CD8 depletion. As shown in Figure 6B, chimerism was achieved in all CD8-replete WT and IL-10 KO recipients, and in the majority of CD8-depleted mice. However, the level of chimerism was slightly lower in both CD8-replete and CD8-depleted IL-10 KO mice compared to their WT counterparts.
Figure 6. Recipient IDO and IL-10 are dispensable for achievement of CD8 T-cell tolerance.
Recipients genetically deficient in IDO (A) or IL-10 (B) were given 3Gy TBI/anti-CD154/allo-BMT, with and without anti-CD8. Percent donor chimerism in the Mac-1+ lineage of the peripheral blood at 4 or 5 weeks post-BMT is depicted. Each symbol represents an individual animal. The dotted line at 5% donor chimerism depicts our cutoff for referring to an animal as a chimera.
Recipient B cells do not require antidonor alloreactivity, and transferred donor B cells cannot substitute for recipient B cells in promoting tolerance of recipient CD8 cells
To determine whether or not cognate recognition of donor antigen by recipient B cells might be involved in their tolerogenic role, we adoptively transferred recipient-type B cells lacking antidonor reactivity into μMT mice at the time of allo-BMT. To generate tolerized B cells, B6 mice were treated with lethal TBI and given T- and B-cell-depleted B6 plus B10.A BM. Four weeks later, B6 B cells, all of which developed de novo posttransplant and therefore should lack reactivity to B6 and B10.A, were isolated from these mixed chimeras and transferred to μMT B6 mice treated with 3 Gy TBI, anti-CD154 and B10.A BMT. As shown in Figure 7A, the tolerant B cells promoted chimerism induction even more effectively than B cells from syngeneically reconstituted control mice and as effectively as those from untreated B6 mice.
Because the result described in the preceding paragraph suggests that cognate recognition of the donor is not required for B cells to promote tolerance, we hypothesized that large numbers of donor B cells might also be able to promote CD8 cell tolerance in our model. However, as shown in Figure 7B, 43 million donor-type B cells did not promote chimerism induction in μMT B6 mice, whereas 25 million recipient-type B cells significantly enhanced chimerism induction in the same experiment. Donor-type B cells were cleared from the recipient between 1 and 2 weeks after transfer. Thus, donor-type B cells cannot substitute for recipient B cells in promoting CD8 T-cell tolerance.
Discussion
In mice receiving BMT with 3 Gy TBI and anti-CD154 mAb, peripheral alloreactive CD8 but not CD4 T-cell tolerance requires recipient MHC class II expression in the periphery, host B cells, host DCs and host CD4 T cells (6,7). We investigated the requirement for MHC and costimulatory molecules on recipient B cells and DCs and discovered an antigen-presenting role for recipient DCs but not recipient B cells in promoting CD8 T-cell tolerance.
B-cell-deficient μMT mice conditioned with 3Gy TBI/anti-CD154 failed to achieve mixed chimerism unless B cells from WT B6 mice were adoptively transferred on the day of BMT (6). B cells from MHC class II-deficient CIITA−/− B6 mice effectively promoted tolerance of CD8 T cells (6). We have now demonstrated that B cells lacking MHC class I, both MHC class I and class II, or CD80/86 all promoted CD8 T-cell tolerance as effectively as WT B cells, suggesting that recipient B cells promote CD8 T-cell tolerance without presenting antigen. In contrast, recipient DCs deficient in both MHC class I and class II or in CD80/86, could not induce CD8 T-cell tolerance. Thus, recipient DCs most likely contribute to CD8 T-cell tolerance through a mechanism involving antigen presentation to T cells. The preservation of CD80 and CD86 expression on recipient DCs but not B cells in mice undergoing tolerance is consistent with the critical antigen-presenting role of the former but not the latter.
B cells are known to be capable of modulating T-cell immunity (15). Possible roles for recipient B cells include production of IL-10 and/or TGFβ that could inhibit donor-reactive CD8 T cells. IL-10 can inhibit CD8 T- cell responses via an indirect effect on APCs (16) and B-cell-derived IL-10 has been shown to suppress autoimmunity (17). However, we found that IL-10-deficient recipients were permissive to the induction of chimerism with anti-CD154, indicating that B cell-derived IL-10 was not an absolute requirement. Nevertheless, the levels of chimerism were reduced in IL-10-deficient compared to WT mice, even when CD8 cells were depleted, suggesting a possible role for recipient IL-10 in enhancing chimerism independent of CD8 cells.
Moreover, large numbers of donor B cells could not replace the requirement for recipient B cells in our model, indicating that recognition of donor antigen on B cells is insufficient to tolerize CD8 T cells. The ability of adoptively transferred tolerant recipient-type B cells to promote CD8 cell tolerance indicates that B cells do not need to respond to donor antigen in order play this role, as the transferred B cells were tolerized via bone marrow chimerism, which leads to deletion or receptor editing of antigen-reactive cells (18–21). Thus, the mechanism by which recipient B cells promote CD8 cell tolerance in our model remains unclear. TGFβ can suppress the cytotoxicity of CD8 T cells (22), and surface-bound TGFβ on lipopolysaccharide-activated B cells can induce CD8 T-cell anergy (23,24). Further exploration of the role of TGFβ is, therefore, warranted.
The ability of either MHC class I or class II expression on recipient DCs to promote CD8 tolerance may be explained by a need for T-cell-dependent reverse signaling to render DCs tolerogenic for CD8 cells. CD80/86 on recipient DCs would be ligated by CD28 on CD8 or CD4 T cells in recipients with DCs expressing only class I or class II, respectively. In recipients lacking both MHC class I and class II on their DCs, there would be no MHC:TCR interaction with either T-cell subset and, therefore, no CD80/86 ligation by CD28. In addition to CD28, CD80/86 could also be ligated by CTLA4 to induce reverse signaling. Consistently, we have previously observed that addition of CTLA4-Ig to a slightly different BMT regimen tolerizes CD8 T cells (25). When a lower dose of anti-CD154 is administered and TBI is given on Day 0 (instead of Day − 1), robust chimerism is consistently achieved in animals depleted of CD8 T cells but not in CD8-replete animals (25). Because addition of CTLA4-Ig to the regimen overcomes CD8 cell-mediated rejection, binding of CTLA4-Ig to CD80/86 may promote CD8 T-cell tolerance by reverse signaling to DCs, as has been described (26). Our previous study indicates that alloreactive CD4 cells are themselves tolerized by interactions of their CTLA4 molecules with recipient CD80/86 (27), and it is possible that these interactions also indirectly support CD8 T-cell tolerance by reverse signaling into DCs. A third possible ligand for inducing reverse signaling through CD80/86 is PD-L1. CD80 can transduce inhibitory signals into DCs when ligated by PD-L1, which is required in our model and could be provided by either CD4 or CD8 T cells when they engage their MHC counterpart (28,29). Thus, we hypothesize that either MHC class I or class II must be expressed on DCs in order for CD80/86 on the DC to be ligated by CD28, CTLA4 or PD-L1. We further hypothesize that reverse signaling into recipient DCs via CD80/86 conditions the DC to tolerize CD8 T cells through an as yet unidentified mechanism. While reverse signaling into DCs through CD80/86 has been shown to transduce inhibitory signals (30), inducing production of IDO (31), our studies show that recipient IDO production is not required to promote CD8-cell tolerance. Production of IL-10 by recipient DCs is also not required, and the role of TGFβ remains to be determined.
In summary, we demonstrate that, although both recipient B cells and recipient DCs are required for CD8 T-cell tolerance, only recipient DCs must present antigen. B cells may contribute to tolerance in this model via suppressive cytokines that remain to be identified. To explain the requirement for CD80/86 and either MHC class I or class II on recipient DCs, we hypothesize that a DC: T-cell interaction must transduce an inhibitory signal into the DC via CD80/86 and that this signal, together with CD154 blockade, leads to CD8 T-cell tolerance via molecules and cell– cell interactions that remain to be elucidated.
Acknowledgments
The authors thank Drs. Thomas Fehr and Luis Fernandez for critical review of this paper, Orlando Moreno for technical assistance and Gena Coleman for expert assistance with the paper.
Funding source: This work was supported by National Institutes of Health Grant R01 HL49915. J.L.M. was supported by the 2008 AST/Wyeth Basic Science Fellowship Grant and a National Institutes of Health Training Grant T32DK007540-21. CLL was supported by the National Defense Science and Engineering Graduate Fellowship. FH was supported by fellowships from the Foundation pour la Recherche Medicale and the American Society of Blood and Marrow Transplantation.
Abbreviations
- BMT
bone marrow transplantation
- DT
diphtheria toxin
- DTR
diphtheria toxin receptor
- TBI
total body irradiation
References
- 1.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. New Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fudaba Y, Spitzer TR, Shaffer J, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: In vivo and in vitro analyses. Am J Transplant. 2006;6:2121–2133. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 3.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi Y, Ito H, Kurtz J, Wekerle T, Ho L, Sykes M. Earlier low-dose TBI or DST overcomes CD8+ T-cell-mediated alloresistance to allogeneic marrow in recipients of anti-CD40L. Am J Transplant. 2004;4:31–40. doi: 10.1046/j.1600-6135.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand [letter]. Nat Med. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 6.Fehr T, Haspot F, Mollov J, Chittenden M, Hogan T, Sykes M. Alloreactive CD8 T cell tolerance requires recipient B cells, dendritic cells and MHC class II. J Immunol. 2008;181:165–173. doi: 10.4049/jimmunol.181.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fehr T, Takeuchi Y, Kurtz J, Sykes M. Early regulation of CD8 T cell alloreactivity by CD4+CD25-T cells in recipients of anti- CD154 antibody and allogeneic BMT is followed by rapid peripheral deletion of donor-reactive CD8 +T cells, precluding a role for sustained regulation. Eur J Immunol. 2005;35:2679–2690. doi: 10.1002/eji.200526190. [DOI] [PubMed] [Google Scholar]
- 8.Taylor PA, Lees CJ, Waldmann H, Noelle RJ, Blazar BR. Requirements for the promotion of allogeneic engraftment by anti-CD154 (anti-CD40L) monoclonal antibody under nonmyeloablative conditions. Blood. 2001;98:467–474. doi: 10.1182/blood.v98.2.467. [DOI] [PubMed] [Google Scholar]
- 9.Koo GC, Peppard JR. Establishment of monoclonal anti-NK-1.1 antibody. Hybridoma. 1984;3:301–303. doi: 10.1089/hyb.1984.3.301. [DOI] [PubMed] [Google Scholar]
- 10.Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- 11.Vugmeyster Y, Glas R, Perarnau B, Lemonnier FA, Eisen H, Ploegh H. Major histocompatiblity complex (MHC) class I KbDb −/− deficient mice possess functional CD8+ T cells and natural killer cells. Proc Natl Acad Sci USA. 1998;95:12492–12497. doi: 10.1073/pnas.95.21.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CH, Guerder S, Hong SC, van Ewijk W, Flavell RA. Mice lacking the MHC class II transactivator (CIITA) show tissue-specific impairment of MHC class II expression. Immunity. 1996;4:167–178. doi: 10.1016/s1074-7613(00)80681-0. [DOI] [PubMed] [Google Scholar]
- 13.Van Gool SW, Vandenberghe P, de Boer M, Ceuppens JL. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol Rev. 1996;153:47–83. doi: 10.1111/j.1600-065x.1996.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 14.Mellor AL, Munn DH. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 15.Katz SI, Parker D, Turk JL. B-cell suppression of delayed hypersensitivity reactions. Nature. 1974;251:550–551. doi: 10.1038/251550a0. [DOI] [PubMed] [Google Scholar]
- 16.Groux H, Bigler M, de Vries JE, Roncarolo M-G. Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J Immunol. 1998;160:3188–3193. [PubMed] [Google Scholar]
- 17.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 18.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes. Nature. 1989;342:385–391. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 19.Goodnow CC, Cyster JG, Hartley SB, et al. Self-tolerance checkpoints in B lymphocyte development. Adv Immunol. 1995;59:279–368. doi: 10.1016/s0065-2776(08)60633-1. [DOI] [PubMed] [Google Scholar]
- 20.Nemazee D, Buerki K. Clonal deletion of autoreactive B lymphocytes in bone marrow chimeras. Proc Natl Acad Sci USA. 1989;86:8039–8043. doi: 10.1073/pnas.86.20.8039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6:728–740. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 22.Chen ML, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci USA. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B cells activated by lipopolysaccharide, but bot by anti-Ig and anti-CD40 antibody, induce anergy in CD8(+) T cells: Role of TGF-beta1. J Immunol. 2003;170:5897–5911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]
- 24.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 25.Wekerle T, Sayegh MH, Hill J, et al. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187:2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 27.Kurtz J, Raval F, Vallot C, Der J, Sykes M. CTLA-4 on alloreactive CD4 T cells interacts with recipient CD80/86 to promote tolerance. Blood. 2009;113:3475–3484. doi: 10.1182/blood-2008-01-133736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007 doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haspot F, Fehr T, Gibbons C, et al. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008;112:2149–2155. doi: 10.1182/blood-2007-12-127449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 31.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4(+) T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]