Abstract
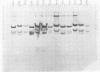
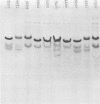
Resistance to the complement-dependent bactericidal activity of normal human serum is found in nearly all Neisseria gonorrhoeae strains causing disseminated gonococcal infection. Transformation of serum-sensitive gonococcal strain NRL 7189 to serum resistance using deoxyribonucleic acid from three separate disseminated-infection gonococci was accompanied by simultaneous structural and antigenic changes in the principal outer-membrane protein (POMP) of the transformants. In each of 10 separate transformations, there was a reduction in subunit molecular weight of the POMP from that in the recipient (39,000) to that in the deoxyribonucleic acid donors (36,500). Also, in each instance the POMP antigenic type, as measured by enzyme-linked immunosorbent assay, converted from that of the recipient to an antigenic type common to each DGI donor strain. This conversion of POMP antigen was reflected in part by changes in the surface fluorescence of the transformed gonococci to the microimmunofluorescence pattern of the donor strains. These results suggested that serum resistance of gonococci is related to the possession of a POMP of characteristic subunit molecular weight and antigenicity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A. Serogrouping of Neisseria gonorrhoeae: identification of four immunologically distinct acidic polysaccharides. J Infect Dis. 1976 Oct;134(4):377–383. doi: 10.1093/infdis/134.4.377. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M. Antigenic heterogeneity of gonococcal pili. J Exp Med. 1975 Jun 1;141(6):1470–1475. doi: 10.1084/jem.141.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Arko R. J. Immunity to gonococcal infection induced by vaccination with isolated outer membranes of Neisseria gonorrhoeae in guinea pigs. J Infect Dis. 1977 Jun;135(6):879–887. doi: 10.1093/infdis/135.6.879. [DOI] [PubMed] [Google Scholar]
- CATLIN B. W. Transformation of Neisseria meningitidis by deoxyribonucleates from cells and from culture slime. J Bacteriol. 1960 Apr;79:579–590. doi: 10.1128/jb.79.4.579-590.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREAVES R. I. Preservation of living cells by freeze-drying. Ann N Y Acad Sci. 1960 Apr 13;85:723–728. doi: 10.1111/j.1749-6632.1960.tb49992.x. [DOI] [PubMed] [Google Scholar]
- Heckels J. E. The surface of Neisseria gonorrhoeae: isolation of the major components of the outer membrane. J Gen Microbiol. 1977 Apr;99(2):333–341. doi: 10.1099/00221287-99-2-333. [DOI] [PubMed] [Google Scholar]
- Inoue K., Akiyama Y., Kinoshita T., Higashi Y., Amano T. Evidence for a one-hit theory in the immune bactericidal reaction and demonstration of a multi-hit response for hemolysis by streptolysin O and Clostridium perfringens theta-toxin. Infect Immun. 1976 Feb;13(2):337–344. doi: 10.1128/iai.13.2.337-344.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Holmes K. K., Gotschlich E. C. The serological classification of Neisseria gonorrhoeae. I. Isolation of the outer membrane complex responsible for serotypic specificity. J Exp Med. 1976 Apr 1;143(4):741–758. doi: 10.1084/jem.143.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp J. S., Holmes K. K. Disseminated gonococcal infections caused by Neisseria gonorrhoeae with unique nutritional requirements. J Infect Dis. 1975 Aug;132(2):204–208. doi: 10.1093/infdis/132.2.204. [DOI] [PubMed] [Google Scholar]
- Mayer L. W., Holmes K. K., Falkow S. Characterization of plasmid deoxyribonucleic acid from Neisseria gonorrhoeae. Infect Immun. 1974 Oct;10(4):712–717. doi: 10.1128/iai.10.4.712-717.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L. W., Schoolnik G. K., Falkow S. Genetic studies on Neisseria gonorrhoeae from disseminated gonococcal infections. Infect Immun. 1977 Oct;18(1):165–172. doi: 10.1128/iai.18.1.165-172.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutchan J. A., Levine S., Braude A. I. Influence of colony type on susceptibility of gonococci to killing by human serum. J Immunol. 1976 Jun;116(6):1652–1655. [PubMed] [Google Scholar]
- Rice P. A., Kasper D. L. Characterization of gonococcal antigens responsible for induction of bactericidal antibody in disseminated infection. J Clin Invest. 1977 Nov;60(5):1149–1158. doi: 10.1172/JCI108867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoolnik G. K., Buchanan T. M., Holmes K. K. Gonococci causing disseminated gonococcal infection are resistant to the bactericidal action of normal human sera. J Clin Invest. 1976 Nov;58(5):1163–1173. doi: 10.1172/JCI108569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966 Nov;92(5):1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink W. W., Keefer C. S. STUDIES OF GONOCOCCAL INFECTION. II. THE BACTERIOLYTIC POWER OF THE WHOLE DEFIBRINATED BLOOD OF PATIENTS WITH GONOCOCCAL ARTHRITIS. J Clin Invest. 1937 Mar;16(2):177–183. doi: 10.1172/JCI100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Sadoff J. C., Wilson C. Variability of the lytic susceptibility of Neisseria gonorrhoeae to human sera. J Immunol. 1977 May;118(5):1843–1851. [PubMed] [Google Scholar]
- WHITE L. A., KELLOGG D. S., Jr NEISSERIA GONORRHOEAE IDENTIFICATION IN DIRECT SMEARS BY A FLUORESCENT ANTIBODY-COUNTERSTAIN METHOD. Appl Microbiol. 1965 Mar;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Holmes K. K., Knapp J. S., Ott S., Kyzer D. D. Immunologic classification of Neisseria gonorrhoeae with micro-immunofluorescence. J Immunol. 1977 Sep;119(3):795–803. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Wiesner P. J., Handsfield H. H., Holmes K. K. Low antibiotic resistance of gonococci causing disseminated infection. N Engl J Med. 1973 Jun 7;288(23):1221–1222. doi: 10.1056/NEJM197306072882308. [DOI] [PubMed] [Google Scholar]