Abstract
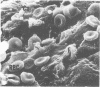
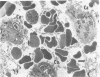
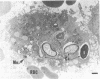
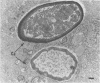
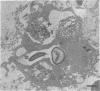
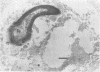
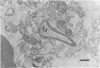
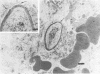
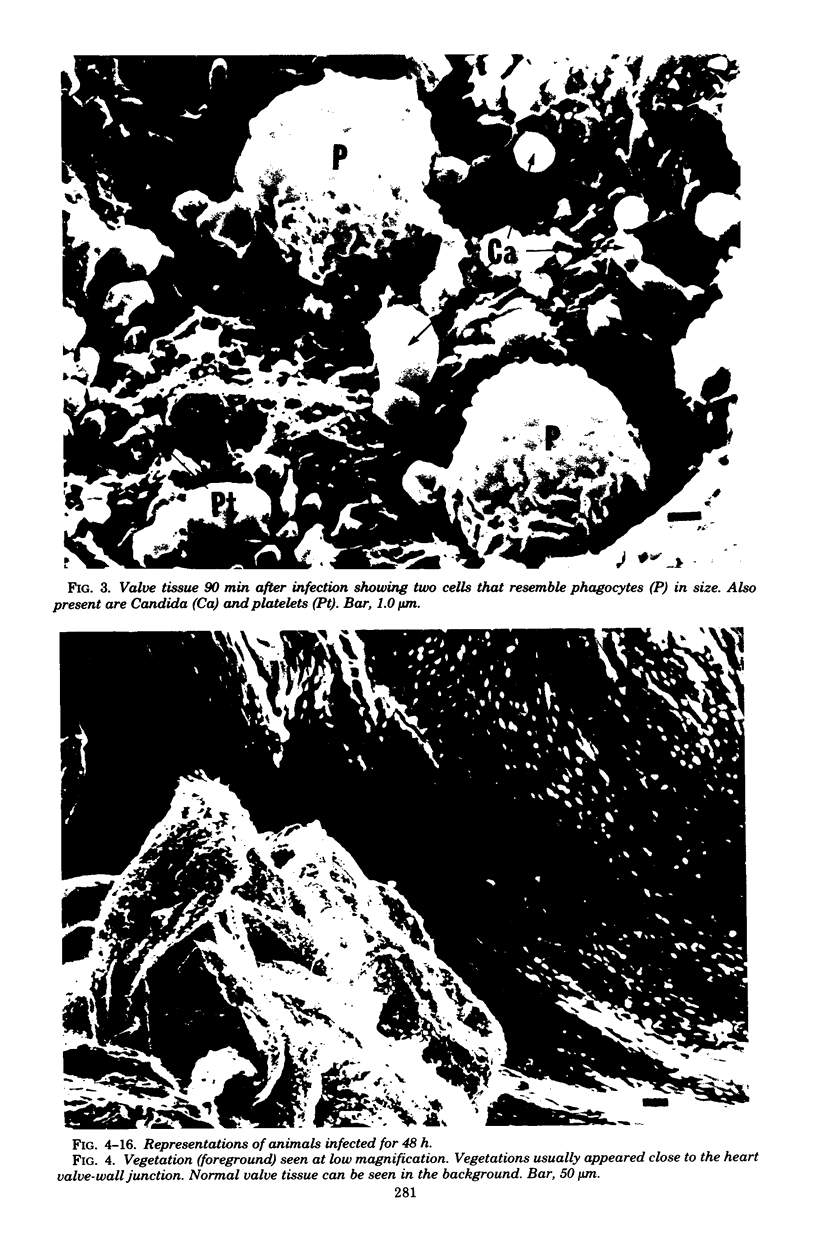
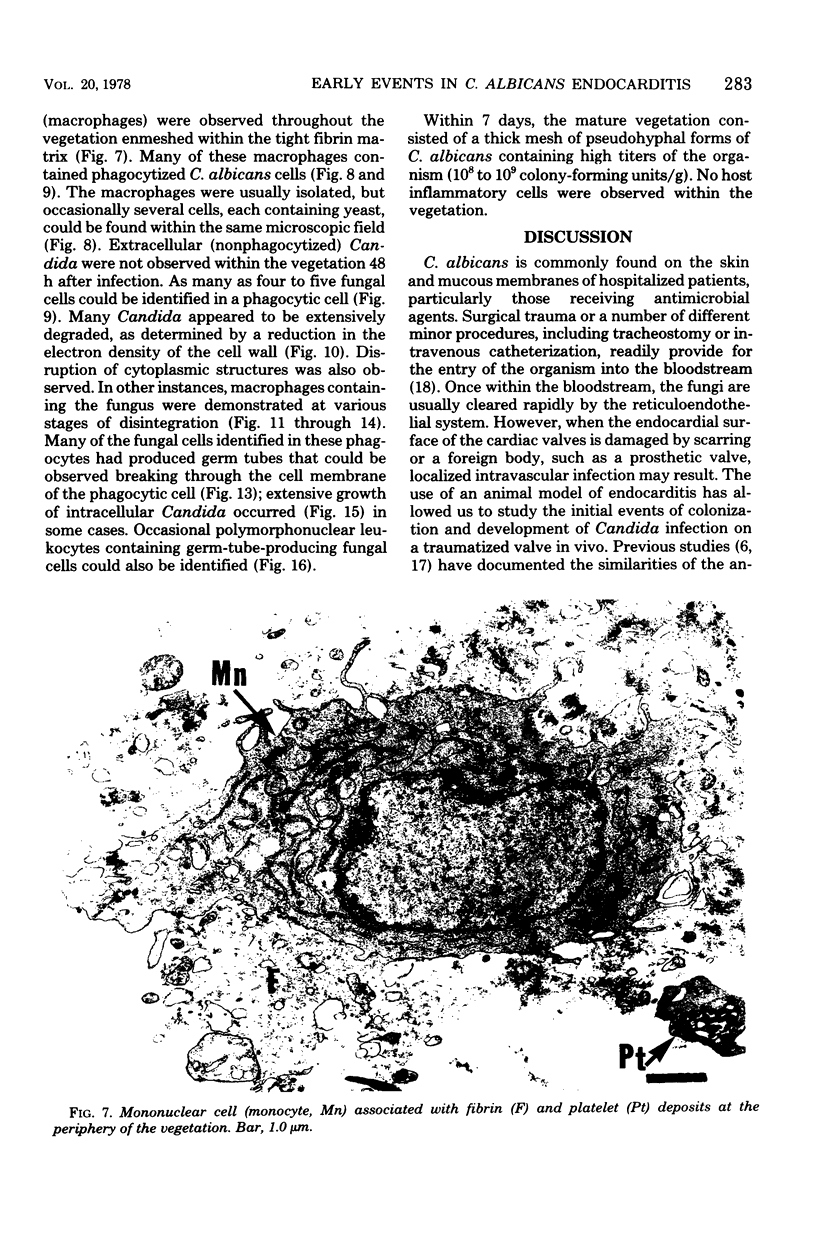
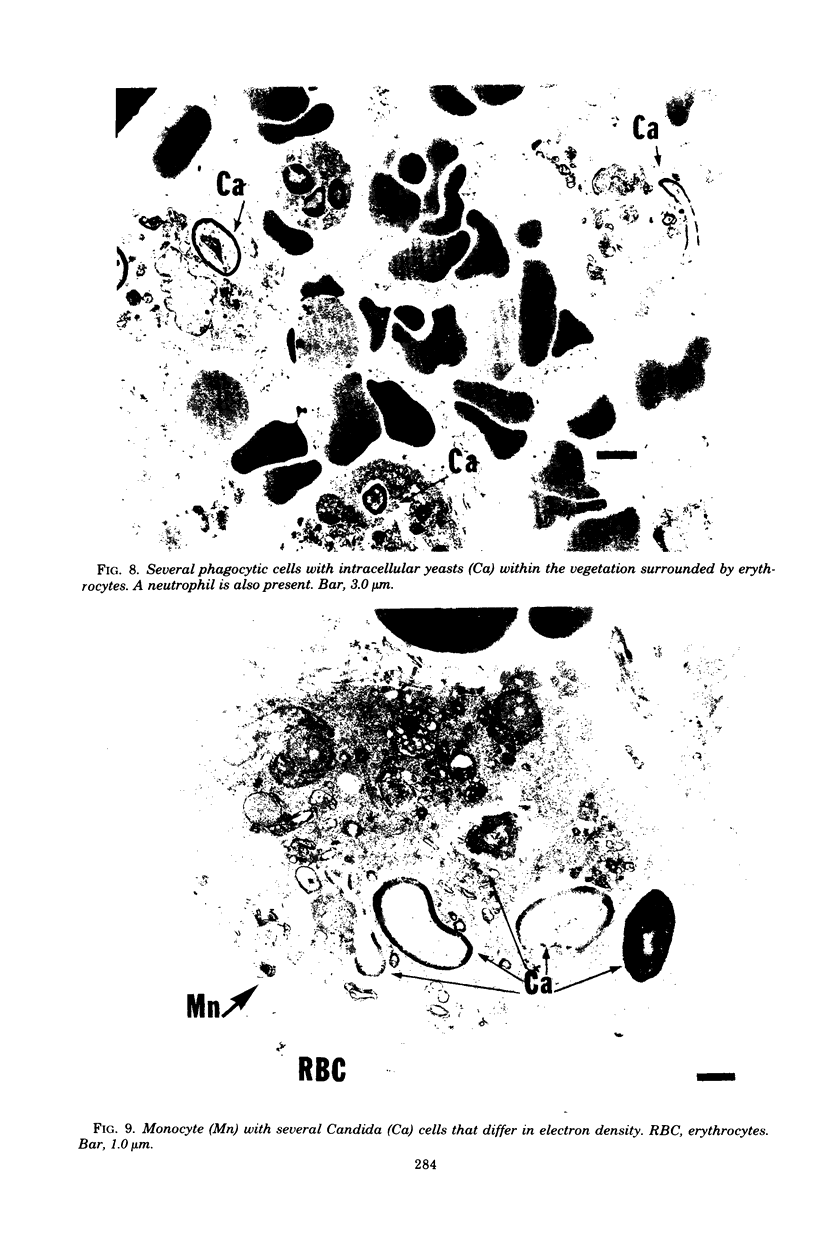
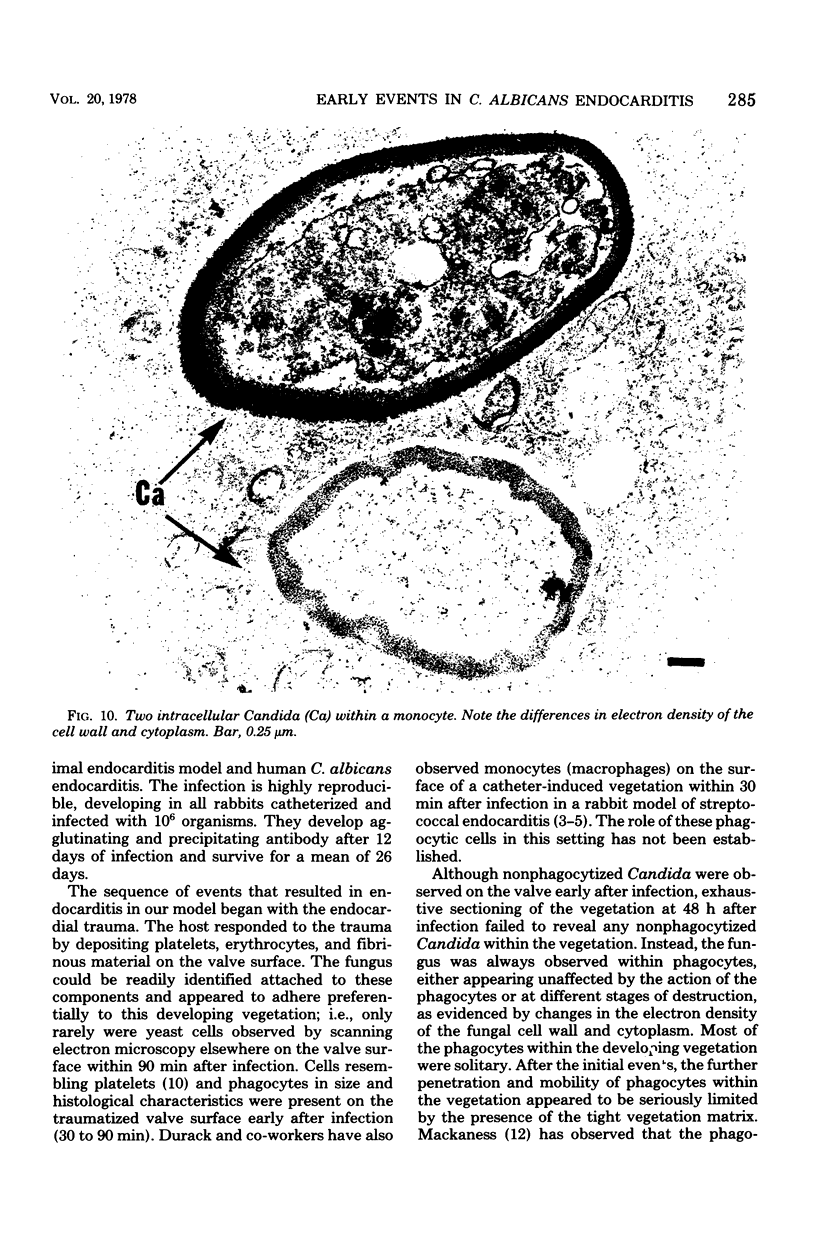
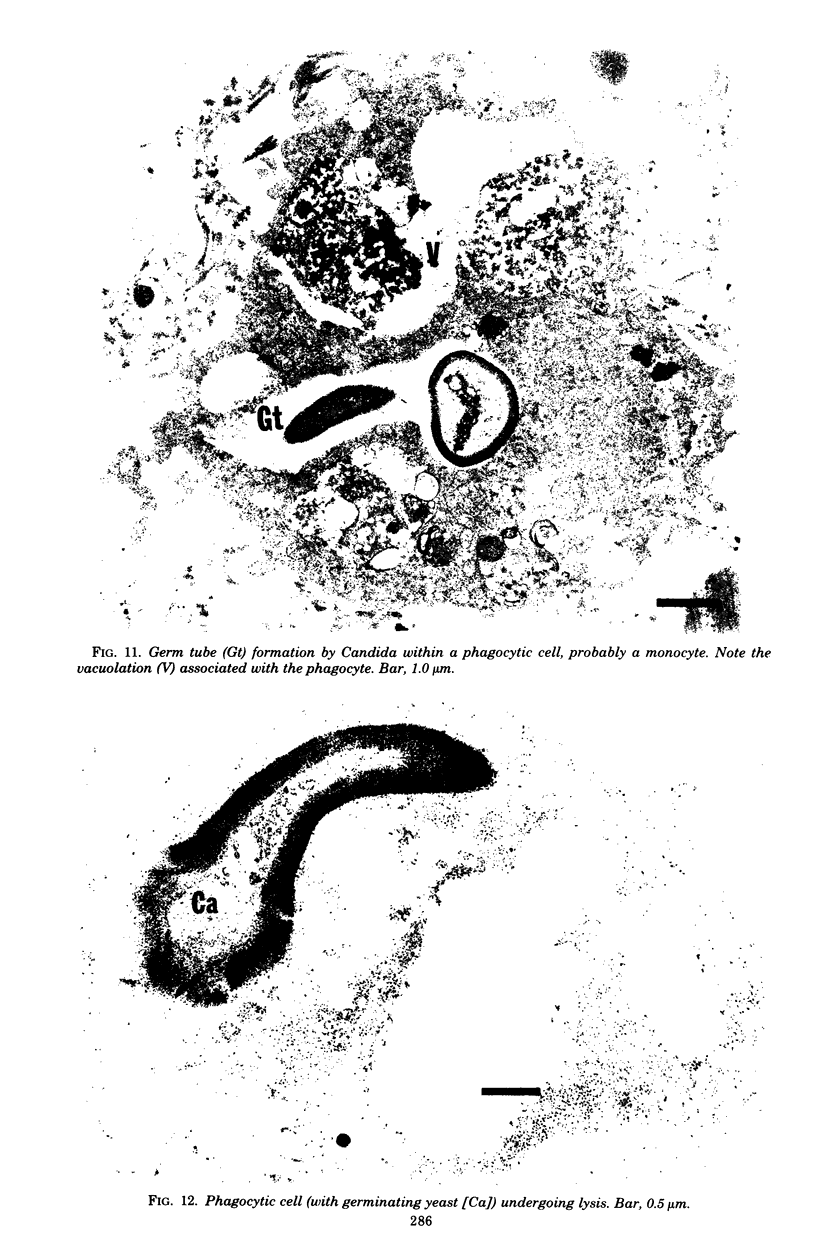
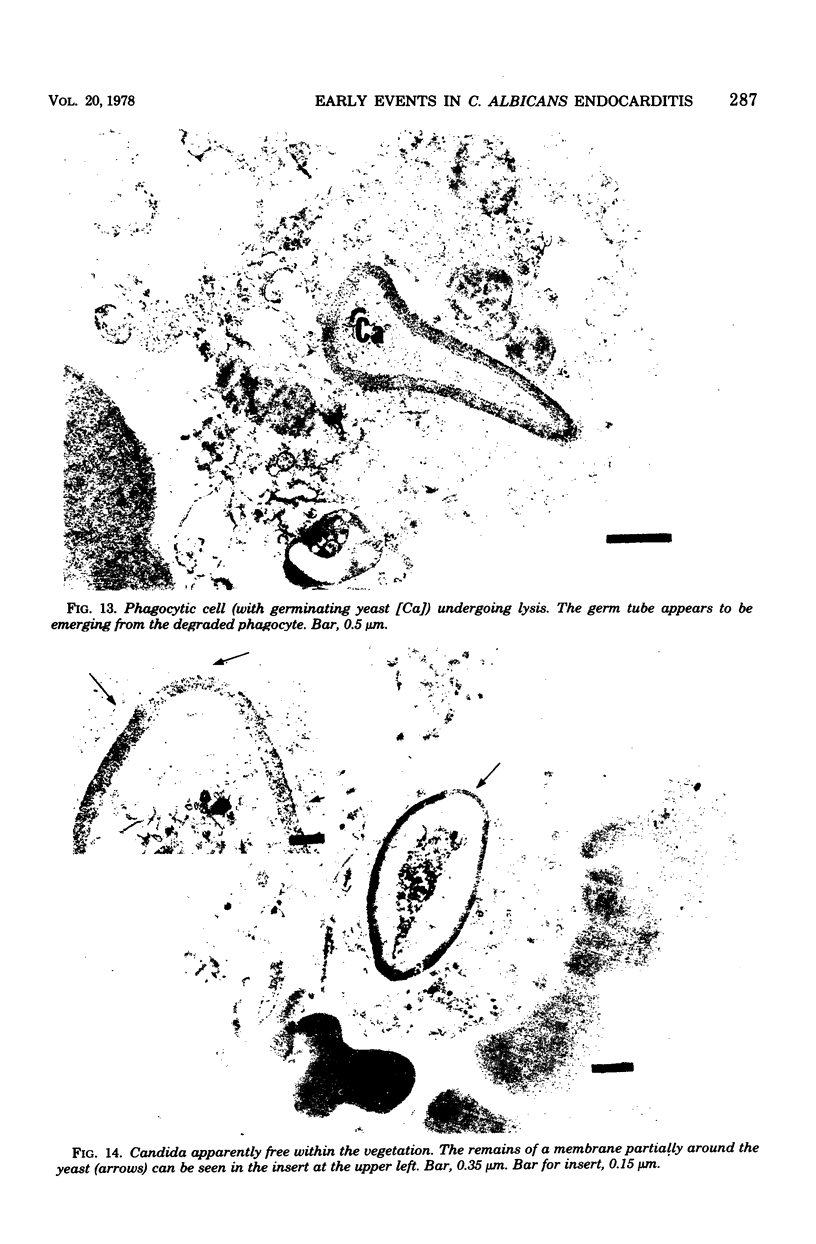
Candida albicans endocarditis was established in rabbits after transaortic catherization. Within 30 to 90 min after infection, C. albicans was observed by scanning electron microscopy on the valve surface. The organisms were predominantly associated with host deposits of erythrocytes, phagocytes, platelets, and fibrinous-appearing material, which collectively appeared on the valve surface in response to trauma. Within 48 h after infection, vegetations composed of these same host components were observed on the heart valves, although Candida cells were not demonstrable on the valve or vegetation surface. When the vegetations were examined by transmission electron microscopy, Candida blastospores were only observed within phagocytic cells (predominantly monocytes) enmeshed within the vegetation matrix. Many of the intracellular organisms were undergoing degradation, as evidenced by a reduction in electron density of the cell wall. Other fungi had highly electron-dense cell walls and germ tubes. Phagocytic cells containing germinating Candida were highly vacuolated and were observed at various stages of cell lysis. After 7 days of infection, the vegetation contained a dense meshwork of fibrin and Candida pseudohyphae with 108 to 109 colony-forming units/g of vegetation. The mature vegetation was devoid of phagocytic cells and continued to grow until the death of the animal.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDRIOLE V. T., KRAVETZ H. M., ROBERTS W. C., UTZ J. P. Candida endocarditis. Clinical and pathologic studies. Am J Med. 1962 Feb;32:251–285. doi: 10.1016/0002-9343(62)90294-2. [DOI] [PubMed] [Google Scholar]
- Carrizosa J., Kohn C., Levison M. E. Experimental aspergillus endocarditis in rabbits. J Lab Clin Med. 1975 Nov;86(5):746–753. [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972 Feb;53(1):44–49. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T., Beeson P. B., Petersdorf R. G. Experimental bacterial endocarditis. 3. Production and progress of the disease in rabbits. Br J Exp Pathol. 1973 Apr;54(2):142–151. [PMC free article] [PubMed] [Google Scholar]
- Durack D. T. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J Pathol. 1975 Feb;115(2):81–89. doi: 10.1002/path.1711150204. [DOI] [PubMed] [Google Scholar]
- Freedman L. R., Johnson M. L. Experimental endocarditis. IV. Tricuspid and aortic valve infection with Candida albicans in rabbits. Yale J Biol Med. 1972 Apr;45(2):163–175. [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Russell E., Jr, Remington J. S. The compromised host and infection. II. Deep fungal infection. J Infect Dis. 1969 Aug;120(2):169–191. doi: 10.1093/infdis/120.2.169. [DOI] [PubMed] [Google Scholar]
- Hook E. W., 3rd, Sande M. A. Role of the vegetation in experimental Streptococcus viridans endocarditis. Infect Immun. 1974 Dec;10(6):1433–1438. doi: 10.1128/iai.10.6.1433-1438.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N. C. A scanning electron microscopic study of platelets of certain animal species. Thromb Diath Haemorrh. 1975 Jun 30;33(3):501–507. [PubMed] [Google Scholar]
- Kammer R. B., Utz J. P. Aspergillus species endocarditis. The new face of a not so rare disease. Am J Med. 1974 Apr;56(4):506–521. doi: 10.1016/0002-9343(74)90483-5. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. The phagocytosis and inactivation of staphylococci by macrophages of normal rabbits. J Exp Med. 1960 Jul 1;112:35–53. doi: 10.1084/jem.112.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister H., Heymer B., Schäfer H., Haferkamp O. Role of Candida albicans in granulomatous tissue reactions. II. In vivo degradation of C. albicans in hepatic macrophages of mice. J Infect Dis. 1977 Feb;135(2):235–242. doi: 10.1093/infdis/135.2.235. [DOI] [PubMed] [Google Scholar]
- Ozato K., Uesaka I. The role of macrophages in Candida albicans infection in vitro. Jpn J Microbiol. 1974 Jan;18(1):29–35. doi: 10.1111/j.1348-0421.1974.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Pearsall N. N., Lagunoff D. Immunological responses to Candida albicans. I. Mouse-thigh lesion as a model for experimental candidiasis. Infect Immun. 1974 Jun;9(6):999–1002. doi: 10.1128/iai.9.6.999-1002.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E. M., Hawley R. J., Calderone R. A. An ultrastructural analysis of protoplast-spheroplast induction in Cryptococcus neoformans. Can J Microbiol. 1976 Oct;22(10):1518–1521. doi: 10.1139/m76-224. [DOI] [PubMed] [Google Scholar]
- Sande M. A., Bowman C. R., Calderone R. A. Experimental Candida albicans endocarditis: characterization of the disease and response to therapy. Infect Immun. 1977 Jul;17(1):140–147. doi: 10.1128/iai.17.1.140-147.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig M. S., Speth C. P., Kozinn P. J., Toni E. F., Taschdjian C. L. Candida endocarditis after cardiac surgery. Clues to earlier detection. J Thorac Cardiovasc Surg. 1973 Apr;65(4):583–601. [PubMed] [Google Scholar]
- Stanley V. C., Hurley R. The growth of Candida species in cultures of mouse peritoneal macrophages. J Pathol. 1969 Feb;97(2):357–366. doi: 10.1002/path.1710970222. [DOI] [PubMed] [Google Scholar]