Abstract
We report an interesting process whereby the formation of nanoparticle assemblies on and nanoporosities within calcite crystals is directed by an intrinsically disordered C-RING mollusk shell nacre protein, AP7. Under mineralization conditions, AP7 forms protein phases that direct the nucleation of ordered calcite nanoparticles via a repetitive protein phase deposition process onto calcite crystals. These organized nanoparticles are separated by gaps or spaces that become incorporated into the forming bulk crystal as nanoporosities. This is an unusual example of organized nanoparticle biosynthesis and mineral modification directed by a C-RING protein phase.
The formation of the mollusk shell nacre layer is believed to be under the control of two protein families. The nacre intracrystalline proteome1−4 is a family of proteins that are occluded within the nacre tablets themselves. In contrast, the framework proteome5,6 is a subset of proteins associated with the macromolecular silk−β-chitin exterior gel-like phase that coats the exterior of the nacre tablets. Together, both proteomes participate in aragonite tablet formation and contribute to the fracture toughening properties of the nacre.1−6 Unfortunately, there is very little information available regarding either proteome or their participation in the nacre mineralization process. Thus, our present understanding of mollusk shell biomineralization is limited, and this in turn prevents us from understanding the shell engineering process.
Recent studies reveal that certain members of the nacre intracrystalline proteome, such as the intrinsically disordered C-RING protein, AP7 (Aragonite Protein-7, 66 amino acids, 7.5 kDa, Haliotis rufescens),2−4 self-assemble to form protein phases that gather amorphous calcium carbonate (ACC) nanoparticles together into hybrid protein–mineral supramolecular networks in solution.4 However, it is not known whether these same protein phases can modify calcium carbonate crystals themselves and, if so, what modifications are introduced into these crystals. Establishing this dual capability of protein phases is an important step in understanding the mollusk shell formation process as well as variations in C-RING functionality.
In this work, we describe the additional ability of C-RING AP7 protein phases to modify calcite crystals in two dramatic ways. First, these protein phases direct the nucleation of highly oriented calcite nanoparticles on calcite crystals formed in standard calcium carbonate mineralization assays (Figure 1). The formation of these nanoparticles mimics the findings obtained in polymer phase-based7 and gel-based8 mineralization systems. Second, these surface nanoparticle assemblies and associated gap regions become incorporated into the bulk crystal during overgrowth, leading to the introduction of nanoporosities within the bulk crystal (Figure 2). Thus, intracrystalline protein phases, such as those generated by C-RING AP7, not only induce mineral assembly in solution4 but also can introduce new physical properties (i.e., nanopatterning and nanoporosities) into crystals.
Figure 1.
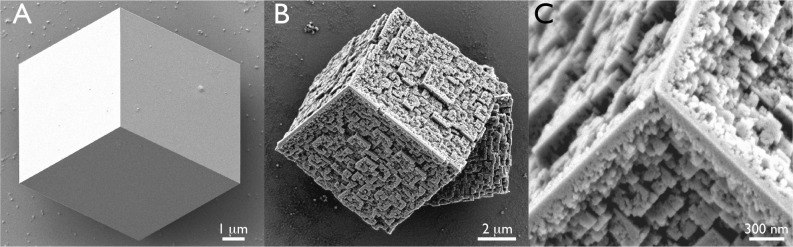
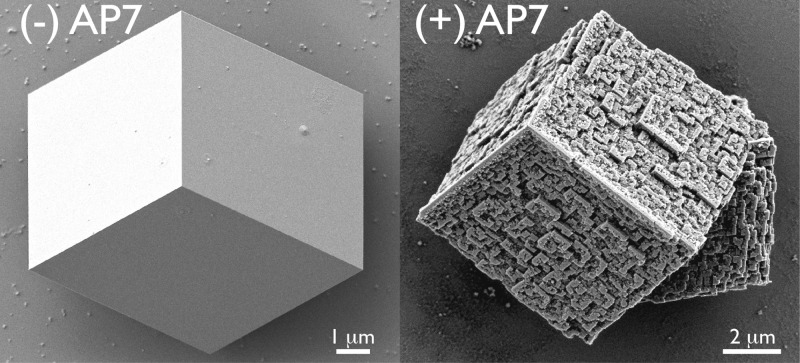
(A) Representative calcite crystals obtained from protein-deficient controls. (B and C) Representative nanoparticle assemblies generated by AP7 in 1 h, revealing three-dimensional nanopatterning and building block construction. Note the occurrence of gaps or spaces between nanoparticles in panel C.
Figure 2.
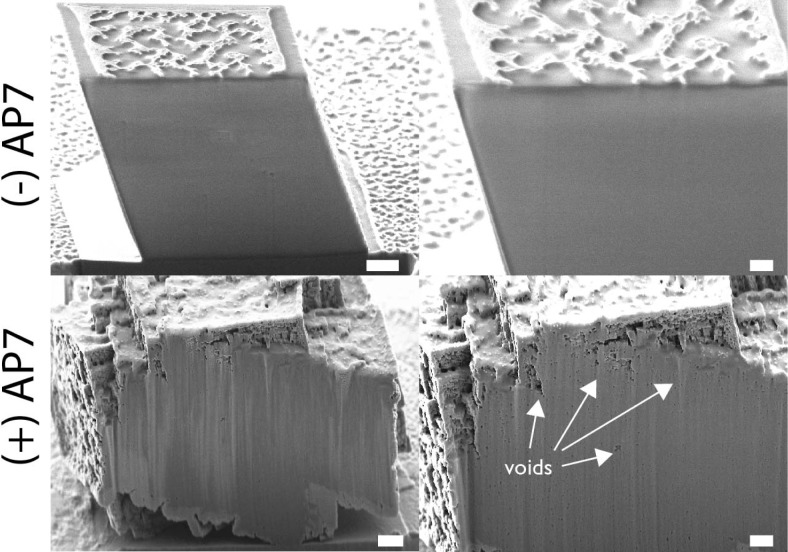
Representative scanning electron microscopy images of focused-ion beam-sectioned Ir-coated crystals obtained from protein-deficient and AP7-containing assays. White arrows denote locations of subsurface voids. Scale bars are 200 nm. Note that the nanopatterning on the control crystal surfaces is generated by bombardment of the sample with a Ga ion beam. Additional images that document subsurface porosities can be found in the Supporting Information.
For the purposes of this study, we utilized sealed carbonate/bicarbonate mineralization assays (Supporting Information) that predominantly generate calcite (>90%) with well-defined {104} surfaces for electron microscopy examination and thus avoid imaging complications arising from vaterite and aragonite. We observe that the AP7 protein directs the nucleation of intricate three-dimensional mineralized structures (Figure 1). These structures consist of highly arranged mineral deposits that are approximately 30–300 nm in size and resemble building blocks. Many of these nanoparticles are separated by gaps or spaces. These novel three-dimensional mineralized structures represent >90% of the total crystals produced by AP7 under these assay conditions and thus represent a dominant in vitro protein-mediated process.
Physical characterization methods revealed that the nanoparticles are single-crystal calcite with some evidence of a polycrystalline calcite component being present (Figures S1–S3 and Table S1 of the Supporting Information). This polycrystalline component may arise from the underlying calcite crystal foundation or may originate from a true polycrystalline component promoted by the protein phase. Using focused-ion beam sectioning, we confirmed that these mineralized structures (Figure 1) possess very interesting interior features (Figure 2 and Figure S4 of the Supporting Information). First, in peripheral regions, we can detect distinct layers of nanoparticles. Second, relative to controls, we note a larger number of subsurface nanometer-sized porosities or voids randomly distributed within the peripheral regions of AP7-treated crystals (Figure S4 of the Supporting Information). We conclude that the AP7 protein phases are responsible for creating both the nanometer-sized calcite crystal clusters at the surface and the subsurface nanoporosities.
We suspected that the nanoparticle assemblies and subsurface modifications were created by AP7 protein phases that were deposited onto calcite crystal surfaces during the assay. Because the AP7 C-RING domain includes four Cys residues,2−4 we employed X-ray microanalyses and confirmed S content at crystal surfaces where protein phases were noted, thus verifying the presence of AP7 (Figures S5 and S6 of the Supporting Information). Similar X-ray microanalyses were performed on subsurface porosities, but because of the low levels of protein and small scanned areas, we were unable to detect evidence of AP7 at this time (data not shown). Nonetheless, the presence of surface-associated AP7 protein phases correlates with the nanoparticle structures that form during the assays.
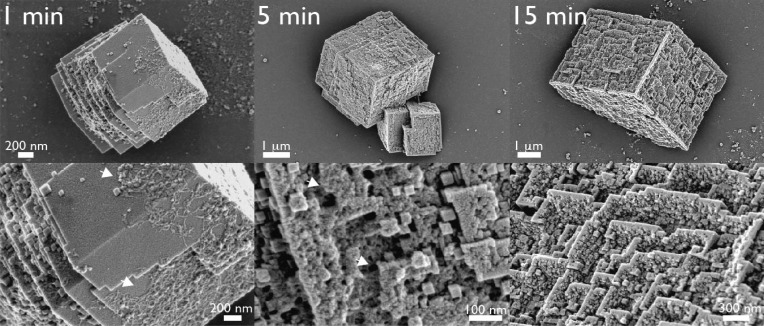
A further confirmation of the deposition of the AP7 protein onto calcite crystals was obtained using time-resolved assays (Figure 3). At 1 min, typical rhombohedral calcite crystals are observed along with AP7 protein phases that were deposited onto the surfaces of the Si wafers and calcite crystals. Nanoparticle formation is initiated on surface regions where the protein phase has coated or migrated over the calcite crystal. After 5 min, we observe ordered nanoparticles coating the exposed surfaces of calcite (Figure 3) and the continued deposition of AP7 protein phases on the Si wafer background and on exposed calcite surfaces. By 15 min, these crystal assemblies are largely complete and exhibit the familiar three-dimensional surface topographies, gaps, and crystal orientations that we observed at 1 h (Figure 1). Thus, these mineral assemblies result from the repetitive deposition of AP7 phases onto a calcite “core”, with each wave of AP7 deposition directing additional nucleation of single-crystal calcite nanoparticles on exposed surfaces.
Figure 3.
Scanning electron microscopy images of mineral deposits and AP7 phases that form in time-resolved mineralization assays. Images in the bottom row represent higher magnifications of important features for each time point. At 1 min, note the resemblance between the crystal-bound protein phases and those deposited on SI wafers (Figure S5 of the Supporting Information). White arrows denote the location of AP7 protein phases captured on crystal surfaces.
Thus, Nature has “tweaked” the intracellular protein–protein and signaling C-RING2−4 sequence of intrinsically disordered AP7 to form phases that serve two purposes: (a) to gather mineral nanoparticles in solution4 and (b) to guide nanoparticle formation on crystal surfaces (Figures 1 and 3). On the basis of available evidence, we conclude that AP7 protein phases allow calcite crystals to initially form, and then in a repetitive cycle, these phases are deposited onto these crystals and dimensionally limit and organize the mineral overgrowth that nucleates on the calcite crystals. We believe that it is the underlying thermodynamically stable calcite mineral phase that dictates the lattice structure of the nucleating nanoparticles (i.e., calcite), and thus, the relatively unstable aragonite phase cannot form, even in the presence of nacre-specific AP7 (Figure 1).3 This protein-guided nucleation process is relevant not only for understanding how proteins organize single-crystal mineral assemblies that support and protect a variety of organisms in Nature but also for developing material-based approaches that utilize organic phases to generate single-crystal nanoparticle coatings for devices and sensors.9
The repetitive deposition of AP7 phases onto crystal surfaces also leads to another phenomenon: the creation of subsurface nanoporosities (Figure 2 and Figure S4 of the Supporting Information). Similar intracrystalline features (termed “nanopatches”) were observed within prismatic calcite crystals in the shell of the mollusk Atrina rigida.10 In general, porous materials exhibit interesting properties, because porosities with controllable dimensions modulate material strength and allow materials to discriminate and interact with molecules and clusters.9 Hence, the AP7-induced nanoporosity phenomenon represents an intriguing model system for understanding the creation and impact of nanoporosities on the physical properties of both biological and synthetic materials. With respect to the process of porosity formation in our systems, we believe that the nanoporosities originate from the spaces or gaps that exist in the peripheral regions (Figures 1 and 2). Normally, as nucleation proceeds, these spaces or gaps would be eliminated as mineral overgrowth overtakes these areas, but if remnant AP7 protein phases were present in these gap regions, then these phases might prevent overgrowth from fully occupying these regions, leading to the formation of nanoporosities that potentially contain the protein phase. Obviously, this is speculative, and additional experimentation will be required to establish this phenomenon more precisely.
Acknowledgments
This paper represents contribution 76 from the Laboratory for Chemical Physics, New York University.
Supporting Information Available
Details of all mineral characterization methods and data (Figures S1–S6 and Table S1) and protein preparation and mineralization assay conditions. This material is available free of charge via the Internet at http://pubs.acs.org.
This research was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Materials Sciences and Engineering, via Grant DE-FG02-03ER46099 to J.S.E. L.A.E. acknowledges support from National Cancer Institute Grant R01 CA173083. The micro Raman studies made use of the Cornell Center for Materials Research Shared Facilities (National Science Foundation MRSEC Program DMR-1120296).
The authors declare no competing financial interests.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Marie B.; Zanella-Cleon I.; Le Roy N.; Becchi M.; Luquet G.; Marin F. (2010) ChemBioChem 11, 2138–2147. [DOI] [PubMed] [Google Scholar]
- Michenfelder M.; Fu G.; Lawrence C.; Weaver J. C.; Wustman B. A.; Taranto L.; Evans J. S.; Morse D. E. (2003) Biopolymers 70, 522–533. [DOI] [PubMed] [Google Scholar]
- Amos F. F.; Evans J. S. (2009) Biochemistry 48, 1332–1339. [DOI] [PubMed] [Google Scholar]
- Amos F. F.; Ndao M.; Ponce C. B.; Evans J. S. (2011) Biochemistry 50, 8880–8887. [DOI] [PubMed] [Google Scholar]
- Nogawa C.; Baba H.; Masaoka T.; Aoki H.; Samata T. (2012) Gene 504, 84–89. [DOI] [PubMed] [Google Scholar]
- Suzuki M.; Saruwatari K.; Kogure T.; Yamamoto Y.; Nishimura T.; Kato T.; Nagasawa H. (2010) Science 325, 1388–1390. [DOI] [PubMed] [Google Scholar]
- Bewernitz M.; Gebauer D.; Long J.; Cölfen H.; Gower L. B. (2012) Faraday Discuss. 159, 291–312. [Google Scholar]
- Gal A.; Habraken H.; Gur D.; Fratzl P.; Weiner S.; Addadi L. (2013) Angew. Chem., Int. Ed. 52, 4867–4870. [DOI] [PubMed] [Google Scholar]
- Lu G. Q., and Zhao X. S. (2004) Nanoporous materials: An overview. In Nanoporous Materials: Science and Engineering (Lu G. Q., and Zhao X. S., Eds.) pp 1–12, World Scientific Publishing, Ltd., Singapore. [Google Scholar]
- Li H.; Xin H. L.; Kunitake M. E.; Keene E. C.; Muller D. A.; Estroff L. A. (2011) Adv. Funct. Mater. 21, 2028–2034. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.