Abstract
Removal of triclocarban (TCC) and triclosan (TCS) from wastewater is a function of adsorption, abiotic degradation, and microbial mineralization or transformation, reactions that are not currently controlled or optimized in the pollution control infrastructure of standard wastewater treatment. Here, we report on the levels of eight transformation products, human metabolites, and manufacturing byproducts of TCC and TCS in raw and treated sewage sludge. Two sample sets were studied: samples collected once from 14 wastewater treatment plants (WWTPs) representing nine states, and multiple samples collected from one WWTP monitored for 12 months. Time-course analysis of significant mass fluxes (α = 0.01) indicate that transformation of TCC (dechlorination) and TCS (methylation) occurred during sewage conveyance and treatment. Strong linear correlations were found between TCC and the human metabolite 2′-hydroxy-TCC (r = 0.84), and between the TCC-dechlorination products dichlorocarbanilide (DCC) and monochlorocarbanilide (r = 0.99). Mass ratios of DCC-to-TCC and of methyl-triclosan (MeTCS)-to-TCS, serving as indicators of transformation activity, revealed that transformation was widespread under different treatment regimes across the WWTPs sampled, though the degree of transformation varied significantly among study sites (α = 0.01). The analysis of sludge sampled before and after different unit operation steps (i.e., anaerobic digestion, sludge heat treatment, and sludge drying) yielded insights into the extent and location of TCC and TCS transformation. Results showed anaerobic digestion to be important for MeTCS transformation (37–74%), whereas its contribution to partial TCC dechlorination was limited (0.4–2.1%). This longitudinal and nationwide survey is the first to report the occurrence of transformation products, human metabolites, and manufacturing byproducts of TCC and TCS in sewage sludge.
Introduction
Triclocarban [3-(4-chlorophenyl)-1-(3,4-dichlorophenyl)urea, TCC] and triclosan [5-chloro-2-(2,4-dichlorophenoxy)phenol, TCS] (Supporting Information (SI) Figure S1) have been used in a plethora of consumer products (including liquid and solid soaps, toothpaste, plastics, fabrics, and clothing apparel) for their broad-range antimicrobial properties for half a century.1 As a result of their frequent and long-term elective use, TCC and TCS are now frequently found in human samples.2−5 Continuous discharge via sewage and incomplete degradation in wastewater treatment plants (WWTPs) contributes to these substances being ubiquitous in the environment and pervasive in animal tissues.6−13 TCS, TCC, and their transformation products are among the most frequently detected organic contaminants in environmental samples.6,7,11,14−16 Concerns with discharged TCC and TCS stem from the fact that both are precursors to known or presumed human carcinogens and toxicants (including chlorinated dibenzo-p-dioxins and chlorinated anilines)17,18 in addition to the parent compounds eliciting a suite of adverse health effects, and potentially influencing natural microbial ecosystems and the emergence of antibiotic-resistant strains.19−30 TCC degrades via aerobic biodegradation and photolysis into toxic chlorinated anilines.17,31,32 TCS degrades into a range of carcinogenic and toxic chlorophenols33,34 as well as dioxin-like compounds (and possibly traces of the toxic 2,3,7,8-tetrachlorodibenzo-p-dioxin).18,35,36 Due to the higher relative toxicity of chloroanilines, chlorophenols, and dioxins compared to TCC and TCS, more controlled and efficient removal is warranted of these chlorinated antimicrobials prior to their environmental discharge via effluent and biosolids (i.e., treated sewage sludge fit for application on land, in accordance with regulatory requirements of the U.S. Environmental Protection Agency (EPA), 40 CFR Part 503).
In typical conventional WWTPs, biodegradation of TCC is believed to be minimal,16,37 whereas for TCS, up to 50% can be degraded into methyl-triclosan, [MeTCS; 5-chloro-2-(2,4-dichlorophenoxy)anisole], as well as other, unknown products via microbial activity and abiotic mechanisms.8,38 Thus, a significant fraction of TCC and TCS (70–90% and 30–70%, respectively) will accumulate in sewage sludge with a lesser portion of the residual load being discharged via effluent.8,11,37,38 After discharge into surface water, TCC and TCS will partition to sediments and/or bioaccumulate in wildlife and microbiota.1,11,39−41 While TCS can be partially degraded, TCC must likely first undergo reductive dechlorination prior to being available for biodegradation of the core carbanilide structure.42 These sequential microbial reactions could potentially be leveraged as part of a biotechnological decontamination strategy in future upgrades of existing WWTP infrastructure, but presently the reactions in the respective removal pathways are minimally efficient and slow.
For TCC and its manufacturing byproduct, 3,3',4,4'-tetrachlorocarbanilide (3′Cl-TCC), these detoxification reactions are their sequential dechlorination via 4,4′-dichlorocarbanilide (DCC) and 1-(3-chlorophenyl)-3-phenylurea (MCC) into carbanilide (NCC) (reaction sequence 1),1,43 averting their undesirable breakdown into toxic chloroanilines, including 3-chloroaniline (3-CA) and/or 3,4-dichloroaniline (reaction 2) (a list of acronyms and the chemical structures are provided in the SI).17,43 The former process is speculated to occur under anaerobic, reducing conditions16 through the action of exclusively anaerobic dechlorinating microorganisms,11 analogous to anaerobic reductive dechlorination of trichloroethene.44 Previously, it has been shown that TCC dechlorination products can be detected in freshwater and brackish sediments downstream of effluent discharge sites,1,11,45 but it remains unknown whether dechlorination occurs before and/or after environmental discharge. The phase-I human metabolites of TCC, 2′-OH-TCC and 3′-OH-TCC (reaction 3), have been described previously46 and are expected to be discharged as phase-II metabolites after human use via black water into sewage. Presently, there is no evidence to suggest sources of 2′-OH-TCC and 3′-OH-TCC other than human metabolism (i.e., environmental sources).
For TCS, the detoxification mechanisms are the microbial degradation into unknown products and the reversible methylation of the free hydroxyl-moiety, both of which occur under aerobic conditions (reaction 4).43,47−51 Even though MeTCS retains some toxicity for microbial activity,52 the methylation of TCS will mitigate microbial growth inhibition and avert ultraviolet light-driven dioxin formation.52−54 MeTCS is also more persistent than its parent compound and is more prone to bioaccumulate in fish;55a more detailed (eco)toxicological evaluation of MeTCS is therefore warranted.
| 1 |
| 2 |
| 3 |
| 4 |
Even though TCC and TCS transformation mechanisms have been described previously and attributed to different stages of WWTPs, little to no research has been performed relating to their geographical distribution, significance at different sites, and stability over time. In addition, there are no reports on the abundance and prevalence of human metabolites of TCC and TCS in WWTPs. Therefore, we aimed to determine via analysis of raw and treated sewage sludge whether TCC and TCS transformation (i) occurs in sewage systems and WWTPs across the United States and is consistent within a WWTP over a 12 month period; (ii) takes place during anaerobic digestion (AD) and heat treatment or drying of the treated sewage sludge; and (iii) is dependent on factors such as environment, geography, climate, sewage-delivery system or WWTP configuration. The extent of TCC and TCS transformation via dechlorination and methylation, respectively, was determined by quantifying their transformation products in untreated sewage sludge and biosolids samples from across the United States. This approach was conceived as an alternative and stepping stone to challenging and time-consuming mass balance studies. In addition, we aimed to inform on promising sampling locations for the future enrichment or isolation of the presently unknown microbial strains performing the transformation of TCC and TCS.42
Experimental Section
Standards and Reagents
All standards and reagents were purchased in the highest purity available. Native solid standards for TCC (99%), TCS (>97%), 1-(3-chlorophenyl)-3-phenylurea (MCC), carbanilide (NCC, 98%), and 3-CA (99%) were purchased from Aldrich (Sigma-Aldrich, St. Louis, MO). 4,4′-Dichlorocarbanilide (DCC) was obtained from Oakwood Products Inc. (West Columbia, SC). Unlabeled MeTCS (99%) and the isotopically labeled 13C12-MeTCS (99%) were purchased from Cambridge Isotope Laboratories (Tewksbury, MA). 13C13-TCC (>99%) and 13C12-TCS (>99%) were obtained from Wellington Laboratories Inc. (Guelph, Ontario, Canada). Oxidative metabolites of TCC were provided by Dr. Bruce Hammock (University of California, Davis) and were manufactured as previously described.46 Their purity was verified by LC-MS/MS upon arrival in the laboratory. The chemical structures of the 10 analytes of interest are presented in SI Figure S1. LC-MS-grade (99%) methanol, water, and acetic acid were obtained from Fluka and LC-MS-grade acetone was obtained from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO). Individual stock solutions of the native and isotopically labeled compounds were prepared in methanol. All stock solutions were stored in glass vials with polytetrafluoroethylene septa at −20 °C. All glassware was washed with detergent, rinsed three times with ultrapure water, and subsequently baked at 550 °C for 4 h.
Sewage Sludge and Biosolids Samples
Most sewage sludge and biosolids samples were collected between March and June, 2009 (SI Table S1). The exceptions included biosolids (B16-1) where longer time series of samples were collected beginning in March or May, 2009, extending through the end of 2009 to early 2010. For site no. 16, both time-course data and averaged data are presented. Biosolids were collected at 14 sludge processing facilities located in nine states (AZ, IA, FL, MD, MT, NY, TX, WI, and VT), with an additional commercially available product from another plant (B16-2) purchased at a nationwide retail store. Untreated sewage sludge samples were obtained from 3 WWTPs, at two of which (nos. 15 and 16) biosolids were also collected following anaerobic digestion. Sampled WWTPs treated a broad range of wastewater flows (<0.25 to >25 million liters of wastewater per day) and employed different biosolids treatment practices.56 Most of the biosolids analyzed were designated as Class B biosolids, many of which have been treated with anaerobic digestion (SI Table S1). We relied on cooperation with WWTPs and U.S. Geological Survey employees to provide the samples studied, which were provided based on condition of nondisclosure of their source. Basic information about the WWTP operations is provided in SI Table S1. At WWTPs 6, 14, and 16, two types of biosolids products were analyzed; a Class B biosolids produced by anaerobic digestion, as well as a Class A biosolids where further treatment involved either high temperature stabilization, or extended storage, and composting. At one site (no. 6), the digested sludge was dewatered after anaerobic digestion. Biosolids from three WWTPs (nos. 7, 8, and 14) and sewage sludge from one WWTP (no. 16) were resampled at least a second time, more than a month apart; for these sites averaged data are presented. The samples were collected as discrete units, then frozen after sampling, thawed, subsampled, shipped to Arizona State University as frozen samples on dry ice in glass jars with polytetrafluoroethylene septa, stored at −80 °C, and homogenized prior to extraction.
Sample Processing and Analysis
The detailed procedures for sample extraction, sample analysis using LC-MS/MS and GC-MS/MS, data processing, and quality assurance and quality control are available in SI. Briefly, triplicate samples of wet sewage sludge samples (approximately 0.5 g) were dried, and extracted with an acidified methanol/acetone solution by sonication. The organic extract was evaporated to dryness, reconstituted, and filtered prior to analysis. All concentrations provided here are on a dry weight basis. All analytes were determined using LC-MS/MS, with the exception of MeTCS, which was quantified using GC-MS/MS.
Results
Time-Series Analysis of Contaminant Levels
The aim of the study was first to determine whether the contaminants occurred in biosolids, and second whether their levels in biosolids grab samples from a single site were consistent during a 12-month period. To achieve these aims, the 10 analytes of interest (SI Figure S1) were monitored for one year using 23 biosolids samples from a single WWTP (no. 16). NCC and 3-CA were not detected in any sample, which left eight principal analytes of interest for this study. As expected for contaminants from a common source, the concentration changes were minimal and rarely differed significantly (α = 0.01) for the parent compounds, human metabolites, and manufacturing byproducts (i.e., TCC, 2′-OH-TCC, 3′-OH-TCC, 3′-Cl-TCC, and TCS) over the course of a year as determined using a moving window analysis (SI Table S6). The percent change per time window (n = 22) (typically a two-week period) ranged from 1 to 16% (mean ± standard deviation [x̅] = 7 ± 5%) for TCC, 1–39% (x̅ = 12 ± 11%) for 2′–OH-TCC, 1–132% (x̅ = 31 ± 31%) for 3′–OH-TCC, 1–39% (x̅ = 13 ± 12%) for 3′-Cl-TCC, and 0.4–26% (x̅ = 7 ± 7%) for TCS (Figure 1), where the ranges and averages were calculated using absolute values of the concentration differences. Conversely, the changes in concentration over different sampling events were typically much more pronounced for the transformation products DCC, MCC, and MeTCS with changes ranging between 4 and 53% (x̅ = 23 ± 14%), 12–180% (x̅ = 54 ± 37%), and 1–800% (x̅ = 76 ± 172%), respectively (Figure 1). The maximum percent change occurred in the same time interval for TCC, 2′-OH-TCC, 3′-OH-TCC, and 3′-Cl-TCC (window 13) and in different windows for DCC, MCC, and MeTCS (windows 19, 8, and 13, respectively). These findings suggest that the levels of transformation products (generated via dechlorination and methylation) in biosolids was more variable compared to the parent compounds (TCC and TCS), the manufacturing byproduct (3′-Cl-TCC), and the human metabolites (2′-OH-TCC and 3′-OH-TCC), presumably due to fluctuations in environmental factors (e.g., season, operational parameters, and microbial communities). We hypothesized that if indeed transformation is dependent on location-specific environmental factors (e.g., climate/season, population or urban characteristics, sewage system and WWTP design, operational parameters, and microbial communities), then significant differences in removal should be observable in biosolids from different WWTPs across the U.S.
Figure 1.
Concentrations of TCC, and its microbial (DCC and MCC) and human metabolites (2′-OH-TCC and 3′-OH-TCC), and manufacturing byproducts (DCC and 3′-Cl-TCC), along with those for TCS and the microbial metabolite MeTCS in biosolids samples from one WWTP (no. 16) sampled during 2009–2010. The error bars represent standard deviations of triplicate extractions calculated from averages of duplicate injections per sample.
Contaminant Concentrations Across the U.S
To assess whether the transformation of TCC and TCS is site-dependent, sewage sludge and biosolids samples from 14 different WWTPs from across the United States were screened for TCS, TCC, their transformation products, human metabolites, and manufacturing byproducts. Figure 2 summarizes the distribution of average biosolids concentrations from these 14 different WWTPs. Data for 3′-OH-TCC is not shown because this chemical was detected only very rarely (at two sites only). When investigating for transformation of TCC and TCS, mass spectrometric analysis revealed that there were significant differences (α = 0.05) in the extent of dechlorination of TCC and methylation of TCS between samples from different WWTPs. The measure chosen to assess removal of TCC was the DCC/TCC ratio. Technical-grade TCC (>99%), which is commonly used in commercial applications, has a DCC-to-TCC ratio of about 0.002.45 Under the assumption that TCC dechlorination rates are slow during wastewater treatment, an increase in the DCC/TCC ratio from the 0.002 base value may be indicative of (incomplete) removal of TCC. We found that the DCC/TCC ratio in biosolids increased significantly at most locations, varying from about 0.001 ± 0.000 to 0.901 ± 0.013 in the WWTP samples (Figure 3), suggesting that the initiation of TCC dechlorination was widespread. Yet, because NCC was never detected, it remains unclear whether TCC dechlorination was indeed slow and incomplete (with no NCC formation), or whether NCC was readily degraded and thus complete dechlorination of TCC may have occurred during wastewater treatment. The samples were also screened for 3-CA, an abiotic transformation product of TCC, DCC, and MCC but that compound was not detected; lack of detection could be due to absence of 3-CA in the sample or lack of partitioning into sludge used for analysis. The TCC transformation efficiency was found to be unrelated to that of TCS, since no relationship was found between the observed DCC/TCC and MeTCS/TCS ratios (Figure 3); the latter being the measure chosen to assess transformation of TCS. In fact, one of the WWTPs where no DCC was detected (B9) had one of the highest MeTCS/TCS ratios (0.215 ± 0.020). Determination of less chlorinated triclosan derivatives was not performed because of the absence of commercially available authentic standards for identification and quantification.
Figure 2.
Box-and-whisker plot of the contaminant concentrations in biosolids from 14 locations from nine states across the United States (this study) respective to the TCC and TCS levels measured previously (i.e., shaded boxplots) in biosolids sampled during the 2001 U.S. EPA National Sewage Sludge Survey (“TCC(’01)” and “TCS(’01)”).59
Figure 3.
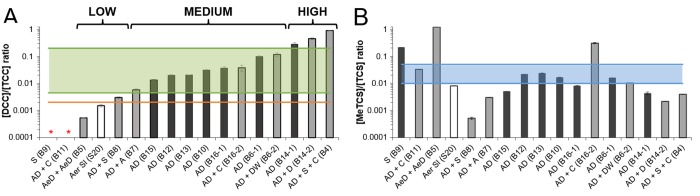
Generation of transformation products of TCC and TCS during sludge treatment presented as average mass ratios of DCC/TCC (A) and MeTCS/TCS (B) in aerobic sludge (white bar), digested sludge (dark bars), and digested sludge with additional treatment (gray bars) from 14 different WWTPs across the United States. The DCC/TCC ratios in sludge are compared to those in technical-grade TCC (>99%)(orange line = 0.002) and the range of ratios detected during a statewide survey of freshwater sediments (green box11). For two WWTPs, no DCC was detected (red asterisk). The blue box in panel B indicates the 0.01–0.05 MeTCS range typically observed in various freshwater environments.49,50,53,62 The WWTP is provided between parentheses (B# or S#). A: aging; AeD: aerobic digestion; Aer Sl: aerobic sludge; AD: anaerobic digestion; C: composting, D: drying; DW: dewatering; S: storing.
Removal by Different Processes
Whereas TCS methylation is commonly observed in aerobic environments,47 dechlorination of TCC, if occurring, is likely located in an oxygen-limiting milieu, assuming the process and microbial ecology are similar to the reductive dechlorination of trichloroethene. Hence, the anaerobic digester is a likely environment for dechlorination to occur in a conventional WWTP, due to the redox conditions required for biogas production. For this reason, contaminant levels were determined in sludge sampled before and after anaerobic digestion from two sites with medium DCC/TCC ratios (nos. 15 and 16). However, data from these two plants revealed that anaerobic digestion of the sludge resulted in a significant accumulation of TCC, TCS, and their transformation products (α = 0.01 and α = 0.05) (Figure 4). These findings suggested that these parent compounds are more persistent relative to the organic matter that is to be gasified and mineralized during the digestion process. In a third WWTP (no. 6), anaerobic digested sludge was dewatered; yet, the dewatering process resulted in only minor removal for TCC and 3-Cl′-TCC, while no significant changes were observed for the other carbanilides (Figure 4C). In a fourth WWTP (no. 14) with a high DCC/TCC ratio (Figure 3), significant removal was observed for nearly all carbanilides (Figure 4D; α = 0.01) after heat treating the digested sludge. Comparison of the occurrences of the transformation products before and after the treatment, indicated good removal for MCC (85%) compared to 49% for TCC. Conversely, the manufacturing byproduct 3′-Cl-TCC was not removed nor did it accumulate significantly after heat treatment of the digested sludge at plant no. 14 (Figure 4D). Overall, treatment of the sludge resulted in an increase in the DCC/TCC ratio for the WWTP. The observed increases were for heat drying of AD sludge: from 0.28 to 0.45 (p < 0.01; α = 0.01), dewatering of AD sludge: from 0.10 to 0.12 (p = 0.208; α = 0.01), and AD of untreated sludge at two plants: from 0.01 to 0.04 (p < 0.01; α = 0.01) and from 0.010 to 0.014 (p < 0.01; α = 0.01).
Figure 4.
Effect of different conventional sludge treatment processes on the concentrations of TCC, its microbial and human metabolites, and production byproducts along with those for TCS and MeTCS in sewage sludge and biosolids from selected WWTPs. #: number of sampled WWTP. *: p < 0.01; **: p < 0.05.
Sewage sludge and biosolids at the same four WWTPs were also screened for removal of TCS and MeTCS (Figure 4). Whereas TCS was not significantly affected by either dewatering or heat treatment (α = 0.05; p > 0.05), TCS accumulated significantly during the digestion process (α = 0.01). Conversely, MeTCS was significantly removed with all treatment processes (α = 0.05) (Figure 4) potentially suggesting either MeTCS transformation or its reversion back to TCS through hitherto unknown mechanisms. The MeTCS removal efficiencies calculated from averaged data were 58%, 37%, 74%, and 71%, during biosolids heat drying, biosolids dewatering, or AD of untreated sludge in the latter two cases, respectively.
Confirmation of Anticipated Relationships
To confirm anticipated relations between certain co-contaminants, correlation analyses were performed using the average concentrations from individual WWTPs (Figure 5). Hence, a strong relation (Pearson’s r = 0.84) was found between free TCC and 2′–OH-TCC (Figure 5A), which are both assumed to originate from sewage. To determine whether TCC dechlorination is driven by the amount of TCC present at the sampling location, we examined the data for a correlation between the concentrations of TCC and DCC. However, no significant correlation was found (Pearson’s r = 0.02) (Figure 5B), suggesting factors other than TCC concentration as potential determinants, such as microbial community composition, which was not examined in this work. Still, a strong relation between the DCC and MCC levels was revealed (Pearson’s r = 0.99) (Figure 5C), suggesting that if TCC dechlorination is initiated during standard wastewater treatment, the second step in the dechlorination of TCC will occur concomitantly, with the formation of MCC likely not being rate limiting. Thus, other factors (such as microbial community composition or redox conditions) likely are at play in the initiation of TCC dechlorination. Finally, a correlation analysis of TCS and MeTCS was performed to determine whether the levels of TCS in biosolids were a predictor of the extent of TCS methylation to MeTCS during standard wastewater treatment. Levels of TCS in biosolids did not represent an adequate predictor for the methylation of TCS (Pearson’s r = 0.01) (Figure 5D), a finding that is consistent with previous reports.47 Statistical analysis of environmental, geographical, climatic factors, sewage-delivery system or WWTP configuration with plant performance could not be performed, as release of such information might provide identifying information.
Figure 5.

Relation between co-contaminants from a common source (A), and parent compound and transformation product (B, C, and D) as plotted through their concentrations in sewage sludge from different WWTPs. Each data point represents the average of triplicate measurements of the analyte concentration in one sewage sludge sample from a WWTP. All data presented in μg/g dry weight.
Discussion
Antimicrobial Release
TCC and TCS have been documented to be ubiquitous contaminants in freshwater environments.11−13,15,45,48,50,53,57,58 In previous studies, TCC and TCS were found to be the most abundant pharmaceuticals and personal care products (PPCPs)16 in archived biosolids samples from 200158 with median concentrations (n = 5) of 29 and 13 μg/g, respectively.59 Here, TCC and TCS were found with median concentration (n = 14) of 17 and 21 μg/g, respectively. The differences between both studies (p < 0.05 and p = 0.18 for TCC and TCS, respectively; α = 0.05) likely stem from a different geographic coverage, sampling dates, usage patterns, plant design and performance, etc. (Figure 2). The typical 2:1 TCC/TCS ratio37,38 was not observed in the present study of 14 WWTPs from nine states as the TCC levels exceeded those of TCS in only 5 of the 14 WWTPs, possibly due to differences between sampling locations, as well as changes in the relative use of both chemicals over the past decade.
Contaminant Ratios in Biosolids as a Measure for Removal Efficiency
Our findings show for the first time that TCC dechlorination does not occur exclusively in freshwater and brackish sediments,1,11 but also in the sewage delivery system, the WWTP, and/or other sewage sludge treatment facilities. The sampling sites were organized in three groups: low, medium, and high (Figure 3) depending on whether their DCC/TCC ratios fell below, within, or above the range of ratios previously detected in freshwater sediments.11 In two biosolids samples, DCC was not detected and no DCC/TCC ratio could be determined. As a result, these two WWTPs could not be classified (Figure 3) since this phenomenon could be indicative of either efficient or inhibited transformation. A similar analysis was performed for TCS transformation using the MeTCS/TCS ratios at all sites (Figure 3) and showed no substantial overlap between TCC and TCS transformation efficiencies. Even though our approach was useful for generating a holistic assessment for removal of hydrophobic contaminants in the sewage system and WWTPs, it only partly provided information on what treatment stages contributed to their transformation.
TCC Transformation
Di- to nonchlorinated carbanilides (i.e., DCC, MCC, and NCC) have been observed previously in WWTPs as well as in bed sediments,1,11,45 where, substantial deviations of the DCC/TCC ratios from the expected 0.2 wt % in the estuary samples first suggested reductive dechlorination of TCC.1 Indeed, a highly efficient anaerobic reductive dechlorinating culture60 was obtained from brackish sediment with elevated DCC/TCC ratios (of up to 5.000 or 5:1).1 Whereas the latter study1 suggested TCC dechlorination in estuarine environments was highly dependent on the local milieu and microbial community, a recent study11 documented less-chlorinated congeners of TCC to be ubiquitous in WWTP-impacted freshwater sediments. It remained unclear, however, whether TCC dechlorination was limited to the sediment environment or whether the WWTPs also contribute to the mitigation of TCC contamination through its dechlorination. The present study is the first to document that TCC dechlorination can, in fact, occur significantly in the sewage system and/or WWTPs but the efficiency of the process is seemingly dependent on various WWTP-specific and geographic/climatic factors (Figure 3A). Our data also document that if the first TCC dechlorination step (from TCC to DCC) occurs, the second step (from DCC to MCC) takes place equivalently (Figure 5C). In addition, the DCC/TCC ratios observed here were in the same range and even exceeded those previously observed in sediments (Figure 3A), where contact times between the microorganisms and contaminants were inherently much longer.1,11 Assuming all lesser chlorinated carbanilides originated from TCC, the extent of TCC transformation could be estimated by calculating 1 minus the ratio of the molar concentration of TCC to the summed molar concentrations of all carbanilide congeners for the sample. Hence, we found removal efficiencies of 1.2 ± 0.01% and 1.0 ± 0.2% in the undigested sludge and 1.6 ± 0.1% and 3.2 ± 0.1% in the biosolids from two ADs (nos. 15 and 16) with medium DCC/TCC ratios (SI Table S7). More substantial removal was observed for ADs in the two WWTPs with high and medium DCC/TCC ratios (nos. 14 and 16), where in digested sludge transformation efficiencies of 29.8 ± 3% and 10.3 ± 0.2% were observed, respectively. At those sites, subsequent treatment of digested sludge with heat treatment and sludge dewatering resulted in total removal efficiencies of 35.0 ± 1.5% and 11.6 ± 0.6%, respectively. Overall, the total TCC transformation efficiencies reported here were in the same range as those reported previously for ADs in Japan.16
Comparing the removal efficiencies before and after digestion for multiple WWTPs documented that dechlorination was limited in the AD, and that transformation was highly dependent on the WWTP (2% versus 30% removal) and its specific processes (additional 5% removal due to heat treatment). By comparison, previous research determined transformation efficiencies between 40 and 94% for deep brackish sediment.1 The removal efficiencies in sediment were presumably elevated due to the extended contact time between microbiota and contaminants that allow for relatively higher abundances of the less-chlorinated carbanilides, MCC and NCC, compared to those observed in samples from the ADs (although elevated NCC levels in sediments may be the result of industrial non-TCC related sources). A previous lab-based soil study reported dechlorination of TCC to NCC, without detecting the intermediate products DCC or MCC.43 This would suggest that the first dechlorination step (from TCC to DCC) may be the rate limiting one, which is consistent with our correlation analyses in Figure 5. The same soil study also reported the detection of the hydrolysis products of TCC, mono- and dichloroaniline, during simulated biosolids amendments.43 Yet, 3-CA was not detected in the sewage sludge or biosolids samples of the present study, likely due to its known aqueous mobility and its rapid biodegradation.43
TCS Transformation
Mass-balance studies document that TCS degrades to a significant extent in the wastewater infrastructure and freshwater environment via biodegradation and photolysis, respectively.53,61 Yet, the formation of MeTCS via biological methylation in different WWTP stages8,48,49,62 apparently limits the extent of total TCS removal because the transformation product is much more resistant to photolysis.48,53 MeTCS is also more lipophilic, persistent, and bioaccumulative relative to TCS, and hence, the environmental behavior and fate of the two compounds is vastly different.48,53 While aqueous TCS is readily degraded in the environment, the relative fraction of MeTCS increases, gradually peaking during summer and nearly equaling the residual TCS concentration in the top layers of surface water.53 Despite the slow reversion back to the parent compound in fish liver and intestine, MeTCS will bioaccumulate upon chronic exposure55 posing a potential health threat to humans as a result of fish consumption. MeTCS is typically found in much lower concentrations than those of TCS with MeTCS/TCS ratios between about 0.01 and 0.05 for effluent, surface water, sludge, and sediment.49,50,53,62 The data in this study, however, documented that the MeTCS/TCS ratio can largely exceed the previously observed thresholds reported for effluent, surface water, sludge, and sediment as it attained 0.30 in commercial compost (B16-2) and 1.21 in aerobically digested biosolids (B5) (Figure 3). Taken together, this study and previous work show that the MeTCS fraction can become substantial in environments where TCS is readily degraded relative to MeTCS (such as at the air–liquid interface of lakes) as well as in aerobic environments with high microbial activity (such as aerobic composters). This transformation of TCS into MeTCS will ultimately increase the environmental persistence of triclosan because the methylation increases the bioaccumulation potential and limits the biodegradation of the total of TCS congeners (i.e., the sum of MeTCS and TCS).
Some environments exhibit a moderately efficient transformation of TCS into MeTCS as well as other unknown transformation products, even without deliberate attempts to optimize these processes. Future research needs to focus on providing a more comprehensive (eco)toxicological evaluation of the transformation products of TCS (including MeTCS), such that efforts may be made to allow for a well-designed, sustainable removal of TCS prior to the release of treated wastewater. Ultimately, the favored strategy for mitigating environmental contamination by TCS will depend on the cost of the process, the relative rates of transformation into their respective transformation products, the relative masses of the transformation products, and the corresponding relative toxicity of the mixture. Such an analysis needs to be holistic, and take into account the environmental fate of the contaminants, their toxicity as well as that of their transformation products, and their persistence.
Even though MeTCS was not enriched in the different treatment stages of a conventional WWTP,8,62 another study showed MeTCS increased during the first 120 h in aerobic sludge cultures.47 Taken together, these findings suggest MeTCS generation likely occurs upstream of the WWTP in the sewage delivery system, which would make the process of methylating triclosan difficult to control. In this study, MeTCS concentrations were found to decrease during different processes (AD, dewatering, and heat treatment) (Figure 4). The decrease of MeTCS concentration after sludge treatment processes coincided with a slight, but significant increase in the TCS concentrations in both digesters (SI Table S7). We emphasize, however, that there was no indication that MeTCS was converted back to TCS, albeit a possibility, and that the removal efficiencies presented here are expected to have slight changes over time (Figure 1). Whereas a significant relation was previously identified between both the rate constants and the final MeTCS concentration with TCS concentration in aerobic experiments,47 no such relation was found for the ADs here (Figure 5D). Finally, our data are consistent with previous research,47,62 since no TCS removal was found to occur in the AD.
Human Metabolism and Excretion
The strong relation (Pearson’s r = 0.84) found between TCC and its human metabolite (Figure 5A) was expected, since free TCC and 2′-OH-TCC are assumed to have the same source, that is., both originating from human use of antimicrobial products. Further, the present study was consistent with previous research on TCC metabolites in human urine,46 in that the same oxidative TCC metabolites (2′-OH-TCC and 3′-OH-TCC) were found. In the present work, the ratios of 2′-OH-TCC/TCC ranged from 0.008 to 0.045 (x̅ = 0.020 ± 0.010) compared to approximately 0.50–1.10 from human excretion via urine after conjugate hydrolysis.46 The reason for this shift can presumably be attributed to dilution of black water containing excreted TCC metabolites with elevated volumes of discharged greywater containing predominantly unmetabolized TCC. The biosolids samples were found to seldom contain detectable concentrations of 3′-OH-TCC, which is consistent with a previous study reporting its detection in human urine is rare.46 Yet, it is currently unknown whether these hydroxylated metabolites are solely low-abundance human metabolites or whether they in part constitute environmental transformation products.
Adsorption of TCC and TCS to sludge will substantially decrease the aqueous concentrations of these antimicrobials and thus, strike a balance between (1) permitting biotransformation by serving as a constant source of growth substrate1,42 and (2) reducing microbial toxicity to subinhibitory levels.26,27,52 Yet, large differences in TCC and TCS transformation at the various WWTPs were found to be independent of their concentrations in sludge and were hypothesized to be due to different microbial communities and the site-specific processes and fluctuations. Future research will need to (i) identify the best combination of variables at WWTPs to optimize methylation and dechlorination, (ii) determine whether transformation to MeTCS or TCS is preferable for mitigating human health hazards by comparing the rate of toxicant formation in both scenarios, (iii) and study the processes that lead to highly variable transformation rates during sewage delivery and treatment.
Acknowledgments
This work was supported in part by the National Institute of Environmental Health Sciences (NIEHS) Award Numbers R01ES015445 and 1R01ES020889 and their respective supplements, and by the USGS Toxics Substances Hydrology Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or the National Institutes of Health (NIH). We thank the many WWTP operators for making samples available and providing specific information pertaining to their WWTP and the numerous USGS personnel that collected samples for this project. We thank Tracy Yager, U.S. Geological Survey, for critically reading the manuscript. The use of trade, firm, or brand names in this paper is for identification purposes only and does not constitute endorsement by the authors or the U.S. Geological Survey.
Supporting Information Available
A detailed description of the extraction procedure is provided in Supporting Information. The chemical structures of all analytes of interest are presented in Figure S1. Detailed information about the WWTPs and samples is provided in Table S1, on the analytical methods in Tables S2–S5, on the long-term monitoring and transformation of chlorinated antimicrobials in Tables S6 and S7, respectively. This material is available free of charge via the Internet at http://pubs.acs.org/.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Miller T. R.; Heidler J.; Chillrud S. N.; DeLaquil A.; Ritchie J. C.; Mihalic J. N.; Bopp R.; Halden R. U. Fate of triclosan and evidence for reductive dechlorination of triclocarban in estuarine sediments. Environ. Sci. Technol. 2008, 42124570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmyr M.; Adolfsson-Erici M.; McLachlan M. S.; Sandborgh-Englund G. Triclosan in plasma and milk from Swedish nursing mothers and their exposure via personal care products. Sci. Total Environ. 2006, 372187–93. [DOI] [PubMed] [Google Scholar]
- Geens T.; Neels H.; Covaci A. Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 2012, 877796–802. [DOI] [PubMed] [Google Scholar]
- Toms L. M.; Allmyr M.; Mueller J. F.; Adolfsson-Erici M.; McLachlan M.; Murby J.; Harden F. A. Triclosan in individual human milk samples from Australia. Chemosphere 2011, 85111682–6. [DOI] [PubMed] [Google Scholar]
- Ye X.; Zhou X.; Furr J.; Ahn K. C.; Hammock B. D.; Gray E. L.; Calafat A. M. Biomarkers of exposure to triclocarban in urine and serum. Toxicology 2011, 2861–369–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney C. A.; Furlong E. T.; Kolpin D. W.; Burkhardt M. R.; Zaugg S. D.; Werner S. L.; Bossio J. P.; Benotti M. J. Bioaccumulation of pharmaceuticals and other anthropogenic waste indicators in earthworms from agricultural soil amended with biosolid or swine manure. Environ. Sci. Technol. 2008, 4261863–70. [DOI] [PubMed] [Google Scholar]
- Langdon K. A.; Warne M. S.; Smernik R. J.; Shareef A.; Kookana R. S. Field dissipation of 4-nonylphenol, 4-t-octylphenol, triclosan and bisphenol A following land application of biosolids. Chemosphere 2012, 86101050–8. [DOI] [PubMed] [Google Scholar]
- Lozano N.; Rice C. P.; Ramirez M.; Torrents A. Fate of Triclocarban, Triclosan and Methyltriclosan during wastewater and biosolids treatment processes. Water Res. 2013, 47134519–27. [DOI] [PubMed] [Google Scholar]
- Pannu M. W.; O’Connor G. A.; Toor G. S. Toxicity and bioaccumulation of biosolids-borne triclosan in terrestrial organisms. Environ. Toxicol. Chem. 2012, 313646–53. [DOI] [PubMed] [Google Scholar]
- Pannu M. W.; Toor G. S.; O’Connor G. A.; Wilson P. C. Toxicity and bioaccumulation of biosolids-borne triclosan in food crops. Environ. Toxicol. Chem. 2012, 3192130–7. [DOI] [PubMed] [Google Scholar]
- Venkatesan A. K.; Pycke B. F.; Barber L. B.; Lee K. E.; Halden R. U. Occurrence of triclosan, triclocarban, and its lesser chlorinated congeners in Minnesota freshwater sediments collected near wastewater treatment plants. J. Hazard. Mater. 2012, 229–230, 29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate F. M. Jr.; Schulwitz S. E.; Stevens K. J.; Venables B. J. Bioconcentration of triclosan, methyl-triclosan, and triclocarban in the plants and sediments of a constructed wetland. Chemosphere 2012, 883323–9. [DOI] [PubMed] [Google Scholar]
- Zhao J. L.; Zhang Q. Q.; Chen F.; Wang L.; Ying G. G.; Liu Y. S.; Yang B.; Zhou L. J.; Liu S.; Su H. C.; Zhang R. Q. Evaluation of triclosan and triclocarban at river basin scale using monitoring and modeling tools: Implications for controlling of urban domestic sewage discharge. Water Res. 2013, 471395–405. [DOI] [PubMed] [Google Scholar]
- Barnes K. K.; Kolpin D. W.; Furlong E. T.; Zaugg S. D.; Meyer M. T.; Barber L. B. A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States—I) groundwater. Sci. Total Environ. 2008, 4022–3192–200. [DOI] [PubMed] [Google Scholar]
- Kolpin D. W.; Furlong E. T.; Meyer M. T.; Thurman E. M.; Zaugg S. D.; Barber L. B.; Buxton H. T. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams, 1999–2000: A national reconnaissance. Environ. Sci. Technol. 2002, 3661202–11. [DOI] [PubMed] [Google Scholar]
- Narumiya M.; Nakada N.; Yamashita N.; Tanaka H. Phase distribution and removal of pharmaceuticals and personal care products during anaerobic sludge digestion. J. Hazard Mater. 2013, 260, 305–12. [DOI] [PubMed] [Google Scholar]
- Gledhill W. E. Biodegradation of 3,4,4′-triclocarbanilide, TCC, in sewage and activated-sludge. Water Res. 1975, 197597. [Google Scholar]
- Anger C. T.; Sueper C.; Blumentritt D. J.; McNeill K.; Engstrom D. R.; Arnold W. A. Quantification of triclosan, chlorinated triclosan derivatives, and their dioxin photoproducts in lacustrine sediment cores. Environ. Sci. Technol. 2013, 4741833–43. [DOI] [PubMed] [Google Scholar]
- Ahn K. C.; Zhao B.; Chen J.; Cherednichenko G.; Sanmarti E.; Denison M. S.; Lasley B.; Pessah I. N.; Kultz D.; Chang D. P.; Gee S. J.; Hammock B. D. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ. Health Perspect. 2008, 11691203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. E.; Franko J.; Kashon M. L.; Anderson K. L.; Hubbs A. F.; Lukomska E.; Meade B. J. Exposure to triclosan augments the allergic response to ovalbumin in a mouse model of asthma. Toxicol. Sci. 2013, 132196–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.; Ahn K. C.; Gee N. A.; Ahmed M. I.; Duleba A. J.; Zhao L.; Gee S. J.; Hammock B. D.; Lasley B. L. Triclocarban enhances testosterone action: A new type of endocrine disruptor?. Endocrinology 2008, 14931173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherednichenko G.; Zhang R.; Bannister R. A.; Timofeyev V.; Li N.; Fritsch E. B.; Feng W.; Barrientos G. C.; Schebb N. H.; Hammock B. D.; Beam K. G.; Chiamvimonvat N.; Pessah I. N. Triclosan impairs excitation-contraction coupling and Ca2+ dynamics in striated muscle. Proc. Natl. Acad. Sci. U.S.A. 2012, 1094016393–16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton E. M. R.; Todd M.; Dowd J. B.; Aiello A. E. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003–2006. Environ. Health Perspect. 2011, 1193390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James M. O.; Li W.; Summerlot D. P.; Rowland-Faux L.; Wood C. E. Triclosan is a potent inhibitor of estradiol and estrone sulfonation in sheep placenta. Environ. Int. 2010, 368942–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. R.; Navone R.; Larson E. L. An unusual epidemic of methemoglobinemia. Pediatrics 1963, 31, 222–225. [PubMed] [Google Scholar]
- Pycke B. F.; Vanermen G.; Monsieurs P.; De Wever H.; Mergeay M.; Verstraete W.; Leys N. Toxicogenomic response of Rhodospirillum rubrum S1H to the micropollutant triclosan. Appl. Environ. Microbiol. 2010, 76113503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycke B. F.; Crabbe A.; Verstraete W.; Leys N. Characterization of triclosan-resistant mutants reveals multiple antimicrobial resistance mechanisms in Rhodospirillum rubrum S1H. Appl. Environ. Microbiol. 2010, 76103116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury B.; Scott J.; Rosi-Marshall E. J.; Kelly J. J. Triclosan exposure increases triclosan resistance and influences taxonomic composition of benthic bacterial communities. Environ. Sci. Technol. 2013, 47158923–30. [DOI] [PubMed] [Google Scholar]
- Ciusa M. L.; Furi L.; Knight D.; Decorosi F.; Fondi M.; Raggi C.; Coelho J. R.; Aragones L.; Moce L.; Visa P.; Freitas A. T.; Baldassarri L.; Fani R.; Viti C.; Orefici G.; Martinez J. L.; Morrissey I.; Oggioni M. R.; Consortium B. A novel resistance mechanism to triclosan that suggests horizontal gene transfer and demonstrates a potential selective pressure for reduced biocide susceptibility in clinical strains of Staphylococcus aureus. Int. J. Antimicrob. Agents 2012, 403210–20. [DOI] [PubMed] [Google Scholar]
- Aiello A. E.; Larson E. L.; Levy S. B. Consumer antibacterial soaps: Effective or just risky?. Clin. Infect. Dis. 2007, 45Suppl 2S137–47. [DOI] [PubMed] [Google Scholar]
- Chhabra R. S.; Huff J. E.; Haseman J. K.; Elwell M. R.; Peters A. C. Carcinogenicity of p-chloroaniline in rats and mice. Food Chem. Toxicol. 1991, 292119–24. [DOI] [PubMed] [Google Scholar]
- Ding S. L.; Wang X. K.; Jiang W. Q.; Meng X.; Zhao R. S.; Wang C.; Wang X. Photodegradation of the antimicrobial triclocarban in aqueous systems under ultraviolet radiation. Environ. Sci. Pollut. Res. Int. 2013, 2053195–201. [DOI] [PubMed] [Google Scholar]
- Son H. S.; Ko G.; Zoh K. D. Kinetics and mechanism of photolysis and TiO2 photocatalysis of triclosan. J. Hazard. Mater. 2009, 1662–3954–960. [DOI] [PubMed] [Google Scholar]
- Sanchez-Prado L.; Llompart M.; Lores M.; Garcia-Jares C.; Bayona J. M.; Cela R. Monitoring the photochemical degradation of triclosan in wastewater by UV light and sunlight using solid-phase microextraction. Chemosphere 2006, 6581338–47. [DOI] [PubMed] [Google Scholar]
- Buth J. M.; Grandbois M.; Vikesland P. J.; McNeill K.; Arnold W. A. Aquatic photochemistry of chlorinated triclosan derivatives: Potential source of polychlorodibenzo-p-dioxins. Environ. Toxicol. Chem. 2009, 28122555–63. [DOI] [PubMed] [Google Scholar]
- Aranami K.; Readman J. W. Photolytic degradation of triclosan in freshwater and seawater. Chemosphere 2007, 6661052–6. [DOI] [PubMed] [Google Scholar]
- Heidler J.; Sapkota A.; Halden R. U. Partitioning, persistence, and accumulation in digested sludge of the topical antiseptic triclocarban during wastewater treatment. Environ. Sci. Technol. 2006, 40113634–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidler J.; Halden R. U. Mass balance assessment of triclosan removal during conventional sewage treatment. Chemosphere 2007, 662362–9. [DOI] [PubMed] [Google Scholar]
- Chalew T. E.; Halden R. U. Environmental exposure of aquatic and terrestrial biota to triclosan and triclocarban. J. Am. Water Works Assoc. 2009, 4514–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. P.; Paesani Z. J.; Chalew T. E.; Halden R. U. Bioaccumulation of triclocarban in Lumbriculus variegatus. Environ. Toxicol. Chem. 2009, 28122580–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coogan M. A.; Edziyie R. E.; La Point T. W.; Venables B. J. Algal bioaccumulation of triclocarban, triclosan, and methyl-triclosan in a North Texas wastewater treatment plant receiving stream. Chemosphere 2007, 67101911–8. [DOI] [PubMed] [Google Scholar]
- Miller T. R.; Colquhoun D. R.; Halden R. U. Identification of wastewater bacteria involved in the degradation of triclocarban and its non-chlorinated congener. J. Hazard. Mater. 2010, 1831–3766–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J. W.; Xia K. Fate of triclosan and triclocarban in soil columns with and without biosolids surface application. Environ. Toxicol. Chem. 2012, 312262–9. [DOI] [PubMed] [Google Scholar]
- Kastner M. Reductive dechlorination of trichloroethylenes and tetrachloroethylenes depends on transition from aerobic to anaerobic conditions. Appl. Environ. Microb. 1991, 5772039–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota A.; Heidler J.; Halden R. U. Detection of triclocarban and two co-contaminating chlorocarbanilides in US aquatic environments using isotope dilution liquid chromatography tandem mass spectrometry. Environ. Res. 2007, 103121–9. [DOI] [PubMed] [Google Scholar]
- Schebb N. H.; Inceoglu B.; Ahn K. C.; Morisseau C.; Gee S. J.; Hammock B. D. Investigation of human exposure to triclocarban after showering and preliminary evaluation of its biological effects. Environ. Sci. Technol. 2011, 4573109–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.; Nielsen J. L.; Furgal K.; Liu Y.; Lolas I. B.; Bester K. Biodegradation of triclosan and formation of methyl-triclosan in activated sludge under aerobic conditions. Chemosphere 2011, 844452–6. [DOI] [PubMed] [Google Scholar]
- Balmer M. E.; Poiger T.; Droz C.; Romanin K.; Bergqvist P. A.; Muller M. D.; Buser H. R. Occurrence of methyl triclosan, a transformation product of the bactericide triclosan, in fish from various lakes in Switzerland. Environ. Sci. Technol. 2004, 382390–5. [DOI] [PubMed] [Google Scholar]
- Bester K. Triclosan in a sewage treatment process--balances and monitoring data. Water Res. 2003, 37163891–6. [DOI] [PubMed] [Google Scholar]
- Bester K. Fate of triclosan and triclosan-methyl in sewage treatment plants and surface waters. Arch. Environ. Contam. Toxicol. 2005, 4919–17. [DOI] [PubMed] [Google Scholar]
- Federle T. W.; Kaiser S. K.; Nuck B. A. Fate and effects of triclosan in activated sludge. Environ. Toxicol. Chem. 2002, 2171330–7. [PubMed] [Google Scholar]
- Farre M.; Asperger D.; Kantiani L.; Gonzalez S.; Petrovic M.; Barcelo D. Assessment of the acute toxicity of triclosan and methyl triclosan in wastewater based on the bioluminescence inhibition of Vibrio fischeri. Anal. Bioanal. Chem. 2008, 39081999–2007. [DOI] [PubMed] [Google Scholar]
- Lindstrom A.; Buerge I. J.; Poiger T.; Bergqvist P. A.; Muller M. D.; Buser H. R. Occurrence and environmental behavior of the bactericide triclosan and its methyl derivative in surface waters and in wastewater. Environ. Sci. Technol. 2002, 36112322–9. [DOI] [PubMed] [Google Scholar]
- Clayborn A. B.; Toofan S. N.; Champlin F. R. Influence of methylation on the antibacterial properties of triclosan in Pasteurella multocida and Pseudomonas aeruginosa variant strains. J. Hosp. Infect. 2011, 772129–33. [DOI] [PubMed] [Google Scholar]
- James M. O.; Marth C. J.; Rowland-Faux L. Slow O-demethylation of methyl triclosan to triclosan, which is rapidly glucuronidated and sulfonated in channel catfish liver and intestine. Aquat. Toxicol. 2012, 124–125, 72–82. [DOI] [PubMed] [Google Scholar]
- Brownawell B. J.; Kinney C. A.; Doherty A. C.; Li X.; Ruggieri J. P.; McHugh D.; Kolpin D. W.; Pycke B. F.; Halden R. U.; Furlong E. T.. Quaternary ammonium compounds in U.S. biosolids and sewage sludges: Compositions and concentrations on a national scale.Unpublished data.
- Adolfsson-Erici M.; Pettersson M.; Parkkonen J.; Sturve J. Triclosan, a commonly used bactericide found in human milk and in the aquatic environment in Sweden. Chemosphere 2002, 469–101485–9. [DOI] [PubMed] [Google Scholar]
- McClellan K.; Halden R. U. Pharmaceuticals and personal care products in archived U.S. biosolids from the 2001 EPA National Sewage Sludge Survey. Water Res. 2010, 442658–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari B. P.; Halden R. U. Validation of mega composite sampling and nationwide mass inventories for 26 previously unmonitored contaminants in archived biosolids from the U.S National Biosolids Repository. Water Res. 2012, 46154814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv-El M.; Delgado A. G.; Yao Y.; Kang D. W.; Nelson K. G.; Halden R. U.; Krajmalnik-Brown R. Development and characterization of DehaloR̂2, a novel anaerobic microbial consortium performing rapid dechlorination of TCE to ethene. Appl. Microbiol. Biotechnol. 2011, 9251063–71. [DOI] [PubMed] [Google Scholar]
- Heidler J.; Halden R. U. Meta-analysis of mass balances examining chemical fate during wastewater treatment. Environ. Sci. Technol. 2008, 42176324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAvoy D. C.; Schatowitz B.; Jacob M.; Hauk A.; Eckhoff W. S. Measurement of triclosan in wastewater treatment systems. Environ. Toxicol. Chem. 2002, 2171323–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





