Abstract

Iron–sulfur clusters are ubiquitous protein cofactors with critical cellular functions. The mitochondrial Fe–S assembly complex, which consists of the cysteine desulfurase NFS1 and its accessory protein (ISD11), the Fe–S assembly protein (ISCU2), and frataxin (FXN), converts substrates l-cysteine, ferrous iron, and electrons into Fe–S clusters. The physiological function of FXN has received a tremendous amount of attention since the discovery that its loss is directly linked to the neurodegenerative disease Friedreich’s ataxia. Previous in vitro results revealed a role for human FXN in activating the cysteine desulfurase and Fe–S cluster biosynthesis activities of the Fe–S assembly complex. Here we present radiolabeling experiments that indicate FXN accelerates the accumulation of sulfur on ISCU2 and that the resulting persulfide species is viable in the subsequent synthesis of Fe–S clusters. Additional mutagenesis, enzyme kinetic, UV–visible, and circular dichroism spectroscopic studies suggest conserved ISCU2 residue C104 is critical for FXN activation, whereas C35, C61, and C104 are all essential for Fe–S cluster formation on the assembly complex. These results cannot be fully explained by the hypothesis that FXN functions as an iron donor for Fe–S cluster biosynthesis, and further support an allosteric regulator role for FXN. Together, these results lead to an activation model in which FXN accelerates persulfide formation on NFS1 and favors a helix-to-coil interconversion on ISCU2 that facilitates the transfer of sulfur from NFS1 to ISCU2 as an initial step in Fe–S cluster biosynthesis.
Sulfur is a critical element for all life forms and is found in a variety of protein cofactors, including molybdopterins, lipoic acid, thiamin, biotin, and iron–sulfur (Fe–S) clusters. Even though the functions of these cofactors are well understood, mechanistic details about how sulfur is incorporated into these biomolecules are just emerging.1,2 Bacteria, archaea, and eukaryotic organelles use one or more of the ISC, NIF, and SUF systems for the biosynthesis of Fe–S clusters, and a common early step for these pathways is the PLP-dependent degradation of l-cysteine to l-alanine by a cysteine desulfurase and the generation of a cysteine desulfurase-bound persulfide species. This activated sulfur is reductively cleaved by distinct acceptor proteins for the biosynthesis of sulfur-containing cofactors and modification of tRNA.1 Redox agents such as cysteine and DTT (in vitro) can also release the terminal sulfur of the persulfide in an often rate-determining step for the cysteine desulfurase that produces hydrogen sulfide.3,4
Studies of the ISC Fe–S assembly system have yet to identify intermediates on the Fe–S assembly complex during cluster biosynthesis or establish if sulfur is viably transferred from the cysteine desulfurase to the scaffold protein before, after, or during iron incorporation. The persulfide intermediate formed on Escherichia coli cysteine desulfurase IscS5 is at residue C328 (equivalent to C381 in human NFS1), and the transfer of sulfur to the scaffold protein IscU can be monitored by radiolabeling and mass spectrometry; however, the persulfide (or polysulfide) species that were bound to IscU did not appear to be viable in Fe–S cluster formation.6−8 IscS C328 is present on a flexible loop that could potentially traverse the ∼30 Å distance between an IscS substrate–PLP adduct and conserved cysteine residues of the scaffold IscU, C37, C63, and C106.9,10 Consistent with this proposed flexibility, IscS C328 can form disulfide cross-links with IscU residues C378 and C63;11 however, a role for IscU C63 in the stimulation of sulfide production by IscS in the presence of reducing agents is controversial.11−13 Proposals for which IscU residues act as sulfane sulfur acceptors include C63,11 C106,14 either C37 or C63,10 and any of the three conserved cysteines.8 Experimental factors contributing to these different proposals include changes in the binding affinities of IscU variants for IscS, the facile reactivity of persulfide species with thiol-containing molecules, and differences in experimental design such as bypassing IscS (chemical reconstitution), using catalytic amounts of IscS, or using conditions consistent with the IscS–IscU complex.
In eukaryotes, recent evidence suggests Fe–S cluster biosynthesis is catalyzed by an assembly complex that exists in at least two forms: the mostly inactive SDU complex, which contains subunits NFS1, ISD11, and ISCU2, and the activated SDUF complex, which additionally contains frataxin (FXN).15,16 In humans, the 94 kDa homodimeric cysteine desulfurase NFS1 (homologue of IscS)3,17 provides sulfur for Fe–S cluster biosynthesis. ISD11 is a eukaryote-specific protein that interacts with, stabilizes, and may activate NFS1.18−23 Cluster assembly is templated by the scaffold protein ISCU2 (homologue of IscU), whereas FXN stimulates the cysteine desulfurase and Fe–S cluster assembly reactions.15,24−26 Defects in FXN are linked to the neurodegenerative disease Friedreich’s ataxia (FRDA),27,28 and FXN variants encoded by FRDA missense mutations have a compromised ability to bind and activate the Fe–S assembly complex.24,25
The primary models for the role of FXN in Fe–S cluster biosynthesis are as an iron donor and as an allosteric activator that participates in sulfur transfer chemistry. We provide experiments to test this second model. The ability of FXN to enhance the cysteine desulfurase and Fe–S assembly activities implies that it is involved in sulfur mobilization and/or transfer chemistry from the cysteine desulfurase NFS1 to the scaffold protein ISCU2. A 35S radiolabeling experiment using Saccharomyces cerevisiae mitochondria revealed a NFS1 persulfide species under iron-depleted conditions and radiolabeled ferredoxin under iron-replete conditions.23 However, few details about the eukaryotic sulfur hand-off mechanism from NFS1 to ISCU2 (see Figure 1 for a model of the human NFS1–ISCU2 complex) and the role of FXN in this process are known. One advantage of this experimental system compared to the bacterial ISC systems is the significant rate enhancement of the cysteine desulfurase reaction upon FXN binding. Here we build upon this observation and present experiments that support a model in which FXN stabilizes a conformation that both accelerates the formation of persulfide on NFS1 and also interprotein sulfur transfer from NFS1 to residue C104 on ISCU2 (equivalent to E. coli IscU C106) as an early step in Fe–S cluster biosynthesis. Furthermore, we establish that the persulfide formed on ISCU2 in the sulfur transfer reaction is viable in a subsequent Fe–S cluster synthesis reaction, consistent with a sulfur-first mechanism.
Figure 1.

Human NFS1–ISCU2 complex modeled from the crystal structure of the analogous IscS–IscU complex (Protein Data Bank entry 3LVL). NFS1 subunits are colored green and cyan, whereas ISCU2 is colored magenta.
Experimental Procedures
Protein Expression and Purification
Eight human ISCU2 cysteine variants (C96S, C35A, C35S, C61A, C61S, C104A, C104S, and C35A/C61A) were created by QuikChange site-directed mutagenesis (Stratagene) using a pET11a-ISCU2 plasmid template,15 and the mutation sites were confirmed by DNA sequencing (Gene Technologies Lab at Texas A&M University). The resulting plasmids containing ISCU2 with the desired mutations were transformed into BL21(DE3) cells and grown at 37 °C until the OD600 reached 0.6. Protein expression was induced at 16 °C with 0.5 mM IPTG, and the cells were harvested 16 h later. The ISCU2 variants along with human NFS1-ISD11 (SD) and FXN were purified as previously described15 with the exception of adding 10% glycerol to the buffers for the C61A and C35A/C61A variants.
A pET9a plasmid (Novagen) encoding human ferredoxin (FDX, gift from J. Markley)29 was transformed into E. coli BL21(DE3) cells and grown at 37 °C until the OD600 reached 0.6. Expression was induced for 16 h with 0.4 mM IPTG, 1 mM cysteine, and 0.1 mg/mL ferric ammonium citrate. Cells were lysed by sonication, and the supernatant was loaded onto an anion exchange column (26/20 POROS 50HQ, Applied Biosystems) and eluted with a linear gradient from 0 to 1000 mM NaCl in 50 mM Tris (pH 7.5). Fractions containing FDX were further purified on a Sephacryl S100 (26/60, GE Healthcare) size exclusion column equilibrated in 50 mM Tris (pH 7.4) and 50 mM NaCl. Apo FDX was prepared by precipitating the purified FDX with a 10% trichloroacetic acid solution containing 10 mM DTT on ice for 10 min, after which the sample was pelleted by centrifugation.30 The protein pellet was rinsed twice with water and then resuspended anaerobically (mBraun glovebox, ∼12 °C and <1 ppm oxygen) in buffer A [50 mM Tris (pH 8.0) and 250 mM NaCl].30 The protein concentration was determined by the Bradford method for reconstituted FDX using an extinction coefficient (ε280) of 2980 M–1 cm–1 for apo FDX.
SDS–PAGE Analysis of Sulfur Transfer
Reaction mixtures (30 μL) for monitoring sulfur transfer included 3 μM SD, 9–120 μM ISCU2, buffer A, and either 0 or 9 μM FXN. A similar experiment was performed in which FRDA FXN variants N146K, Q148R, I154F, W155R, and R165C (each at 9 μM), which were purified as previously described,24,25 were substituted for native FXN. “Hot” l-cysteine (100 μM) was prepared by adding 50 Ci/mmol [35S]cysteine (American Radiolabeled Chemicals Inc.) to a 1 mM “cold” cysteine stock solution. The hot l-cysteine was added to the samples and reacted for 2 min at 37 °C, and the reactions were terminated by centrifugation through a Micro Bio-Spin P-6 gel filtration column (Bio-Rad). The spin column flow-through was combined with nonreducing SDS–PAGE sample loading buffer and then loaded on a nonreducing 14% SDS–PAGE gel. The gel was dried on chromatography paper in a gel-drying oven at 60 °C under vacuum before a 12 h exposure to a phosphor screen. Incorporation of the 35S label was visualized using a Phosphorimager (Typhoon Trio, GE Healthcare).
Tracking Sulfur from l-Cysteine through the SDUF Complex to a Fe–S Cluster on FDX
To test if the 35S radiolabel from the substrate l-cysteine can be transferred to ISCU2 and then to the [2Fe-2S] cluster of FDX, a sample that contained 80 μM SD, 240 μM ISCU2, 240 μM FXN, and buffer A in a total volume of 400 μL was prepared. The reaction was initiated via the addition of 20 Ci/mmol l-[35S]cysteine and allowed to proceed for 40 min anaerobically at 10 °C. The sample was then loaded onto a 1 mL HisTrap HP column (GE Healthcare) equilibrated in 50 mM Tris (pH 7.5), 200 mM NaCl, and 5 mM imidazole and eluted with a linear gradient from 5 to 500 mM imidazole. To evaluate [35S]persulfide incorporation, 30 μL of each fraction from the 1 mL HisTrap HP column was centrifuged through a Micro Bio-Spin P-6 gel filtration column (Bio-Rad), combined with 8 μL of nonreducing SDS gel loading dye, and the proteins were separated on a 14% SDS–PAGE gel that was dried, exposed, and imaged as described above. Fractions containing labeled NFS1 and ISCU2 were concentrated to 70 μL, of which 20 μL was analyzed for SDUF complex formation using a 6.5% native gel.15 The remaining 50 μL of the radiolabeled SDUF complex was incubated anaerobically at 10 °C with 600 μM Fe(NH4)2(SO4)2, 160 μM l-cysteine, and 1 mM DTT for 1 h. After incubation, the excess reaction components were removed using a Micro Bio-Spin P-6 column that was buffer exchanged into buffer A. The material that flowed through the Bio-Spin P-6 column was incubated with 200 μM apo FDX anaerobically at 10 °C for 1 h. The sample was then loaded and eluted from a 1 mL HisTrap HP column as described above, except that the absorbance was monitored at 405 nm rather than 280 nm. The flow-through fractions corresponding to non-His-tagged FDX were concentrated to a volume of 20 μL before being loaded onto a nonreducing 6.5% native gel.
In a separate experiment, we assessed the ability of reductants to cleave persulfide species from the SDUF complex. Reaction mixtures (30 μL) that included 5 μM SD, 15 μM ISCU2, and 15 μM FXN (SDUF sample) or just 15 μM ISCU2 were prepared in 50 mM HEPES (pH 7.5) and 250 mM NaCl and reacted with 100 μM l-[35S]cysteine (described above) for 4 min at 14 °C. The samples were then treated with 10 mM reductant (DTT, GSH, BME, or TCEP) for 1 min, and the reactions were terminated by centrifugation through a Micro Bio-Spin P-6 gel filtration column (Bio-Rad). The spin column flow-through was combined with nonreducing SDS–PAGE sample loading buffer and then loaded on a nonreducing 14% SDS–PAGE gel. The incorporation of the radiolabel was analyzed with a phosphorimager as described above.
Binding of ISCU2 to and Stimulation of the Fe–S Assembly Complex
Protein titrations, monitored by cysteine desulfurase activity measurements, were performed to determine the number of ISCU2 and FXN equivalents to overcome any loss of binding affinity for the SDUF complex due to ISCU2 mutations. For the titration of ISCU2 variants, the reactions were initiated with 100 μM l-cysteine and included 0.5 μM SD, 2 mM DTT, 10 μM PLP, and buffer A. Sulfide production was measured using a previously described methylene blue assay.15,31,32 The concentration of the ISCU2 variant required to no longer change or saturate the cysteine desulfurase activities of the SD complex was determined to be 1.5 μM for native ISCU2, 1.5 μM for the C35A and C104A variants, 5 μM for the C104S variant, 10 μM for the C35S variant, 15 μM for the C96S variant, 40 μM for the C61A and C61S variants, and 100 μM for the C35A/C61A variant. Once the saturating amounts of the ISCU2 variants had been determined, additional titrations were performed to determine the concentration of FXN needed to maximize the activity of the respective SDU complexes. The required FXN concentration was determined to be 1.5 μM for the native SDU complex, 2.5 μM for the SDUC96S complex, 15 μM for the SDUC104A complex, 25 μM for the SDUC104S complex, 40 μM for the SDUC35A and SDUC35S complexes, 100 μM for the SDUC61A and SDUC61S complexes, and 200 μM for the SDUC35A/C61A complex. The numbers of equivalents (relative to the SD concentration) of the ISCU2 variants and FXN that are required to form the different SDUF complexes are summarized in Table 1. After the number of equivalents of FXN needed to maximize the cysteine desulfurase activity had been determined, additional equivalents of ISCU2 variants were added and the cysteine desulfurase activity was measured. The cysteine desulfurase activity did not further increase, confirming saturation of ISCU2 subunits in the SDUF complex.
Table 1. Kinetic Data for ISCU2 Variant Complexes.
| complex | ISCU2 (equiv) | FXN (equiv) | kcat (min–1)a | Fe-based kcat enhancementb |
|---|---|---|---|---|
| SDU | 3 | not applicable | 0.8 ± 0.1 | 0 |
| SDUF | 3 | 3 | 10.7 ± 0.8 | 1.4 |
| SDUC96SF | 30 | 5 | 9.9 ± 0.9 | 1.2 |
| SDUC35AF | 3 | 80 | 11.1 ± 0.6 | 1.3 |
| SDUC35SF | 20 | 80 | 9.4 ± 0.5 | 1.6 |
| SDUC61AF | 80 | 200 | 9.6 ± 0.2 | 2.3 |
| SDUC61SF | 80 | 200 | 5.9 ± 0.2 | 2.0 |
| SDUC104AF | 3 | 30 | 0.9 ± 0.1 | 0 |
| SDUC104SF | 10 | 50 | 2.7 ± 0.1 | 0 |
| SDUC35A/C61AF | 200 | 400 | 6.4 ± 0.2 | 1.4 |
Kinetics performed with 10 equiv of Fe2+.
Calculated by dividing the kcat for assays with iron by kcat for assays without iron (provided in Table S1 of the Supporting Information).
Cysteine Desulfurase and Fe–S Cluster Assembly Activities for ISCU2 Variant Complexes
Reaction mixtures included 0.5 μM SD, 2 mM DTT, 10 μM PLP, buffer A, and a saturating amount of the ISCU2 variant and FXN (determined above) under standard conditions [in the presence or absence of 5 μM Fe(NH4)2(SO4)2]. The samples were incubated in a glovebox for 30 min before initiation of the reaction with 12.5–1000 μM l-cysteine. The amount of sulfide generated was quantified as previously described,15 and the kcat values for the different ISCU2 variants were determined by fitting to the Michaelis–Menten equation using KaleidaGraph (Synergy Software). The cysteine desulfurase activities for the different SDU complexes were also determined in triplicate using a physiological l-cysteine concentration of 0.1 mM with 0.5 μM SD, 2 mM DTT, 10 μM PLP, buffer A, and saturating amounts of the different ISCU2 variants (above). Similar experiments were also performed with a saturating level of FXN in the absence or presence of 5 μM Fe(NH4)2(SO4)2.
The Fe–S cluster assembly activity was measured using a standard UV–visible assay15 and also with a circular dichroism (CD) assay. For the UV–visible assay, the reaction mixture contained 8 μM SD, 5 mM DTT, 200 μM Fe(NH4)2(SO4)2, 100 μM l-cysteine, and buffer A in a total volume of 0.2 mL. In addition, saturating amounts of the ISCU2 variants with or without saturating amounts of FXN were added to the assay mixture. Specifically, the SDUF complex includes 24 μM ISCU2 and FXN. The SDUC35AF complex includes 24 μM C35A ISCU2 and 640 μM FXN. The SDUC61AF complex includes 640 μM C61A and 1600 μM FXN. The SDUC96SF complex includes 240 μM C96S and 40 μM FXN. The SDUC104AF complex includes 24 μM C104A and 240 μM FXN. The ISCU2 variants were incubated for 1 h with 5 mM DTT in an anaerobic glovebox before addition of the remaining reaction components in an anaerobic cuvette. The reactions were initiated by injecting 100 μM l-cysteine with a gastight syringe. Fe–S cluster formation was monitored at 456 nm and room temperature for 3000 s at 20 °C. Anaerobic CD-monitored Fe–S cluster assembly assay mixtures contained 10 μM SD, saturating amounts of ISCU2 variants and FXN, 200 μM Fe(NH4)2(SO4)2, 1 mM l-cysteine, and buffer A in a total volume of 0.4 mL. The reactions were initiated with injection of 1 mM l-cysteine, and the ellipticity was measured over the wavelength range of 300–600 nm for 60 min at 20 °C using a CD spectrometer (Chirascan).
Results
FXN Increases the Level of Incorporation of Sulfur on NFS1 and ISCU2
Binding of FXN dramatically increases the cysteine desulfurase and Fe–S cluster biosynthesis activities of the SDU complex.15 To test the hypothesis that FXN activation promotes the formation of persulfide species on ISCU2, we monitored the transfer of radioactive sulfur from an l-[35S]cysteine substrate to ISCU2 in the presence of the SD complex and the presence or absence of FXN (see Experimental Procedures). The samples were incubated with the radiolabeled substrate; excess label was removed anaerobically with a desalting column, and then the individual subunits of the SDU or SDUF complexes were analyzed via nonreducing SDS–PAGE. Labeling of both NFS1 and ISCU2 indicated the formation of a covalent adduct [likely a persulfide species (see Discussion)] and was promoted by the addition of FXN (Figure 2). SDUF samples exhibited >3-fold larger amounts of label on NFS1 and >2-fold larger amounts of label on ISCU2 than SDU samples (Figure S1 of the Supporting Information). Next, the abilities of FRDA FXN variants were tested using the same radiolabeling procedure. Samples of the SDU and SDUF complexes were compared to SDUF samples containing the N146K, Q148R, I154F, W155R, and R165C FRDA FXN variants (Figure 3). FXN variants exhibited 50–70% lower levels of label incorporation on NFS1 and 45–76% lower levels of label incorporation on ISCU2 relative to the SDUF sample (Figure S2 of the Supporting Information). Interestingly, the R165C FXN variant was also labeled in this experiment, although this is probably a nonphysiological event. Together, these results indicate an increased level of covalent incorporation of sulfur into both NFS1 and ISCU2 in the presence of FXN and that this level of incorporation is reduced for FRDA FXN variants. Alone, these data do not distinguish if the FXN-dependent increase in the level of incorporation of sulfur into ISCU2 is solely due to the promotion of the NFS1 cysteine desulfurase activity or also to the acceleration of the transfer of sulfur between NFS1 and ISCU2.
Figure 2.
FXN enhances the accumulation of sulfur on NFS1 and ISCU2. Radiolabeled sulfur incorporation from l-[35S]cysteine substrate on NFS1 with subsequent transfer to ISCU2 was monitored by nonreducing SDS–PAGE separation coupled to phosphor imaging. Samples of 3 μM SD and 3–40 equiv of ISCU2 (relative to SD) without FXN and with 9 μM FXN were incubated for 2 min with l-[35S]cysteine and analyzed by SDS–PAGE. The first three lanes correspond to SD, ISCU2, or FXN controls that were incubated for 2 min with l-[35S]cysteine.
Figure 3.
FRDA variants decrease the level of accumulation of sulfur on NFS1 and ISCU2. SD (3 μM) was reacted with 9 μM ISCU2 and 9 μM FXN variants (native FXN, N146K, Q148R, I154F, W155R, and R165C) and analyzed as described in the legend of Figure 2.
Persulfide-Bound Intermediate in Human Fe–S Cluster Biosynthesis
To establish that the 35S-labeled ISCU2 can function as a Fe–S cluster assembly intermediate, we tracked the progression of the 35S label in a two-step reaction equivalent. In the first step, we generated a 35S-labeled form of ISCU2 by adding l-[35S]cysteine, 3 equiv of ISCU2, and 3 equiv of FXN to SD that contained NFS1 labeled with a six-His tag (see Experimental Procedures). The reaction mixture was applied to a HisTrap column and washed with buffer, and proteins associated with six-His NFS1 were eluted with imidazole (Figure 4A). The 35S label was primarily in bound fractions (fractions 11–13) and corresponded to both radiolabeled NFS1 and radiolabeled ISCU2 (Figure 4B). Very little radioactivity was associated with the noncomplexed flow-through fractions (Figure 4B, bottom). A nonreducing native gel showed that the radioactivity was linked to a slower migrating band (Figure 4C) that had been previously shown15 to be associated with the SDUF complex (Figure S3 of the Supporting Information).
Figure 4.
35S radiolabel tracking from a cysteine substrate to a Fe–S cluster on FDX. The SDUF complex was reacted (see Experimental Procedures) with l-[35S]cysteine and (A) fractionated with a HisTrap column. (B) Fractions were analyzed for protein (top) and radioactivity (bottom) via nonreducing 14% SDS–PAGE, and (C) fractions 11–13 corresponding to [35S]SDUF were combined and analyzed for protein (top) and radioactivity (bottom) via nonreducing 6.5% Native PAGE. [35S]SDUF was then reacted with iron (see Experimental Procedures) and (D) fractionated on a second HisTrap column. (E) Fractions 2 and 3 from panel D were combined and analyzed via native PAGE in the absence (labeled 1) and presence (labeled 2) of DTT. Standards SD, ISCU2, FXN, FDX, and SDU were included for the native gels; proteins were stained using Coomassie blue, and radioactivity was detected using a Phosphorimager. The absorbance (blue) at 280 (A) or 405 nm (D) was overlaid with a 5 to 500 mM imidazole gradient (pink).
In the second step, we followed the progression of the 35S label onto the human ferredoxin (FDX) substrate. The purified 35S-labeled SDUF complex (fractions 11–13 from the HisTrap column) was incubated with ferrous iron, nonradioactive l-cysteine, and DTT as an electron source, which are conditions consistent with synthesizing Fe–S clusters (see below). Notably, a separate experiment established that the radiolabel on ISCU2, but not NFS1, is resistant to DTT-dependent cleavage (Figure S4 of the Supporting Information). A desalting column was used to remove any generated sulfide and unreacted substrates, and the sample was incubated with apo-FDX and loaded onto a HisTrap column, washed with buffer, and eluted with imidazole. Flow-through fractions 2 and 3 had absorbance at 405 nm (Figure 4D), consistent with a Fe–S cluster, as did fractions 11–13, which likely represents NFS1-bound PLP and potentially some untransferred Fe–S clusters associated with the SDUF complex. Bands in flow-through fractions 2 and 3 migrated similarly on a native gel as a Fe–S cluster-containing FDX standard (Figure 4E, top). Moreover, phosphorimaging analysis revealed the 35S label was associated with the same fractions and was resistant to the addition of DTT, consistent with an FDX-bound Fe–S cluster (Figure 4E, bottom). Thus, these data support a persulfide-bound ISCU2 species being a viable intermediate and the terminal sulfur of the persulfide species being converted into the inorganic sulfide of a Fe–S cluster during cofactor biosynthesis.
ISCU2 C104 Variants Disrupt Cysteine Desulfurase Activation by FXN
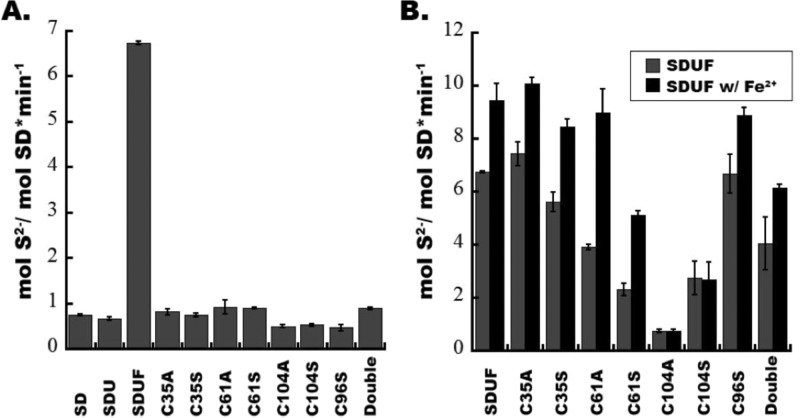
To identify the roles of the nonconserved cysteine (C96) and the conserved cysteines (C35, C61, and C104) in ISCU2, we introduced substitutions of the cysteines to serine and alanine, which has no ability to function in sulfur transfer or cluster ligation. As these surface substitutions could affect the binding affinity of ISCU2 and/or FXN for the SD complex, we first determined the ability of these ISCU2 variants to form the SDUF complex using native gel analysis. Native gel analysis revealed that the SDUC35AF, SDUC61AF, and SDUC104AF complexes formed the slower-migrating band that is characteristic of the four-protein SDUF complex (Figure S3 of the Supporting Information).15 Interestingly, the slower-migrating band was less defined for the C61A variant, suggesting this variant may have a larger impact on binding to the complex. Second, we determined the number of equivalents of ISCU2 and FXN that maximized changes in cysteine desulfurase activity as measured by a methylene blue assay (Table 1). In particular, C61 variants appeared to have the largest effect on binding, whereas mutations affecting C104 and the nonconserved C96 had modest binding effects. Saturating amounts of ISCU2 and FXN were used for subsequent activity measurements to remove complications from changes in binding affinity due to the incorporated ISCU2 mutations.
Next, we determined that assembly complexes that included C104 variants behaved differently than the other cysteine variants with respect to sulfur transfer chemistry. In the absence of FXN, all of the SDU variant complexes exhibited typical unstimulated cysteine desulfurase activities that were similar to the native SDU complex (Figure 5A). In the presence of FXN, the SDUC96SF, SDUC35AF, SDUC35SF, and SDUC61AF complexes exhibited kcat values similar (9.4–11.1 min–1) to the value of 10.7 min–1 for the native SDUF complex (Table 1). The kcat values for the SDUC61SF (5.9 min–1) and SDUC35A/C61AF (6.4 min–1) complexes were slightly compromised compared to that of the native SDUF complex, whereas complexes for the C104A (0.9 min–1) and C104S (2.7 min–1) variants were more similar to samples that lacked FXN (0.8 min–1). Previously, iron was found to further increase the FXN-based stimulation of the cysteine desulfurase activity and was observed for reactions with the activated SDUF but not unactivated SDU Fe–S assembly complex.15 Here, all SDUF variant complexes, except those that include substitutions at C104, exhibited this characteristic iron-based stimulation of the cysteine desulfurase activity (Figure 5B and Table 1). The loss of Fe-based stimulation under standard conditions was not simply due to the increased concentrations of the ISCU2 variant or FXN, which may compete for iron binding, but was specifically associated with the C104 variants. For example, the SDUC61AF complex exhibited the most dramatic Fe-based stimulation (Figure 5B and Table 1) and includes ∼27-fold more ISCU2 and ∼7-fold more FXN than the SDUC104AF complex. CD spectroscopy established that the C104 substitutions did not impart a global change in the ISCU2 structure (Figure S5 of the Supporting Information). These data revealed that mutations at position C104, but not the other cysteine residues, were compromised in their FXN-associated stimulation of the cysteine desulfurase reaction and suggested a link between ISCU2 residue C104 and FXN activation.
Figure 5.
Cysteine desulfurase activity for Fe–S assembly complexes containing different ISCU2 variants. (A) Cysteine desulfurase activity for SDU complexes with different ISCU2 variants compared to the native SDUF complex. The double mutant is ISCU2 variant C35A/C61A. (B) Cysteine desulfurase activity for the SDUF complexes with saturating amounts of FXN and the ISCU2 variant in the presence and absence of 5 μM Fe(NH4)2(SO4)2. Error bars in panels A and B are for three independent measurements. All assays were performed with 100 μM l-cysteine.
ISCU2 Conserved Cysteines Are Critical for Enzymatic Fe–S Cluster Assembly
We then measured Fe–S cluster formation rates for ISCU2 variants using two different spectroscopic assays. First, the increase in absorbance at 456 nm was used as a measure of the Fe–S cluster assembly activity. The ISCU2 C96S variant, which eliminates the nonconserved cysteine, had a Fe–S cluster assembly activity similar to those of the native SDU and SDUF complexes (Figure 6A), indicating that this residue is essential neither for cluster ligation nor for the mechanism of cluster formation. In contrast, SDUF complexes containing the C35A, C61A, and C104A ISCU2 variants had a dramatic loss of activity relative to that of native SDUF (Figure 6A). Interestingly, there is a low-level increase in absorbance for samples containing the C35A, C61A, and C104A variants or native ISCU2 in the absence of FXN, which would be consistent with sulfide- and iron-dependent solution chemistry. Consistent with this idea, the samples with the lowest cysteine desulfurase activity, SDU and SDUC104AF (Table 1), exhibited the slowest increase in absorbance (Figure 6A). Second, we monitored the 300–600 nm region under DTT-free conditions using circular dichroism (CD) spectroscopy, which is sensitive to the PLP cofactor and to protein-bound Fe–S cluster species. A CD signal with maxima at 330 and 430 nm developed for samples of SDUF containing the native ISCU2 or the C96S variant (Figure 6B) that appeared to be similar to the [2Fe-2S] cluster bound to bacterial IscU.14 The development of this Fe–S signal was absent for assembly complex samples that contained the C35A, C61A, and C104A variants. These samples had a CD signal with a maximum at 420 nm, but this signal is due to the PLP cofactor of NFS1 rather than an Fe–S cluster. Together, these data indicate that C96 is not required for the Fe–S assembly reaction and that all three conserved cysteines (C35, C61, and C104) are essential for enzymatic Fe–S cluster formation.
Figure 6.

Conserved cysteines are critical for Fe–S cluster formation on ISCU2. (A) Fe–S cluster formation was monitored at 456 nm by UV–vis spectroscopy as a function of time. (B) Fe–S cluster formation was monitored by CD spectroscopy, and the 60 min time point is displayed. Samples include SDU (yellow), SDUF (red), SDUC35AF (blue), SDUC61AF (black) SDUC96SF (purple), and SDUC104AF (green).
Discussion
Despite many excellent studies of the ISC biosynthetic system, details of the sulfur transfer mechanism and intermediates in Fe–S cluster biosynthesis remain unknown. Currently, three minimalist models have been proposed for Fe–S cluster biosynthesis that differ in the order of substrate addition: the sulfur-first, iron-first, and persulfide radical mechanisms.33,34 In the sulfur-first mechanism, the first step is the transfer of the terminal sulfur of a persulfide species onto the cysteine desulfurase (bacterial IscS or human NFS1) to a cysteine residue on the scaffold protein (bacterial IscU or human ISCU2).35 This is followed, in an undefined order, by a second sulfur transfer event, the incorporation of two ferrous irons, and the addition of two electrons to form a [2Fe-2S]2+ intermediate. This model is supported by the ability of E. coli and Azotobacter vinelandii IscS to transfer sulfur from l-cysteine to form persulfide- or polysulfide-bound IscU.6,7 In the Fe-first mechanism, ferrous iron binds to the cysteines of the scaffold protein as an initiating step in forming the [2Fe-2S]2+ intermediate. This model is supported by the ability of Thermotoga maritima IscU to bind ferrous iron36 and by the stabilization of Haemophilus influenza IscU by binding of divalent metal ions such as zinc to the active site cysteines.37,38 The third mechanism is based on the observation that reduced ferredoxin is oxidized upon the addition of cysteine to IscS and postulates the presence of a stable persulfide radical intermediate that is transferred to IscU and then further reduced by iron to generate a ferric sulfide intermediate.34,39 However, there is no direct experimental support for the persulfide radical species, and neither the persulfide-bound nor iron-bound IscU has been demonstrated to be a viable intermediate in Fe–S cluster biosynthesis.
Even less is known about the sulfur transfer mechanism in eukaryotes, and two additional components (FXN and ISD11) are implicated in modulating the cysteine desulfurase activity of NFS1.15,18−23 Here our objective was to directly test if a persulfide generated on ISCU2 is viable in forming a Fe–S cluster. Our strategy was to track the 35S radiolabel from a cysteine substrate to a persulfide species on the human Fe–S cluster assembly complex and then show that this persulfide species is viable in forming an iron–sulfur cluster on an acceptor protein. The addition of an l-[35S]cysteine substrate to the SDUF complex resulted in comigration of the radiolabel with both NFS1 and ISCU2 on a nonreducing SDS–PAGE gel (Figure 4), consistent with the formation of a covalent adduct. As the labeling experiments were performed under anaerobic conditions and the excess label was removed before the analysis via SDS–PAGE, the covalent adduct is likely a persulfide species rather than a cystine formed by oxidative disulfide bond formation between a radiolabeled cysteine and a cysteine residue on NFS1 or ISCU2. This SDUF complex with a persulfide-bound ISCU2 subunit was reacted with iron, DTT as an electron source, and additional nonradiolabeled cysteine. The sample was desalted to remove any generated sulfide or unreacted reagents and then incubated with apo-FDX. Our results indicate the radiolabel was transferred to FDX and was associated with a DTT-resistant species that absorbs at 405 nm (Figure 4), which is consistent with a Fe–S cluster. We favor persulfide reduction on ISCU2 after iron addition to generate the inorganic sulfide of the [2Fe-2S] cluster, consistent with the resistance of the persulfide species on ISCU2 to reductants in the absence of iron (Figure S4 of the Supporting Information). Together, these experiments show that a persulfide species can be formed on the ISCU2 subunit of the SDUF complex and that this species has properties consistent with an intermediate in a sulfur-first mechanism for eukaryotic Fe–S cluster biosynthesis.
In addition, these results help clarify the role of FXN in Fe–S cluster biosynthesis. FXN accelerates both the in vitro cysteine desulfurase (sulfide production) and Fe–S assembly (increase in 456 nm absorbance) activities of the human SDU complex.15 FXN could stimulate the rates of these reactions by inducing a conformational change that (i) increases active site accessibility, (ii) enhances a step associated with PLP chemistry on NFS1, and/or (iii) facilitates interprotein transfer of sulfur from NFS1 to ISCU2. The first mechanism suggests a FXN-dependent increase in active site accessibility that allows DTT to intercept a catalytic intermediate and generate sulfide, which could drive nonenzymatic assembly of Fe–S species.40 This explanation would predict that the rates of the cysteine desulfurase and Fe–S assembly reactions should be correlated, which is generally true for SDUF samples containing FRDA variants24,25 and for the SDUC104AF complex that exhibits activities similar to those of samples that lack FXN. However, this is not true for the SDUC35AF and SDUC61AF complexes that exhibit nativelike cysteine desulfurase activities (Table 1) but compromised Fe–S cluster assembly activities (Figure 6). This suggests that the absorbance assay is sensitive to enzymatic Fe–S cluster biosynthesis and is not just a reflection of the cysteine desulfurase activity that drives nonenzymatic Fe–S solution chemistry. A model in which a FXN-induced conformational change increases active site accessibility would also not explain the increase in the level of accumulation of the radiolabel on ISCU2 (Figures 2 and 3) or the FXN depletion phenotype that results in the loss of Fe–S cluster activity. Thus, these results indicate a functional role for FXN in directly or indirectly facilitating sulfur transfer chemistry that promotes Fe–S cluster biosynthesis.
FXN induces a SDU conformational change that stimulates the in vitro cysteine desulfurase and Fe–S cluster activities by affecting the PLP chemistry on NFS1. This second mechanism, involving enhancement of persulfide formation on NFS1, suggests that FXN but not FRDA FXN variants should increase the level of accumulation of the 35S radiolabel on NFS1, which was observed (Figures 2 and 3). This result is also consistent with the effects of FXN on the KM for l-cysteine in the cysteine desulfurase reaction,15,26 and with recent results in S. cerevisiae.41 To be consistent with this mechanism, the C104 ISCU2 variants, which are compromised in FXN-based stimulation, would need to adopt a conformation different from that of either native ISCU2 or the other ISCU2 variants. We disfavor this hypothesis as the exclusive cause of the loss of activation due to the conservative nature of the C104 substitutions, the similar CD spectra for ISCU2 and C104 variants in the absence of SD and FXN (Figure S5 of the Supporting Information), and the lack of correlation between the cysteine desulfurase activation level and relative ISCU2 and FXN binding to the SD complex [C35 and C61 variants exhibit weaker binding but greater activation than C104 (Table 1)], which is an indirect reporter of conformational state. Nevertheless, these results further support a role for FXN as an allosteric activator that affects the PLP-dependent cysteine desulfurase chemistry on NFS1.
FXN may also function in facilitating the transfer of sulfur from NFS1 to ISCU2. The third mechanism, involving FXN-dependent acceleration of interprotein sulfur transfer, is more difficult to separate from acceleration of a rate-limiting formation of a persulfide on NFS1, despite the increase in the level of accumulation of the 35S radiolabel on ISCU2 (Figure 2). However, we also observe a correlation between ISCU2 residue C104 and FXN-based activation. This effect could be explained by FXN-mediated sulfur transfer to C104, which then partitions between rapid DTT cleavage that enhances the cysteine desulfurase activity and intraprotein sulfur transfer that prepares ISCU2 for iron incorporation and another round of sulfur transfer. We excluded an alternate activation model, the transfer of sulfur from NFS1 to ISCU2 residue C35 or C61 followed by intraprotein transfer of sulfur to C104 and reductive cleavage to generate sulfide, by determining that the C35A/C61A variant exhibited a kcat for the cysteine desulfurase reaction that was more similar to that of native ISCU2 than to those of the C104 variants (Table 1). Thus, we favor a model in which C104 acts as a primary rather than secondary sulfur acceptor, consistent with conclusions from a bacterial ISC system,14 and this sulfur species partitions in our assays between being intercepted and reductively cleaved to produce sulfide (cysteine desulfurase assay), functioning as an intermediate in Fe–S cluster formation (Fe–S biosynthesis assay) on the assembly complex. Taken together, these data are consistent with a role for FXN in accelerating both the formation of persulfide on NFS1 and interprotein sulfur transfer to ISCU2.
Accumulating evidence suggests FXN functions at the sulfur transfer step in eukaryotic Fe–S cluster biosynthesis,15,26,41 and that this sulfur transfer reaction initiates Fe–S cluster biosynthesis (Figure 4). Moreover, these FXN-dependent effects cannot be explained by the iron donor hypothesis and are consistent with the loss of Fe–S enzyme activity phenotype upon FXN depletion. We propose that a key aspect of the FXN-based activation of the cysteine desulfurase and Fe–S biosynthesis reactions revolves around the conformation of the C-terminal α-helix of ISCU2 (Figure 7). We propose an equilibrium mixture between nonfunctional (helix) and functional (coil) conformational states of this α-helix and that FXN functions as an allosteric activator by binding and stabilizing the coil conformation. This proposal is reminiscent of the different conformations for the C-terminal helix in the Aquifex aeolicus IscU crystal structure,42 and the mixture of ordered and disordered states proposed for E. coli IscU by the Markley group.43,44 More specifically, we suggest this conformational change is responsible for the enhanced PLP-dependent chemistry on NFS1 and may also facilitate the transfer of sulfur from NFS1 C381 to ISCU2 C104 as an initiating step in Fe–S cluster biosynthesis. Finally, we hypothesize that iron binds to the activated (coil) conformation of the SDU complex and is incorporated into the active site after the transfer of sulfur from NFS1 to ISCU2 residue C104, consistent with the link between C104 and the iron-based stimulation in the cysteine desulfurase activity (Table 1). Future experiments will focus on testing and expanding upon this model with the ultimate goal of developing new strategies for treating FRDA.
Figure 7.

Cartoon model of FXN activation of the Fe–S assembly complex. (A) SDU complexes exist as an equilibrium mixture between a stable inactive (helix) and less stable active (coil) conformation. (B) FXN binds to the coil conformation for the C-terminal helix and shifts the equilibrium from the inactive to active form. (C) NFS1 reacts with l-cysteine to form a persulfide species on residue C381. (D) Sulfur is transferred from NFS1 to ISCU2 residue C104. (E) Addition of the remaining substrates results in [2Fe-2S] cluster formation on ISCU2. (F) The Fe–S cluster is transferred to an apo target, and the active SDUF assembly complex is re-formed. This last step may involve subunit dissociation and/or chaperone proteins.
Acknowledgments
We thank Christopher D. Putnam, James Vranish, D. J. Martin, and Shachin Patra for helpful discussion and suggestions, D. J. Martin for generating Figure S4 of the Supporting Information, and Shachin Patra for collecting the CD spectra for Figure S5 of the Supporting Information.
Glossary
Abbreviations
- BME
2-mercaptoethanol
- DTT
dithiothreitol
- FRDA
Friedreich’s ataxia
- FXN
frataxin
- GSH
glutathione
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- IPTG
isopropyl β-d-1-thiogalactopyranoside
- PLP
pyridoxal 5′-phosphate
- SD
protein complex composed of NFS1 and ISD11
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- CD
circular dichroism
- SDU
protein complex composed of NFS1, ISD11, and ISCU2
- SDUF
protein complex composed of NFS1, ISD11, ISCU2, and frataxin
- TCEP
tris(2-carboxyethyl)phosphine
- Tris
tris(hydroxymethyl)aminomethane.
Supporting Information Available
Figures S1–S5 and Table S1. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
J.B.-R., N.G.F., and C.-L.T. contributed equally to this work.
This work was supported in part by Texas A&M University and by Grant A-1647 from the Robert A. Welch Foundation and Grant 1R01GM096100 from the National Institutes of Health (D.P.B.).
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Kessler D. (2006) Enzymatic activation of sulfur for incorporation into biomolecules in prokaryotes. FEMS Microbiol. Rev. 30, 825–840. [DOI] [PubMed] [Google Scholar]
- Mueller E. G. (2006) Trafficking in persulfides: Delivering sulfur in biosynthetic pathways. Nat. Chem. Biol. 2, 185–194. [DOI] [PubMed] [Google Scholar]
- Zheng L.; White R. H.; Cash V. L.; Dean D. R. (1994) Mechanism for the desulfurization of l-cysteine catalyzed by the nifS gene product. Biochemistry 33, 4714–4720. [DOI] [PubMed] [Google Scholar]
- Behshad E.; Parkin S. E.; Bollinger J. M. (2004) Mechanism of cysteine desulfurase Slr0387 from Synechocystis sp. PCC 6803: Kinetic analysis of cleavage of the persulfide intermediate by chemical reductants. Biochemistry 43, 12220–12226. [DOI] [PubMed] [Google Scholar]
- Cupp-Vickery J.; Urbina H. D.; Vickery L. (2003) Crystal structure of IscS, a cysteine desulfurase from Escherichia coli. J. Mol. Biol. 330, 1049–1059. [DOI] [PubMed] [Google Scholar]
- Urbina H. D.; Silberg J. J.; Hoff K. G.; Vickery L. (2001) Transfer of sulfur from IscS to IscU during Fe/S cluster assembly. J. Biol. Chem. 276, 44521–44526. [DOI] [PubMed] [Google Scholar]
- Smith A. D.; Agar J.; Johnson K. A.; Frazzon J.; Amster I. J.; Dean D. R.; Johnson M. (2001) Sulfur transfer from IscS to IscU: The first step in iron-sulfur cluster biosynthesis. J. Am. Chem. Soc. 123, 11103–11104. [DOI] [PubMed] [Google Scholar]
- Smith A. D.; Frazzon J.; Dean D. R.; Johnson M. (2005) Role of conserved cysteines in mediating sulfur transfer from IscS to IscU. FEBS Lett. 579, 5236–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinoni E. N.; de Oliveira J. S.; Nicolet Y.; Raulfs E. C.; Amara P.; Dean D. R.; Fontecilla-Camps J. C. (2012) (IscS-IscU)2 complex structures provide insights into Fe2S2 biogenesis and transfer. Angew. Chem., Int. Ed. 51, 5439–5442. [DOI] [PubMed] [Google Scholar]
- Shi R.; Proteau A.; Villarroya M.; Moukadiri I.; Zhang L.; Trempe J.-F.; Matte A.; Armengod M. E.; Cygler M. (2010) Structural basis for Fe-S cluster assembly and tRNA thiolation mediated by IscS protein-protein interactions. PLoS Biol. 8, e1000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S.-I.; Mihara H.; Kurihara T.; Takahashi Y.; Tokumoto U.; Yoshimura T.; Esaki N. (2002) Cys-328 of IscS and Cys-63 of IscU are the sites of disulfide bridge formation in a covalently bound IscS/IscU complex: Implications for the mechanism of iron-sulfur cluster assembly. Proc. Natl. Acad. Sci. U.S.A. 99, 5948–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridwell-Rabb J.; Iannuzzi C.; Pastore A.; Barondeau D. P. (2012) Effector role reversal during evolution: The case of frataxin in Fe-S cluster biosynthesis. Biochemistry 51, 2506–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannuzzi C.; Adinolfi S.; Howes B. D.; Garcia-Serres R.; Clémancey M.; Latour J.-M.; Smulevich G.; Pastore A. (2011) The Role of CyaY in Iron Sulfur Cluster Assembly on the E. coli IscU Scaffold Protein. PLoS One 6, e21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomi F.; Iametti S.; Morleo A.; Ta D. T.; Vickery L. E. (2011) Facilitated transfer of IscU-[2Fe2S] clusters by chaperone-mediated ligand exchange. Biochemistry 50, 9641–9650. [DOI] [PubMed] [Google Scholar]
- Tsai C. L.; Barondeau D. P. (2010) Human frataxin is an allosteric switch that activates the Fe-S cluster biosynthetic complex. Biochemistry 49, 9132–9139. [DOI] [PubMed] [Google Scholar]
- Schmucker S.; Martelli A.; Colin F.; Page A.; Wattenhofer-Donzé M.; Reutenauer L.; Puccio H. (2011) Mammalian Frataxin: An Essential Function for Cellular Viability through an Interaction with a Preformed ISCU/NFS1/ISD11 Iron-Sulfur Assembly Complex. PLoS One 6, e16199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L.; White R. H.; Cash V. L.; Jack R. F.; Dean D. R. (1993) Cysteine desulfurase activity indicates a role for NIFS in metallocluster biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 90, 2754–2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam A. C.; Bornhovd C.; Prokisch H.; Neupert W.; Hell K. (2006) The Nfs1 interacting protein Isd11 has an essential role in Fe/S cluster biogenesis in mitochondria. EMBO J. 25, 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Y.; Napoli E.; Cortopassi G. (2007) Mitochondrial frataxin interacts with ISD11 of the NFS1/ISCU complex and multiple mitochondrial chaperones. Hum. Mol. Genet. 16, 929–941. [DOI] [PubMed] [Google Scholar]
- Shi Y.; Ghosh M.; Tong W. H.; Rouault T. A. (2009) Human ISD11 is essential for both iron-sulfur cluster assembly and maintenance of normal cellular iron homeostasis. Hum. Mol. Genet. 18, 3014–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann N.; Urzica E.; Guiard B.; Muller H.; Lohaus C.; Meyer H. E.; Ryan M. T.; Meisinger C.; Muhlenhoff U.; Lill R.; Pfanner N. (2006) Essential role of Isd11 in mitochondrial iron-sulfur cluster synthesis on Isu scaffold proteins. EMBO J. 25, 184–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A.; Golla R.; Yoon H.; Dancis A.; Pain D. (2012) Persulfide formation on mitochondrial cysteine desulfurase: Enzyme activation by a eukaryote-specific interacting protein and Fe-S cluster synthesis. Biochem. J. 448, 171–187. [DOI] [PubMed] [Google Scholar]
- Pandey A.; Yoon H.; Lyver E. R.; Dancis A.; Pain D. (2012) Identification of a Nfs1p-bound persulfide intermediate in Fe-S cluster synthesis by intact mitochondria. Mitochondrion 12, 539–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. L.; Bridwell-Rabb J.; Barondeau D. P. (2011) Friedreich’s Ataxia Variants I154F and W155R Diminish Frataxin-Based Activation of the Iron-Sulfur Cluster Assembly Complex. Biochemistry 50, 6478–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridwell-Rabb J.; Winn A. M.; Barondeau D. P. (2011) Structure-function analysis of Friedreich’s ataxia mutants reveals determinants for frataxin binding and activation of the Fe-S assembly complex. Biochemistry 50, 7265–7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin F.; Martelli A.; Clemancey M.; Latour J. M.; Gambarelli S.; Zeppieri L.; Birck C.; Page A.; Puccio H.; Ollagnier de Choudens S. (2013) Mammalian Frataxin Controls Sulfur Production and Iron Entry during de Novo Fe4S4 Cluster Assembly. J. Am. Chem. Soc. 135, 733–740. [DOI] [PubMed] [Google Scholar]
- Campuzano V.; Montermini L.; Moltè M. D.; Pianese L.; Cossee M.; Cavalcanti F.; Monros E.; Rodius F.; Duclos F.; Monticelli A.; Zara F.; Cañizares J.; Koutnikova H.; Bidichandani S. I.; Gellera C.; Brice A.; Trouillas P.; De Michele G.; Filla A.; De Frutos R.; Palau F.; Patel P. I.; Di Donato S.; Mandel J. L.; Cocozza S.; Koenig M.; Pandolfo M. (1996) Friedreich’s ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427. [DOI] [PubMed] [Google Scholar]
- Lane D. J. R.; Richardson D. R. (2010) Frataxin, a molecule of mystery: Trading stability for function in its iron-binding site. Biochem. J. 426, e1–e3. [DOI] [PubMed] [Google Scholar]
- Xia B.; Cheng H.; Bandarian V.; Reed G. H.; Markley J. L. (1996) Human ferredoxin: Overproduction in Escherichia coli, reconstitution in vitro, and spectroscopic studies of iron-sulfur cluster ligand cysteine-to-serine mutants. Biochemistry 35, 9488–9495. [DOI] [PubMed] [Google Scholar]
- Bonomi F.; Iametti S.; Ta D. T.; Vickery L. (2005) Multiple turnover transfer of [2Fe2S] clusters by the iron-sulfur cluster assembly scaffold proteins IscU and IscA. J. Biol. Chem. 280, 29513–29518. [DOI] [PubMed] [Google Scholar]
- Marelja Z.; Stöcklein W.; Nimtz M.; Leimkühler S. (2008) A novel role for human Nfs1 in the cytoplasm: Nfs1 acts as a sulfur donor for MOCS3, a protein involved in molybdenum cofactor biosynthesis. J. Biol. Chem. 283, 25178–25185. [DOI] [PubMed] [Google Scholar]
- Siegel L. M. (1965) A direct microdetermination for sulfide. Anal. Biochem. 11, 126–132. [DOI] [PubMed] [Google Scholar]
- Fontecave M.; Choudens S. O. d.; Py B.; Barras F. (2005) Mechanisms of iron-sulfur cluster assembly: The SUF machinery. JBIC, J. Biol. Inorg. Chem. 10, 713–721. [DOI] [PubMed] [Google Scholar]
- Kim J. H.; Frederick R. O.; Reinen N. M.; Troupis A. T.; Markley J. L. (2013) [2Fe-2S]-Ferredoxin binds directly to cysteine desulfurase and supplies an electron for iron-sulfur cluster assembly but is displaced by the scaffold protein or bacterial frataxin. J. Am. Chem. Soc. 135, 8117–8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint D. H. (1996) Escherichia coli contains a protein that is homologous in function and N-terminal sequence to the protein encoded by the nifS gene of Azotobacter vinelandii and that can participate in the synthesis of the Fe-S cluster of dihydroxy-acid dehydratase. J. Biol. Chem. 271, 16068–16074. [PubMed] [Google Scholar]
- Mansy S. S.; Wu G.; Surerus K. K.; Cowan J. A. (2002) Iron-sulfur cluster biosynthesis. Thermatoga maritima IscU is a structured iron-sulfur cluster assembly protein. J. Biol. Chem. 277, 21397–21404. [DOI] [PubMed] [Google Scholar]
- Liu J.; Oganesyan N.; Shin D.-H.; Jancarik J.; Yokota H.; Kim R.; Kim S.-H. (2005) Structural characterization of an iron-sulfur cluster assembly protein IscU in a zinc-bound form. Proteins 59, 875–881. [DOI] [PubMed] [Google Scholar]
- Ramelot T. A.; Cort J. R.; Goldsmith-Fischman S.; Kornhaber G. J.; Xiao R.; Shastry R.; Acton T. B.; Honig B.; Montelione G. T.; Kennedy M. A. (2004) Solution NMR structure of the iron-sulfur cluster assembly protein U (IscU) with zinc bound at the active site. J. Mol. Biol. 344, 567–583. [DOI] [PubMed] [Google Scholar]
- Kim J. H.; Bothe J. R.; Frederick R. O.; Holder J. C.; Markley J. L. (2014) Role of IscX in Iron-Sulfur Cluster Biogenesis in Escherichia coli. J. Am. Chem. Soc. 136, 7933–7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H.; Holm R. H.; Münck E. (1997) Iron-sulfur clusters: Nature’s modular, multipurpose structures. Science 277, 653–659. [DOI] [PubMed] [Google Scholar]
- Pandey A.; Gordon D. M.; Pain J.; Stemmler T. L.; Dancis A.; Pain D. (2013) Frataxin Directly Stimulates Mitochondrial Cysteine Desulfurase by Exposing Substrate-binding Sites, and a Mutant Fe-S Cluster Scaffold Protein with Frataxin-bypassing Ability Acts Similarly. J. Biol. Chem. 288, 36773–36786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y.; Wada K.; Fukuyama K.; Takahashi Y. (2008) The Asymmetric Trimeric Architecture of [2Fe-2S] IscU: Implications for Its Scaffolding during Iron-Sulfur Cluster Biosynthesis. J. Mol. Biol. 383, 133–143. [DOI] [PubMed] [Google Scholar]
- Kim J. H.; Tonelli M.; Markley J. L. (2012) Disordered form of the scaffold protein IscU is the substrate for iron-sulfur cluster assembly on cysteine desulfurase. Proc. Natl. Acad. Sci. U.S.A. 109, 454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Z.; Tonelli M.; Markley J. L. (2012) Metamorphic Protein IscU Changes Conformation by cis-trans Isomerizations of Two Peptidyl-Prolyl Peptide Bonds. Biochemistry 51, 9595–9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.