Abstract

Methods capable of exhaustively screening for crystal polymorphism remain an elusive goal in solid-state chemistry. Particularly promising among the new generation of approaches is polymer-induced heteronucleation (PIHn), a tool utilizing hundreds of unique polymers for granting kinetic access to polymorphs. Here PIHn is redeployed in a high density format in which 288 distinct polymers, each acting as a heteronucleant, are arrayed on one substrate. This format allows determining the outcome of thousands of crystallizations in an automated fashion with only a few milligrams of sample. This technology enables the study of a broader range of targets, including preclinical candidates, facilitating determination of polymorphism propensity much earlier in the drug development process. Here the efficacy of this approach is demonstrated using four pharmaceutically relevant compounds: acetaminophen, tolfenamic acid, ROY, and curcumin.
Keywords: pharmaceuticals, Raman spectroscopy, polymer-induced heteronucleation
The recognition that pharmaceuticals often exist in multiple crystalline forms solely differing in the arrangement of molecules, crystalline polymorphs,1 has led to an increase in activity directed towards efficiently screening for solid form diversity. The ideal technique should facilitate formation and identification of all possible polymorphs of a molecule while utilizing minimal amounts of the target compound and automated form identification. This goal remains elusive in part because the nucleation of a specified polymorph is influenced by a wide array of factors, making polymorph discovery an often time-consuming, Edisonian process. A traditional screen typically involves changes in variables, such as solvent, temperature, and degree of supersaturation. These variables have empirically been shown to influence the polymorphic form obtained from a crystallization trial, albeit through a mechanism that is obscure. More sophisticated approaches involving heterogeneous nucleation, where a foreign surface is present that can interact with the crystallizing material in solution2 are emerging. For example, self-assembled monolayers (SAMs),3−6 crystalline heteronucleants,7,8 and amorphous polymers9−13 have all been employed with varying degrees of success. In particular, polymer-induced heteronucleation (PIHn) has proven to be a powerful discovery method utilizing hundreds of unique amorphous polymers as crystallization directors for obtaining novel solid forms.14−16 The polymer selectively promotes the growth of one form above others through a kinetic mechanism involving selective stabilization at the stage of nucleation.17,18 It has been established that functional group interactions at the polymer-crystal interface are responsible for directing and controlling the nucleation of different crystal phases on specific polymer heteronucleants.17,18 Recently nonamorphism in the anti-inflammatory compound flufenamic acid was demonstrated using PIHn, setting a new record for the organic compound with the most structurally characterized polymorphs.19
Although PIHn has been extremely successful in both form selection and in obtaining novel polymorphs, there are still several challenges that must be overcome to improve screening efficiency and accuracy. Raman spectroscopy, an analytical technique used to study the vibrational modes in a material, is often employed to distinguish among polymorphs because of its short analysis times, minimal sample preparation requirements, and high sensitivity. However, the relatively large amount of polymer heteronucleant present often leads to problematic levels of background Raman scattering; this can obscure the Raman spectrum of the compound of interest and hamper automated analysis. Furthermore, PIHn relies on relatively large amounts of sample, limiting polymorph screening to compounds that are readily available.
Previous work on high-throughput platforms focused on the creation of polymer microarrays by a piezo jet-printer.20 This system employed hundreds of soluble commercial polymers and a few synthesized cross-linked and linear copolymers as polymer heteronucleants and demonstrated some success in form selection.20 Here PIHn is adapted into a high density format in which hundreds of distinct amorphous, insoluble cross-linked terpolymers are arrayed on a single substrate by using simple pin tools, making automated, high throughput screening possible. The cross-linked terpolymers used in this study are readily generated from simple feedstocks of monomer solutions which are combined in various ratios, allowing for diversity and flexibility in the composition of the cross-linked terpolymers that are utilized as the heteronucleants in this high density platform. This new format is distinct from traditional PIHn in that the amount of polymer, the platform on which the crystallizations occur, the volume of solvent used for crystallization, and the total amount of material used for the crystallization have been dramatically decreased. The reduction in scale is advantageous for a number of reasons. The reduction in polymer thickness yields Raman spectra of compounds with minimal spectral interference from the polymer heteronucleant, enabling completely automated analysis. The amount of material needed has been considerably reduced (to ∼1 mg) as compared to the amounts previously needed for polymorph discovery with PIHn (∼300 mg). Hence, screening newly synthesized compounds for which typically only small quantities are available becomes feasible. Here the efficacy of this new, high density format using the compounds acetaminophen,14 tolfenamic acid,16 ROY,21 and curcumin22 is demonstrated. Furthermore, the consequence of this reduction in scale on polymorph selection efficacy, as compared with PIHn deployed in a traditional format, is explored.
Most high throughput crystallizations are currently conducted using 96, 384, or 1536 well microtiter plates due to their high densities and compatibility with liquid handling robotics. However, using these plates for polymorph screening can be problematic for several reasons. In situ Raman analysis is challenging due to the high aspect ratio and narrow width of the wells in these microtiter plates. When laser light from the Raman spectrometer is focused on a crystal at the bottom of a well, it is hindered from reaching the sample because of the refraction of light at the top of the well arising from its narrow diameter. This also results in an increase in the focal volume of the laser.23 Even for the portion of the laser light reaching the sample, the light does not scatter directly upward but rather will scatter off of the opaque walls of the plate, limiting the amount of light that reaches the detector. These issues effectively reduce sample throughput by increasing the time needed to collect individual spectra. To quantitatively understand these effects, an experiment was performed with a Delrin aperture (hole diameter of 3.30 mm with a 6.0 mm height) placed above a crystal of the nutraceutical piperine, monitoring the signal intensity as the number of Delrin pieces was increased. When one Delrin aperture was used the signal was diminished by 41%; when two were used (effectively mimicking the depth in a standard 384 microtiter plate) the signal was diminished by 72% as compared to having the same crystal on a planar substrate. This experiment demonstrates how the signal in Raman spectroscopy is affected by the depth and narrowness of a well (see Supporting Information). Direct interrogation of crystals within a microtiter plate by X-rays is not possible because of the geometric requirements for diffraction. The geometry of the microtiter plates also makes it very difficult to manually manipulate crystals for ex situ analysis. After examining all of these disadvantages, it is apparent that microtiter plates are not optimal for conducting efficient polymorph screening.
To overcome the limitations of current approaches to high throughput polymorph screening, a platform which takes advantage of the benefits of a high density microtiter plate but limits the drawbacks currently associated with them was devised. A CO2 laser was utilized to create an array of 288 depressions approximately 300 μm deep on a standard quartz microscope slide (75 mm × 25 mm × 1 mm). This geometry eliminates any constraints to in situ analysis and crystal harvesting (Figure 1) (see Supporting Information). This precisely defined array possesses the spacing of a 1536 well plate (2.25 mm from the center of one depression to another) maintaining compatibility with liquid handling robots. For demonstration purposes the three distinct polymer libraries commonly employed in PIHn studies were chosen; these are characterized by the functionalities of their constituent monomers: acidic, nonpolar aromatic, and polar nitrogen.14 For each of these libraries, there are 96 cross-linked polymers, for a total of 288 unique cross-linked polymers. Therefore, the three libraries can be deposited on a single quartz slide with a unique polymer in each depression. This manipulation was accomplished by taking advantage of the geometry of a 1536 well plate relative to a 384 well plate. On a 384 microtiter plate the spacing from the center of one well to another is 4.5 mm (exactly double the spacing in a 1536 well plate). With this in mind, a custom pin tool24 was fabricated composed of five Delrin combs held together in a poly(methyl methacrylate) lattice (Figure 2). This pin tool enables rapid contact-printing of up to 80 distinct monomer solutions simultaneously from a 384 well plate containing the monomer solutions onto the individual depressions on the laser-etched quartz slide. The number of monomer solutions printed onto the quartz slide can be easily changed by removing a comb from the lattice; depending on the number of combs present, 16–80 distinct monomer solutions can be dispensed at one time. Immediately after each print from the 384 well plate onto the quartz slide, the monomer solutions were photopolymerized, yielding thin polymer films in each depression. Four applications of the printing tool were required to print all 288 distinct monomer solutions (see Supporting Information) and after polymerization was completed, the μPIHn plate was applied to crystallization studies. An additional comb was then used to dispense the crystallization solution of the molecule to be investigated onto the μPIHn plate. This contact printing leads to very low volume transfer (∼0.3 μL per well) and therefore small sample requirements. The extremely thin polymer films allow for analysis of polymorphs directly on the plate without significant signal interference from the polymer heteronucleant, thus enabling automated Raman microscopy mapping. The efficacy of this platform was demonstrated with four model polymorphic compounds: acetaminophen (ACM), tolfenamic acid (TA), ROY, and curcumin.
Figure 1.
Schematic of quartz slide with an array of depressions (1 mm wide) with a 2.25 mm spacing from center of one depression to another, implemented in this study as the crystallization platform.
Figure 2.
Pin tool used for deposition of material onto a μPIHn plate.
Acetaminophen
Acetaminophen is typically found in one of two stable polymorphic forms: form I (monoclinic) and form II (orthorhombic).14 Previously, when PIHn was used to study the polymorphism of ACM, both the monoclinic and orthorhombic forms were found utilizing roughly half of a gram of material for one screen.14 With μPIHn both forms I and II of ACM were obtained using less than one milligram of material (Figure 3). Form I of ACM was crystallized by room temperature evaporation of aqueous solutions in the presence of acidic polymers whereas form II nucleated on polymers within the nonpolar aromatic library (see Supporting Information).
Figure 3.

Raman spectra of acetaminophen forms I and II obtained directly from crystals on the μPIHn plate.
ROY
ROY, an intermediate in the production of the pharmaceutical olanzapine, is known for the color of its red, orange, and yellow polymorphs.21 Using μPIHn, four of the seven structurally characterized forms were obtained: red prism (R), yellow needle (YN), orange needle (ON), and yellow prism (Y) (Figure 4). Red and yellow prisms nucleated on polymers within the polar nitrogen library. However, polymers in the nonpolar aromatic library facilitated the formation of yellow needles. Orange needles were found on polymers in the acidic library (see Supporting Information).
Figure 4.
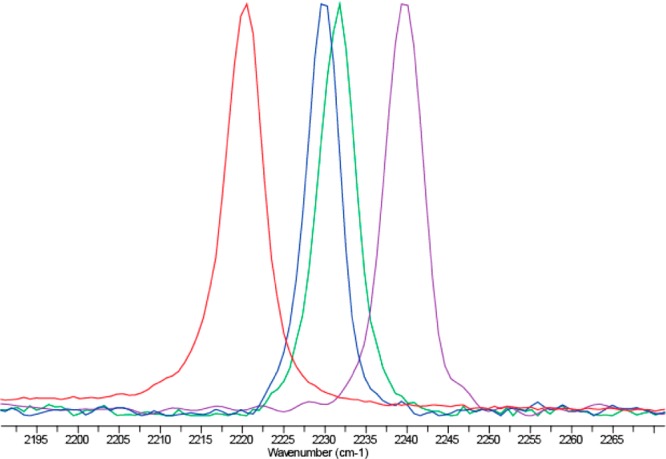
Raman spectra of the diagnostic nitrile region for ROY, in order from left to right: red prism, yellow needles, orange needles, yellow prims, obtained directly from crystals on a μPIHn plate.
Tolfenamic Acid
Tolfenamic acid (TA) is a pentamorphic, nonsteroidal anti-inflammatory drug.16 Previously, when TA was subjected to traditional PIHn screening, five polymorphs were found, with three forms discovered for the first time.16 Now with μPIHn all five known forms of TA were obtained using only 0.2 mg of TA (Figure 5). Forms I, II, and V of TA were found to nucleate on polymers within the polar nitrogen library, whereas forms III and IV nucleated on polymers in the nonpolar aromatic library (see Supporting Information).
Figure 5.

Raman spectra of tolfenamic acid forms I, II, III, VI, and V, obtained directly from crystals on a μPIHn plate.
Curcumin
Curcumin is the primary curcuminoid in the spice turmeric. Curcumin has been found to act as an anti-inflammatory, anticancer, and anti-HIV agent.25 Nangia and co-workers discovered two new polymorphs of curcumin while attempting to form cocrystals.22 All three polymorphs of curcumin were found in the present study (Figure 6). Form I and II formed on polymers within the polar nitrogen library, whereas form III nucleated on polymers within the acidic library (see Supporting Information).
Figure 6.

Raman spectra of curcumin forms I, II, and III, obtained directly from crystals on a μPIHn plate.
In the present study, automated Raman mapping was used to identify all of the pharmaceutical polymorphs. The above results illustrate that using ∼1 mg is viable for efficient polymorph screening for all of the compounds studied with μPIHn. However, the polymers responsible for promoting the formation of a particular polymorph in some cases were different from those of traditional PIHn. For example, with μPIHn, forms II and V of tolfenamic acid were found to nucleate on polymers within the polar nitrogen library whereas with traditional PIHn, these forms were obtained exclusively on polymers within the aromatic library. This difference may arise from the dramatic increase in the rate of evaporation of the crystallizing solution with μPIHn as compared with traditional PIHn. This enhanced evaporation rate is a direct result of the extremely small amount of solvent that is printed into each depression (∼0.3 μL) and the relatively open nature of conducting crystallization on an open plate. Despite this drastic difference in the kinetics of the crystallization, the efficacy of PIHn was still maintained.
The above results have important implications for the stage at which comprehensive polymorph discovery can take place. Solid form screening, as currently practiced, requires substantial sample quantities and it has thus far not been feasible to perform solid form screening as an early stage selection criterion for choosing which bioactive compounds to advance in the pipeline. Hence, the process by which a drug candidate is chosen neglects solid form considerations until a rather late stage where the cost of failure is greater.26,27 With μPIHn only a small amount of material is needed in order to study the potential polymorphism of a newly synthesized compound. Therefore, this new polymorph discovery platform can shift solid form considerations to an earlier stage in the pharmaceutical development process.
PIHn has been transformed into a high density format in which hundreds of distinct polymers are arrayed on one substrate, making automated, high throughput analysis possible. This new format is dissimilar from traditional PIHn in that the amount of polymer, the substrate on which the crystallizations occur, the volume of solvent utilized for crystallization, and the total amount of material used for the crystallization (∼1 mg) have been decreased dramatically. The reduction in polymer thickness yields Raman spectra with minimal spectral interference from the polymer heteronucleant, enabling completely automated analysis.
From the present study, it is apparent that although aspects of the crystallizations with μPIHn have changed from traditional PIHn, the method’s efficacy has been maintained. This is a direct result of the mechanism of PIHn: it is a surface-mediated process dominated by functional group interactions at the polymer-crystal interface, and is therefore independent of the amount of polymer present.17,18 μPIHn can now be implemented to study the potential of polymorphism in newly synthesized compounds. As a result of the unique configuration of this platform, countless crystallization conditions can be explored in the presence of hundreds of distinct polymers including, but not limited to, varying parameters such as the temperature,9 the degree of supersaturation, and solvent, enabling the structural landscape of a compound to be thoroughly explored. Although it is not possible to determine if all of the polymorphs of a compound have been found, by conducting a comprehensive experimental screening in combination with modern methods for computationally predicting which polymorphs are viable on the crystal energy landscape, one can have high confidence that all relevant polymorphs have been discovered. By considering all possible solid forms early in the drug development process, knowledge of solid form diversity can be leveraged to select which drug candidates to advance in the pipeline.
Experimental Procedures
Preparation of the Polymer Libraries
The components used to build the nonpolar aromatic polymer library were 4-acetoxystyrene (AOS), n-butyl methacrylate (n-BuMA), tert-butyl methacrylate (t-BuMA), benzyl methacrylate (BzMA), methyl methacrylate (MMA), styrene (STY), and divinylbenzene (DVB). The components used to build the polar nitrogen polymer library were 2-methyl-2-nitropropyl methacrylate (MNPMA), methacrylonitrile (MAN), 2-(dimethylamino)ethyl methacrylate (DMAEMA), N,N-dimethylmethacrylamide (DMMAA), 2-vinylpyridine (2VP), 4-vinylpyridine (4VP), and divinylbenzene (DVB). The components used to build the acidic polymer library are methyl methacrylate (MMA), acrylic acid (AA), methacrylic acid (MAA), 2-hydroxyethyl methacrylate (HEMA), 2-ethoxyethyl methacrylate (EEMA), styrene (STY), and divinylbenzene (DVB). For each library six 1:1 (v/v) monomer solutions in ethanol were dispensed as 90 pair wise combinations of varied ratios (86:14, 71:29, 57:43, 43:57, 29:71, and 14:86) and six pure monomer solutions by a Gilson 215 liquid handler to a volume of 120 μL. To this was added 40 μL of a 1:1 solution of DVB in ethanol containing 2 mol % 2,2′-Azobis(2-methylpropionitrile) (AIBN) with respect to DVB. The three 96 well plates containing the monomer solutions were transferred into a flat bottom 384 well plate by using an Eppendorf epmotion 5070 liquid handling robot. Using a pin tool composed of Delrin combs in a PMMA lattice four prints were performed from a 384 well plate containing monomer solutions onto the depressions on the laser etched quartz slide. In order to print all 288 monomer solutions four prints were performed from the 384 well plate onto the quartz slide (see Supporting Information). After each print the monomer solutions were photopolymerized with four 15 W UVA bulbs in an atmosphere of N2 for 1 min. Following polymerization, the μPIHn plates were annealed at 85 °C under vacuum for 2 h to produce the cross-linked polymer libraries.
Supporting Information Available
Pin tool preparation, the printing procedure implemented to produce the μPIHn plate, crystallization procedure for each compound, the results of the crystallizations, that is, which polymers facilitated the formation of each polymorph, the Raman spectra for the experiment examining the effect of well depth on Raman intensity, and the Adobe Illustrator files used for making all of the pin tools. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported by the National Institute of Health Grant Number RO1 GM106180.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Bernstein J.Polymorphism in Molecular Crystals; Oxford University Press: New York, 2002. [Google Scholar]
- Davey R.; Garside J.. From Molecules to Crystallizers; Oxford University Press: New York, 2000. [Google Scholar]
- Lee A. Y.; Ulman A.; Myerson A. S. Crystallization of amino acids on self-assembled monolayers of rigid thiols on gold. Langmuir 2002, 18155886–5898. [Google Scholar]
- Kang J. F.; Zaccaro J.; Ulman A.; Myerson A. Nucleation and growth of glycine crystals on self-assembled monolayers on gold. Langmuir 2000, 1683791–3796. [Google Scholar]
- Capacci-Daniel C.; Gaskell K. J.; Swift J. A. Nucleation and growth of metastable polymorphs on siloxane monolayer templates. Cryst. Growth Des. 2010, 102952–962. [Google Scholar]
- Ulman A.; Kang J. F.; Shnidman Y.; Liao S.; Jordan R.; Choi G. Y.; Zaccaro J.; Myerson A. S.; Rafailovich M.; Sokolov J.; Fleischer C. Self-assembled monolayers of rigid thiols. J. Biotechnol. 2000, 743175–88. [DOI] [PubMed] [Google Scholar]
- Mitchell C. A.; Yu L.; Ward M. D. Selective nucleation and discovery of organic polymorphs through epitaxy with single crystal substrates. J. Am. Chem. Soc. 2001, 1234410830–10839. [DOI] [PubMed] [Google Scholar]
- Munroe A.; Croker D.; Hodnett B. K.; Seaton C. C. Epitaxial growth of polymorphic systems: The case of sulfathiazole. CrystEngComm 2011, 13195903–5907. [Google Scholar]
- McKellar S. C.; Urquhart A. J.; Lamprou D. A.; Florence A. J. Polymer templating of supercooled indomethacin for polymorph selection. ACS Comb. Sci. 2012, 143155–159. [DOI] [PubMed] [Google Scholar]
- Lee M. K.; Lee H.; Kim I. W.; Lee J. Novel polymorphic form of adefovir dipivoxil derived from polymer-directed crystallization. Pharmazie 2011, 6610766–770. [PubMed] [Google Scholar]
- Sudha C.; Nandhini R.; Srinivasan K. Polymer-induced selective nucleation of mono or ortho polymorphs of paracetamol through swift cooling of boiled aqueous solution. Cryst. Growth Des. 2014, 142705–715. [Google Scholar]
- Xu A. W.; Dong W. F.; Antonietti M.; Colfen H. Polymorph switching of calcium carbonate crystals by polymer-controlled crystallization. Adv. Funct. Mater. 2008, 1881307–1313. [Google Scholar]
- Chen J. H.; Shao M.; Xiao K.; He Z. R.; Li D. W.; Lokitz B. S.; Hensley D. K.; Kilbey S. M.; Anthony J. E.; Keum J. K.; Rondinone A. J.; Lee W. Y.; Hong S. Y.; Bao Z. A. Conjugated polymer-mediated polymorphism of a high performance, small-molecule organic semiconductor with tuned intermolecular interactions, enhanced long-range order, and charge transport. Chem. Mater. 2013, 25214378–4386. [Google Scholar]
- Price C. P.; Grzesiak A. L.; Matzger A. J. Crystalline polymorph selection and discovery with polymer heteronuclei. J. Am. Chem. Soc. 2005, 127155512–5517. [DOI] [PubMed] [Google Scholar]
- Grzesiak A. L.; Matzger A. J. Selection and discovery of polymorphs of platinum complexes facilitated by polymer-induced heteronucleation. Inorg. Chem. 2007, 462453–457. [DOI] [PubMed] [Google Scholar]
- Lopez-Mejias V.; Kampf J. W.; Matzger A. J. Polymer-induced heteronucleation of tolfenamic acid: Structural investigation of a pentamorph. J. Am. Chem. Soc. 2009, 131134554–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Mejias V.; Knight J. L.; Brooks C. L.; Matzger A. J. On the mechanism of crystalline polymorph selection by polymer heteronuclei. Langmuir 2011, 27127575–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland A. A.; Lopez-Mejias V.; Matzger A. J.; Chen Z. Peering at a buried polymer-crystal interface: Probing heterogeneous nucleation by sum frequency generation vibrational spectroscopy. Langmuir 2011, 2762162–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Mejias V.; Kampf J. W.; Matzger A. J. Nonamorphism in flufenamic acid and a new record for a polymorphic compound with solved structures. J. Am. Chem. Soc. 2012, 134249872–9875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberski A. R.; Tizzard G. J.; Diaz-Mochon J. J.; Hursthouse M. B.; Milnes P.; Bradley M. Screening for polymorphs on polymer microarrays. J. Comb. Chem. 2008, 10124–27. [DOI] [PubMed] [Google Scholar]
- Yu L. A. Polymorphism in molecular solids: An extraordinary system of red, orange, and yellow crystals. Acc. Chem. Res. 2010, 4391257–1266. [DOI] [PubMed] [Google Scholar]
- Sanphui P.; Goud N. R.; Khandavilli U. B. R.; Bhanoth S.; Nangia A. New polymorphs of curcumin. Chem. Commun. 2011, 47175013–5015. [DOI] [PubMed] [Google Scholar]
- Leiws I.; Edwards H.. Handbook of Raman Spectroscopy; Marcel Dekker, Inc: New York, 2001; Vol. 28. [Google Scholar]
- Barbulovic-Nad I.; Lucente M.; Sun Y.; Zhang M. J.; Wheeler A. R.; Bussmann M. Bio-microarray fabrication techniques—A review. Crit. Rev. Biotechnol. 2006, 264237–259. [DOI] [PubMed] [Google Scholar]
- Hatcher H.; Planalp R.; Cho J.; Tortia F. M.; Torti S. V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65111631–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almarsson O.; Zaworotko M. J. Crystal engineering of the composition of pharmaceutical phases. Do pharmaceutical co-crystals represent a new path to improved medicines?. Chem. Commun. 2004, 17, 1889–1896. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Xi H. M.; Ediger M. D.; Richert R.; Yu L., Diffusion-controlled and “diffusionless” crystal growth near the glass transition temperature: Relation between liquid dynamics and growth kinetics of seven ROY polymorphs. J. Chem. Phys. 2009, 131, (7). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



