Abstract
Objective
We compare different dosimetric parameters in cervical cancer patients receiving concurrent chemotherapy and three-dimensional conformal radiotherapy (3DCRT) or intensity-modulated radiation therapy (IMRT) and explore the incidence of hematological toxicity (HT) in these patients.
Methods
Twenty patients receiving 3DCRT or IMRT and 4 weekly doses of cisplatin (25 mg/m2/w) were studied. The volumes of bone marrow receiving 10, 20, 30, 40 and 50 Gy or greater (V10, V20, V30, V40, and V50, respectively) were calculated. The HT was graded according to the guidelines of the Radiation Therapy Oncology Group system. The associations between dosimetric parameters and HT and chemotherapy delivery were analyzed.
Results
The bone marrows V30, V40, and V50 were lower in the IMRT group than in the 3DCRT group (62.93% vs 76.91%, 31.36% vs 39.60%, and 9.79% vs 15.44%, respectively). No statistical difference was observed for both V10 and V20. Acute hematologic toxicity occurred in both groups but was more frequent in the 3DCRT group. The percentage of patients with grade 2 and worse leukopenia and neutropenia was 90% and 80% in the 3DCRT group, whereas it was 80% and 40% in the IMRT group. The median nadir of white blood cells and the absolute neutrophil count were significantly lower in the 3DCRT group than in the IMRT group (1.96 × 109 vs 2.72 × 109 and 1.09 × 109 vs 1.86 × 109, respectively).
Conclusion
The IMRT reduced the volume of bone marrow irradiated at the higher doses and the incidence and severity of acute hematologic toxicity in cervical cancer patients undergoing concurrent chemoradiotherapy.
Key Words: Hematological toxicity, Cervical cancer, Concurrent chemoradiotherapy, Three-dimensional conformal radiotherapy, Intensity-modulated radiation therapy, Bone marrow
Cisplatin-based concurrent chemoradiotherapy is the standard treatment practice for locally advanced cervical cancers because it is more effective at improving survival than radiotherapy alone. However, adverse reactions, especially a high incidence of acute hematologic toxicity, often result in delayed or missed chemotherapy treatments, which impact the prognosis.1 Therefore, it is imperative to reduce the incidence of acute hematologic toxicity in the clinical application of chemoradiotherapy.
More than 50% of the activity of the bone marrow (BM) is located in the lumbar sacrum, ilium, ischium, pubis, and proximal femur,2 and these regions are subject to varying degrees of exposure during pelvic radiotherapy for cervical cancer. Most studies have confirmed that the myelosuppression observed in the patients receiving concurrent chemotherapy and pelvic radiation therapy is related to the volume of BM receiving 10 or 20 Gy. Therefore, it is possible to reduce the incidence of acute hematologic toxicity by reducing the volume to low-dose irradiation.3,4 Three-dimensional conformal radiotherapy (3DCRT) and intensity-modulated radiation therapy (IMRT) are 2 techniques that are currently used for the treatment of pelvic cancer. The IMRT reduces the dose to normal tissues for whole pelvic RT,3,5,6 and smaller volumes of BM were exposed to radiation in IMRT compared with 3DCRT7; however, no consensus was reached on the ability of IMRT to reduce hematological toxicity (HT) compared with 3DCRT techniques. Herein, we retrospectively analyzed the dosimetric parameters of 20 patients who received 3DCRT or IMRT for the treatment of cervical cancer and analyzed incidence of hematologic toxicity to determine whether it is advantageous to use IMRT in concurrent chemoradiotherapy for the treatment of cervical cancer in the clinic.
MATERIALS AND METHODS
Patients
We analyzed the clinical records of 40 patients with cervical carcinoma undergoing treatment with concurrent cisplatin-based chemoradiotherapy at our institution between January 2013 and December 2013. Twenty-one of the tumors were stage II, and 19 were stage III according to the International Federation of Gynecology and Obstetrics staging criteria (2009). All the tumors were confirmed as squamous cell carcinomas. Twenty of the patients received 3DCRT, and the other 20 patients received IMRT. All patients received concurrent chemotherapy with cisplatin 25 mg/m2/w from the second week of radiotherapy. High-dose-rate intracavitary brachytherapy was performed after pelvic radiotherapy (4–5 fractions of 5 Gy per fraction to point A).
Patient Positioning and Computed Tomography Scan
Virtual simulation in supine position was performed using Philips 16-slice spiral computed tomography (CT) scanning with a section thickness of 5 mm. The CT scans were obtained from the L2 vertebral body to the lower edge of the ischial tuberosity. Images were transferred to the Pinnacle3 V7.0 workstations for analysis.
Target Delineation
The target volumes were defined according to the International Commission on Radiation Units and Measurement Reports 62. Gross tumor volume consisted of primary tumor and metastasis in the lymph nodes. In general, pelvic lymph node metastasis was defined by the presence of isolated lymph nodes with a diameter of greater than 10 mm or clusters of lymph nodes. The clinical target volume (CTV) consisted of the upper one half of the vagina, parametrial tissues, uterus, and regional lymph nodes including common, internal, and external iliac nodes (with abdominal aortic bifurcation as the CTV superior margin). Planning target volume was defined as a uniform 5-mm expansion of the CTV boundary. The organs at risk were delineated, including the small intestine, rectum, bladder, BM, and bilateral femoral heads. For each patient, the external contour of all bones in the target was delineated on the planning CT and was used as a surrogate for the pelvic BM.2 The treatment targets for the 40 patients were modified and confirmed by the same senior physician. The treatment protocol was executed by the same physicist.
Treatment Planning
Dose prescription of the pelvic external beam radiation by 3DCRT and IMRT were set at 50 Gy/5 weeks (10-MV x-ray), and all plans were normalized to cover 95% of the planning target volume with 100% of the prescribed dose. After external beam radiation, intracavitary implants using high-dose-rate brachytherapy (192Ir) were given in all cases with a point A dose of DT24-30Gy for 4 to 5 fractions.
The BM volumes receiving 10 or more, 20, 30, 40, and 50 Gy (V10, V20, etc) from pelvic radiation were quantified, and the dose to pelvic BM from brachytherapy was considered negligible.
Chemotherapy
Patients were administered cisplatin (25 mg/m2) weekly concurrently with radiotherapy, which started on the second week of treatment. Patients received 4 cycles of chemotherapy. Complete blood counts were performed once a week from the start of radiotherapy until 2 weeks after completion of the radiotherapy. If the response to chemotherapy was significant, the side effects were evaluated immediately. Cisplatin administration was continued provided the following values were observed: white blood cell (WBC) count 2.0 × 109/L or less, absolute neutrophil count (ANC) of 1.0 × 109/L or less, or platelet (PLT) count of 50 × 109/L or less.
HT End Points
The HT was graded according to the Radiation Therapy Oncology Group acute radiation morbidity scoring criteria.8 The baseline nadir counts of the WBC, ANC, hemoglobin (Hgb), and PLT were recorded.
Statistical Analysis
Data analysis was performed using SPSS 17.0 software (SPSS Inc, Chicago, IL). Two-tailed t tests were performed to compare groups. A P < 0.05 was considered statistically significant.
RESULTS
Clinical Data
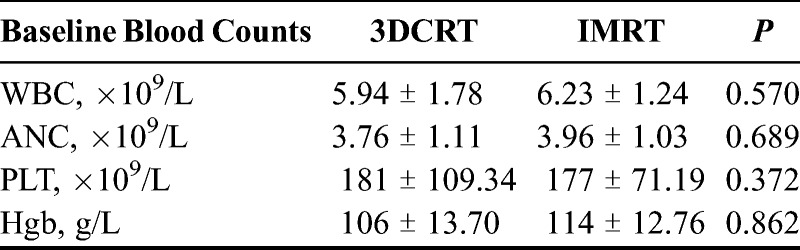
Forty patients (median age, 52 years; age range, 41–64 years) with stage II or III cervical cancer received concurrent chemoradiotherapy. Of these patients, 20 received 3DCRT, and the other 20 received IMRT. No significant differences were observed between the 3DCRT and IMRT groups in the baseline counts of leukocytes (5.94 × 109/L vs 6.23 × 109/L), neutrophils (3.76 × 109/L vs 3.96 × 109/L), PLT (181 × 109/L vs 177 × 109/L), and Hgb (106 vs 114 g/L) (Table 1). There was no incidence of delays or breaks in pelvic RT (range, 35–40 days), and all patients were administered high-dose brachytherapy. Three patients in the 3DCRT group had their chemotherapy treatment interrupted because of the presentation of HT after 1 to 3 cycles of chemotherapy. In contrast, all the patients in the IMRT group completed the 4 cycles of chemotherapy without any interruptions in treatment.
TABLE 1.
The baseline blood counts for 3DCRT and IMRT
Dosimetric Parameters
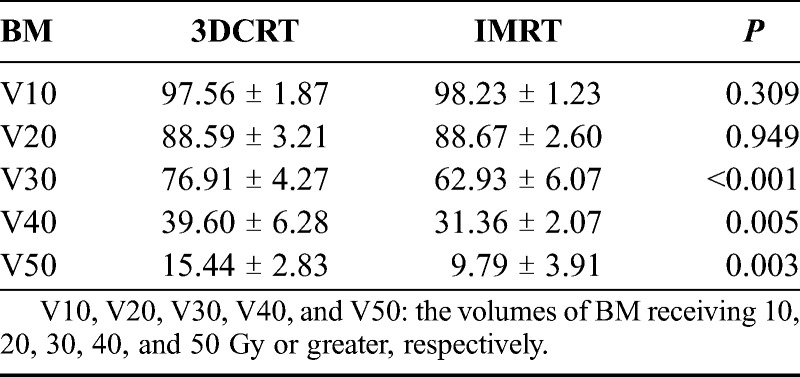
As shown in Table 2, the means of BM V30, V40, and V50 in the IMRT group were 62.93%, 31.36%, and 9.79%, respectively, which were significantly lower than those in the 3DCRT group (76.91%, 39.60%, and 15.44%, respectively). No statistical differences were observed for V10 and V20.
TABLE 2.
BM volumes irradiated at different dosages of 3DCRT and IMRT
Hematologic Toxicity
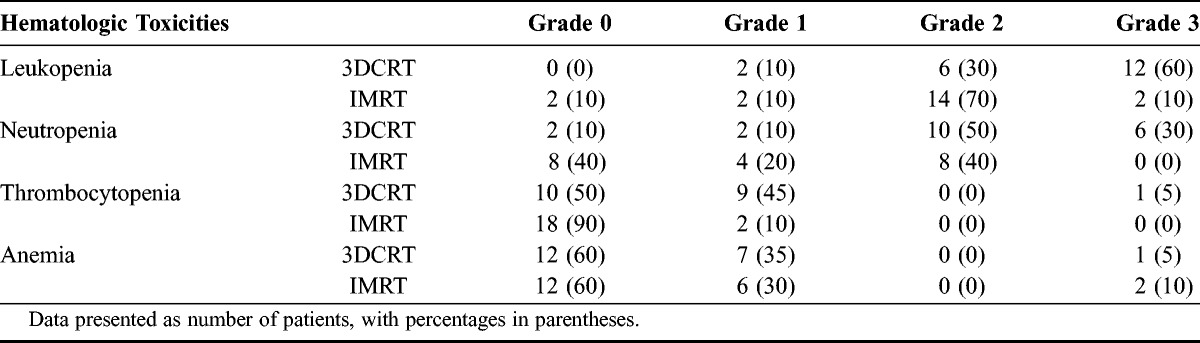
Leukopenia and neutropenia were commonly observed in the patients, whereas the incidence of anemia and thrombocytopenia was not significantly different between the groups. The percentages of patients developing either acute leukopenia, neutropenia, anemia, or thrombocytopenia were 100%, 90%, 50%, and 40%, respectively, in the 3DCRT group and 90%, 60%, 10%, and 30%, respectively, in the IMRT group. The percentages of patients with grade 2 or higher leukopenia and neutropenia were 90% and 80%, respectively, in the 3DCRT group and only 80% and 40%, respectively, in the IMRT group as seen in Table 3.
TABLE 3.
Incidence and severity of acute hematologic toxicity in patients receiving 3DCRT or IMRT
Hematologic Nadirs
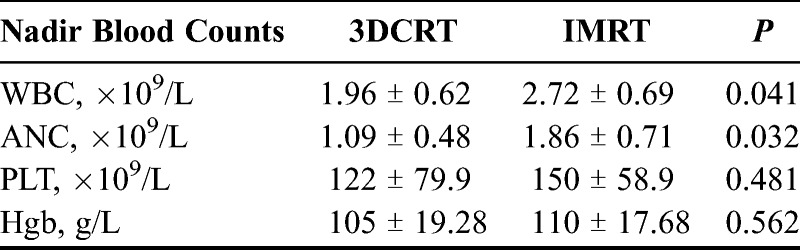
The median WBC and ANC nadirs of patients in the 3DCRT group were significantly lower than those observed in the IMRT group. No significant differences (P > 0.05) were observed for the PLT and Hgb values between the 2 groups as shown in Table 4.
TABLE 4.
Blood cell counts of patients receiving concurrent chemoradiotherapy with 3DCRT or IMRT
DISCUSSION
Acute hematologic toxicity is a common problem in patients receiving concurrent chemoradiotherapy for treatment of cervical cancer. Previous studies have suggested that techniques designed to reduce BM irradiation, such as IMRT, could reduce the incidence of HT.2,9,10 In this study, we analyzed and compared the incidence of acute HT in patients receiving IMRT or 3DCRT. We found that there were significant differences in the WBC counts and ANC nadirs between the 2 groups, consistent with previous reports.2,10 In addition, the patients receiving 3DCRT treatment experienced more drastic drops in WBC and ANC counts than the patients receiving IMRT. In the event of grade 3 HT, understandably, patients had trouble tolerating chemotherapy. In our study, we found that the percentages of patients with grade 3 leukopenia or neutropenia were 60% and 30%, respectively, in the 3DCRT group, compared with 10% and 0%, respectively, in the IMRT group. In addition, the median WBC and ANC nadirs of patients in the 3DCRT group were 1.96 × 109/L and 1.09 × 109/L, respectively, compared with 2.72 × 109/L and 1.86 × 109/L, respectively, in the IMRT group.
Parker and colleagues11 reported that distant metastasis after concurrent chemoradiotherapy in cervical cancer patients was more common in those patients undergoing less cycles of chemotherapy due to the delays or breaks in the treatment cycle caused by acute hematologic toxicity. Therefore, by reducing the BM damage using the IMRT technique, it is possible to improve the treatment tolerance in patients being administered with concurrent chemoradiotherapy.
Despite published reports2,9,12 on the relationship between HT and the volume of pelvic BM receiving 10 Gy or greater and 20 Gy (V10 and V20, respectively) in patients undergoing concurrent chemotherapy and pelvic RT, we did not observe this correlation in our study. Specifically, we analyzed the dosimetric parameters of BM and found that the BM V30, V40, and V50 in the IMRT group were significantly lower than those in the 3DCRT group, suggesting that IMRT effectively reduces the volume of the pelvic BM exposed to the higher doses of irradiation. However, there was no significant difference in the BM volume receiving the lower doses of irradiation between the 2 groups. One possible explanation is that more radiation fields are used in IMRT to improve the dose distribution uniformity and conformity in the target, which results in a larger exposure of normal tissues to the lower doses of radiation. The RTOG 0418 phase II clinical trial13 showed that the hematologic toxicity of chemoradiotherapy for cervical cancer is related to the mean dose and the BM volume receiving a dose greater than 40 Gy, although it was thought that the plan did not specify a limit radiation dose to the BM itself might be the reason. Mell and co-workers6 compared the BM-sparing IMRT and conventional (4-field box and anteroposterior-posteroanterior) techniques in the treatment of cervical cancer and also found that IMRT can reduce the volume of BM receiving high doses of radiation.
Some studies have reported that BM volumes receiving more than 30 to 50 Gy of radiation needed an extended time to recover and sometimes experienced irreversible damage.14,15 In some instances, chemotherapy may be delayed for several months or even 1 year because of this long-term BM suppression.16 Therefore, reducing the volume of BM irradiated at the higher doses is paramount. Although we did not observe a linear correlation between the incidence of acute hematologic toxicity and the volume of tissue receiving a high dose of irradiation, the incidence of grade 2 or 3 hematologic toxicity in the IMRT group was significantly lower than that observed in the 3DCRT group. In addition, the nadirs of WBC and ANC were also higher than those observed in the 3DCRT group. These findings suggest that IMRT reduced the volume of tissue exposed to high doses of radiation and, to a certain extent, preserved the hematopoietic function of BM or its ability to repair damage, an observation that requires further analysis.
Recently, there have been several reports on the applications of BM-sparing IMRT, which was thought to effectively reduce the irradiated volume of BM compared with conventional techniques. However, consensus for the BM delineation and its significance is lacking. Lujan and co-workers5 suggested that the pelvic BM with hematopoietic activity was mostly located in the ala of ilium, so it is advisable to limit the exposure of this tissue to radiation. In a parallel study, Mell and co-workers2 divided the pelvic bone into 3 regions and analyzed the incidence of clinical HT. They found that the radiation dose to the sacral vertebrae and the low pelvic regions were closely linked to the occurrence of acute hematologic toxicity. However, because both areas are near the irradiated target, they cannot be spared without affecting the target dose distribution. The distribution of BM containing red (active) and yellow (inactive) marrow is significantly different among individuals.17 Therefore, conventional CT scanning cannot distinguish between the yellow and red BMs, whereas functional imaging techniques such as magnetic resonance imaging, positron emission tomography, and single-photon computed emission tomography can identify the red BM.18–20 To date, however, the clinical studies on these functional imaging techniques all used small sample patient populations, so these techniques have not been widely used in the clinic. Therefore, more studies are needed to accurately and effectively locate the active BM in patients.
This study has some limitations. First, the entire bones were used as a surrogate for BM. As mentioned before, contouring methods based on functional imaging techniques, which can accurately and effectively locate the active BM, are warranted. Second, the clinical benefits of IMRT for reducing HT need to be further confirmed in randomized or prospective studies. Future studies are needed to improve the delivery and efficacy of IMRT.
In summary, compared with 3DCRT, IMRT represents a promising treatment approach to reduce the volume of BM receiving a high dose of radiation and the incidence of acute hematologic toxicity in cervical cancer patients undergoing concurrent chemoradiotherapy.
Footnotes
Written informed consent was obtained from all patients before the study, and the study was approved by the ethics committee of the First Affiliated Hospital of Xi’an Jiao Tong University.
Supported by the National Natural Science Foundation of China (no. 81071838) and the Department of Health Key Program of Shaanxi Province (no. 2010A02).
The authors declare no conflicts of interest.
REFERENCES
- 1. Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001; 358: 781– 786. [DOI] [PubMed] [Google Scholar]
- 2. Mell LK, Kochanski JD, Roeske JC, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2006; 66: 1356– 1365. [DOI] [PubMed] [Google Scholar]
- 3. Mutyala S, Thawani N, Vainshtein J, et al. Dose constraint recommendations and a predictive nomogram of incidence of hematological toxicity for cervix cancer patients treated with concurrent cisplatin and intensity modulated radiation therapy (IMRT). Int J Radiat Oncol Biol Phys. 2008; 72: S359– S360. [Google Scholar]
- 4. Rose BS, Aydogan B, Liang Y, et al. Normal tissue complication probability modeling of acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2011; 79: 800– 807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lujan AE, Mundt AJ, Yamada SD, et al. Intensity-modulated radiotherapy as a means of reducing dose to bone marrow in gynecologic patients receiving whole pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2003; 57: 516– 521. [DOI] [PubMed] [Google Scholar]
- 6. Mell LK, Tiryaki H, Ahn K-H, et al. Dosimetric comparison of bone marrow-sparing intensity-modulated radiotherapy versus conventional techniques for treatment of cervical cancer. Int J Radiat Oncol Biol Phys. 2008; 71: 1504– 1510. [DOI] [PubMed] [Google Scholar]
- 7. Erpolat O, Alco G, Caglar H, et al. Comparison of hematologic toxicity between 3DCRT and IMRT planning in cervical cancer patients after concurrent chemoradiotherapy: a national multi-center study. Eur J Gynaecol Oncol. 2014; 35: 62. [PubMed] [Google Scholar]
- 8. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995; 31: 1341– 1346. [DOI] [PubMed] [Google Scholar]
- 9. Mell LK, Schomas DA, Salama JK, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008; 70: 1431– 1437. [DOI] [PubMed] [Google Scholar]
- 10.Collaboration CfCCM-A. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta-analysis of individual patient data from 18 randomized trials. J Clin Oncol. 2008; 26: 5802– 5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parker K, Gallop-Evans E, Hanna L, et al. Five years’ experience treating locally advanced cervical cancer with concurrent chemoradiotherapy and high-dose-rate brachytherapy: results from a single institution. Int J Radiat Oncol Biol Phys. 2009; 74: 140– 146. [DOI] [PubMed] [Google Scholar]
- 12. Albuquerque K, Giangreco D, Morrison C, et al. Radiation-related predictors of hematologic toxicity after concurrent chemoradiation for cervical cancer and implications for bone marrow–sparing pelvic IMRT. Int J Radiat Oncol Biol Phys. 2011; 79: 1043– 1047. [DOI] [PubMed] [Google Scholar]
- 13. Klopp AH, Moughan J, Portelance L, et al. Hematologic toxicity in RTOG 0418: a phase 2 study of postoperative IMRT for gynecologic cancer. Int J Radiat Oncol Biol Phys. 2013; 86: 83– 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sacks EL, Goris ML, Glatstein E, et al. Bone marrow regeneration following large field radiation. Influence of volume, age, dose, and time. Cancer. 1978; 42: 1057– 1065. [DOI] [PubMed] [Google Scholar]
- 15. Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995; 31: 1319– 1339. [DOI] [PubMed] [Google Scholar]
- 16. Lhommé C, Fumoleau P, Fargeot P, et al. Results of a European Organization for Research and Treatment of Cancer/Early Clinical Studies Group phase II trial of first-line irinotecan in patients with advanced or recurrent squamous cell carcinoma of the cervix. J Clin Oncol. 1999; 17: 3136– 3142. [DOI] [PubMed] [Google Scholar]
- 17. Berg BCV, Malghem J, Lecouvet FE, et al. Magnetic resonance imaging of the normal bone marrow. Skeletal Radiol. 1998; 27: 471– 483. [DOI] [PubMed] [Google Scholar]
- 18. Basu S, Houseni M, Bural G, et al. Magnetic resonance imaging based bone marrow segmentation for quantitative calculation of pure red marrow metabolism using 2-deoxy-2-[F-18] fluoro-d-glucose-positron emission tomography: a novel application with significant implications for combined structure–function approach. Mol Imaging Biol. 2007; 9: 361– 365. [DOI] [PubMed] [Google Scholar]
- 19. Roeske JC, Mundt AJ. Incorporation of magnetic resonance imaging into intensity modulated whole-pelvic radiation therapy treatment planning to reduce the volume of pelvic bone marrow irradiated [Abstract]. Proceedings the 18th International Congress on Computer Assisted Radiology and Surgery. New York: Elsevier; 2004: 307– 312. [Google Scholar]
- 20. Roeske JC, Lujan A, Reba RC, et al. Incorporation of SPECT bone marrow imaging into intensity modulated whole-pelvic radiation therapy treatment planning for gynecologic malignancies. Radiother Oncol. 2005; 77: 11– 17. [DOI] [PubMed] [Google Scholar]


