Abstract
The present study aims at evaluating the impact of diatoms and copepods on microbial processes mediating nitrate removal in fine-grained intertidal sediments. More specifically, we studied the interactions between copepods, diatoms and bacteria in relation to their effects on nitrate reduction and denitrification. Microcosms containing defaunated marine sediments were subjected to different treatments: an excess of nitrate, copepods, diatoms (Navicula sp.), a combination of copepods and diatoms, and spent medium from copepods. The microcosms were incubated for seven and a half days, after which nutrient concentrations and denitrification potential were measured. Ammonium concentrations were highest in the treatments with copepods or their spent medium, whilst denitrification potential was lowest in these treatments, suggesting that copepods enhance dissimilatory nitrate reduction to ammonium over denitrification. We hypothesize that this is an indirect effect, by providing extra carbon for the bacterial community through the copepods' excretion products, thus changing the C/N ratio in favour of dissimilatory nitrate reduction. Diatoms alone had no effect on the nitrogen fluxes, but they did enhance the effect of copepods, possibly by influencing the quantity and quality of the copepods' excretion products. Our results show that small-scale biological interactions between bacteria, copepods and diatoms can have an important impact on denitrification and hence sediment nitrogen fluxes.
Introduction
Over the past century anthropogenic activities have dramatically increased the amount of reactive nitrogen on Earth [1]. It has been estimated that nitrogen inputs have increased as much as ten-fold in coastal ecosystems [2], [3]. As a result, these often nitrogen-limited areas [4] have experienced severe eutrophication, resulting in anoxia and changes in community structure [5]. Denitrification and anaerobic ammonium oxidation (anammox) are capable of countering eutrophication by removing reactive nitrogen from the ecosystem as nitrous oxide (N2O) or nitrogen gas (N2) [6]. In contrast, during dissimilatory nitrate reduction to ammonium (DNRA), nitrate (NO3 -) and nitrite (NO2 -) are reduced to ammonium (NH4 +), preserving reactive nitrogen in the system. In coastal environments, anammox, denitrification and DNRA are all catalysed in the anoxic sediment, but by different microbial assemblages [7].
Denitrification and DNRA are carried out by a different but diverse range of mostly heterotrophic microorganisms [8] and are assumed to be in situ mutually exclusive processes determined by the C/N ratio of the system [9]. DNRA is thought to be favoured in nitrate-limited environments rich in labile carbon [9], since the energy yield per nitrate reduced is higher for nitrate ammonification than for denitrification (the reduction of nitrate to ammonia consumes eight electrons rather than five in denitrification, thus more carbon can be oxidised per nitrate reduced; [10]). Anammox is apparently only conducted by members of the Planctomycetes group [11] and probably is a less important nitrogen sink than denitrification in nutrient-loaded coastal areas [12].
In the past decades, strong efforts have been made to unravel which benthic organisms affect nitrogen cycling in intertidal sediments and how they do this (e.g. [13], [14]). Macrofauna for example, has been shown to impact DNRA and denitrification by turbating the sediment (e.g. [15], [16]). Other studies focussed on the impact of microphytobenthos (e.g. [17], [18]) on denitrification. The effect of meiofauna (e.g. nematodes and copepods), the intermediate trophic level, on nitrogen fluxes has to date been almost completely neglected [19]. Although the effects of the meiofaunal bioturbation – confined to the superficial sediments – will be far less pronounced than those of macrofaunal bioturbation [20], these organisms can potentially impact benthic nitrate reduction in other ways. Meiofauna is, for instance, capable of eating its body weight equivalent in microorganisms each day [21]. By grazing on microphytobenthos and bacteria, meiofauna will not only counteract the effects of the microphytobenthos and bacteria on nitrate reduction, but also release high amounts of organic nitrogen and carbon [22] into the interstitial environment, thus potentially impacting the C/N ratio. We hypothesized that the meiofauna can impact nitrogen reduction in marine sediments through their grazing activity. The aim of this study was therefore to investigate the impact of meiofauna and its interactions with its food sources, diatoms and bacteria, on denitrification in marine sediments. For this purpose an experiment was setup in which all possible combinations of meiofauna, diatoms and bacteria were included. Harpactecoid copepods were used as meiofauna representative since they occur in high densities at the study site (230±194 ind. 10 cm-2, [23]) and have been well-studied in terms of both composition [23] and feeding ecology (e.g. [24]–[26]) in this tidal flat.
In the experiment, both nitrate reduction (the combined activity of denitrification, DNRA and anammox) and denitrification as such were measured as these biochemical reactions are relevant and important ecosystem functions in coastal sediments. Furthermore, nitrate reduction and denitrification can serve as proxies for the overall functioning of the benthic microbial community. In microcosm experiments with sediment, harpacticoid copepods (Crustacea, Copepoda) and diatoms from an intertidal flat (Paulina Polder, Westerschelde estuary, The Netherlands), nutrient dynamics and the potential for nitrate reduction and denitrification were monitored. Nitrate reduction and denitrification rates could not be measured in situ, as they are largely anaerobic processes whilst copepods are strictly aerobic. Both rates were therefore measured indirectly by making a subsample of the homogenised microcosm anaerobic and measuring the potential rates under non-carbon or -nitrogen limiting conditions.
Methodology
Field sampling
Silty sediment was collected from the intertidal mudflat Paulina (Westerschelde estuary, The Netherlands; 51°20′ N, 3°43′ E) in February 2013 by scraping the top layer (0–3 cm) of the sediment at low tide. Seawater (salinity: 19.3; 1.85±1.11 µM NO2; 122.02±8.17 µM NO3 -; 2.50±1.70 µM NH4 +; 0.73±0.60 µM PO4 3-; 88.89±0.16 µM Si4+; N = 3) was collected from the same site and was filtered over a 0.22 µm filter (Corning 500 mL Bottle Top Vacuum Filter) and stored in the dark at 4°C (filtered seawater: FSW). No permits were required for the sampling nor were there any endangered or protected species involved.
Experimental setup
Collected sediment was washed over a 250 µm sieve to remove all benthic fauna. The sieved sediment (average median grain size: 56.89±0.25 µm; N = 3) was divided in equal aliquots of 80 g in polyethylene containers (microcosms). The microcosms with ±2.5 cm of sieved sediment were stored frozen (−20°C) to kill all the remaining fauna. A microcosm was defrosted two days before the start of a treatment. After adding 60 ml of filtered seawater (FSW), the thawed sediment was thoroughly mixed. Right before the start of a treatment, the FSW was drained off.
The experimental design included a blank: the defaunated sediment in which only bacteria were present. To verify the effect of copepods and diatoms, independently of one another, on the bacteria, they were added separately to the microcosm. To cover the interaction effects between copepods and diatoms, both of them were added to the microcosm. In order to discriminate between the effects of the activity of the copepods themselves and the waste products that they produce, a treatment was included in which the spent medium from copepods was added to the microcosm.
Together with the positive control (increased NO3 -), this resulted in a total of six different treatments.
Each treatment starting with a microcosm containing 80 g of defaunated and thawed sediment: (1) Blank: +70 ml FSW; (2) Increased NO3 -: +0.1 mmol KNO3 in 70 ml FSW; (3) Copepod: +200 phototactic copepods collected from Paulina Polder in 70 ml FSW; (4) Diatom: +4×105 cells of Navicula sp. (36.27±2.30 µm; isolated from the study site in 2012) in 70 ml FSW (corresponds to chlorophyll a concentrations observed in the study site.); (5) Copepod+diatom: +4×105 cells of Navicula sp. and 200 copepods (as above) in 70 ml FSW; (6) Spent medium:+70 ml of FSW, in which 200 copepods were fed 4×105 Navicula cells over a period of one week. A visual (microscopic) screening of the treatments revealed that most diatoms had been eaten by the copepods after one week. Prior to the start of the treatment this spent medium was stored at −20°C after manually removing the copepods.
One hour after the start of the experiment, 10 ml of water was extracted for nutrient analysis (initial nutrient concentration) from each microcosm. All microcosms were then incubated for 7.5 days, at 15°C.
To study the effects of the photosynthetic activity of the diatoms on the nitrogen fluxes, all treatments were run (1) under a diurnal (12 h/12 h) light regime and (2) in the dark. Cold-white fluorescent lamps provided the necessary light at a rate of 20–25 µmol photons m−2 s−1. Four replicates were used for each treatment under each light condition. To avoid depletion of active nitrogen in the microcosms, half of the SW was renewed on day 5 and 6 of the experiment. At the end of the incubation period, 10 ml of SW was stored for nutrient analysis (final nutrient concentration).
An additional experiment was setup in which the blank and copepod+diatom treatment were repeated to verify the effects of copepods and diatoms on the oxygen pentration depths (Text S1).
Denitrification rates were measured using the so-called acetylene inhibition method [27] [16] (cf. Fig S1 which illustrates the design of the experiment). In the presence of acetylene the final reaction of denitrification, in which N2O is converted to N2, is inhibited, causing N2O to accumulate. The easily quantifiable N2O can then be measured as a proxy for denitrification. The rate at which NO3 - is consumed is a good proxy for the combined activity of all three reduction pathways.
At the end of each treatment, a serum vial was filled with 30 g (wet weight) sediment and 20 ml incubation water (collected from the treatments; Fig S1). To prevent nitrogen and carbon limitation, the water was supplemented with 0.5 mmol KNO3 and 1 mmol α-D-glucose. After vigorous shaking, 1 ml was extracted to determine the initial NO3 -/NO2 - concentration (t0). The vials were hermetically sealed and flushed five times with helium to remove oxygen. After adding 10% acetylene, the vials were incubated at 25°C under a constant stirring rate of 90 rpm. The N2O concentrations were measured every two hours by injecting 1 ml of headspace in a GC-TCD (Gas Chromatography- Thermal Conductivity Detector; MICRO E-0391, Interscience; LOD 13.55 ppm N2O). This was done at four time points (t1–t4) for each serum vial (Fig. S1). N2O concentrations were corrected for headspace volume changes, pressure and dissolving of the gas into the liquid phase. To ascertain potential side-effects of acetylene [28], the process was repeated with a technical replicate without acetylene (data not shown). At the second (t2) and final sampling event (t4) from the replicate without acetylene, 800 µl of fluid was extracted for later NO3 -/NO2 - determination (indicative for the NO3 - reduction activity; Fig. S1).
Nutrient analysis
Nutrient concentrations (NO3 -, NO2 -, NH4 + and PO4 -) of the samples collected at the start and the end of the incubation period (initial and final nutrient concentration) were analysed with an automatic chain (SAN plus segmented flow analyser, SKALAR) according to Beyst et al. [29].
Samples extracted from the serum vials for NO3 -/NO2 - determination were analysed differently because the above used method required higher sample volumes. The samples were centrifuged (14000 rpm, 5 min) and the supernatants were stored frozen (−20°C) prior to analysis. Analysis of NO3 - and NO2 - was based on a colorimetric method as described by Cataldo et al. [30], based on Griess [31] with adjustments from Navarro-Gonzálvez et al. [32].
Data analysis
The software package R 2.15.0. was used for data analysis. Differences in initial and final nutrient concentrations between the treatments and light conditions were detected using a two-way ANOVA on the rank transformed concentrations, performed in the software package R 2.15.0. Pairwise differences were unravelled using Dunnett's Modified Tukey-Kramer Pairwise Multiple Comparison Test (DTK) [33] using 95% confidence limits.
N2O production rates and NO3
- reduction rates were calculated according to both Magalhaes et al. [34], assuming no bacterial growth between the N2O, respectively NO3
-/NO2
-, samplings (i.e. between t0 and t4), and Stenström et al. [35], assuming that bacterial growth does occur. Magalhaes et al. [34] obtained the N2O production rates by dividing the N2O concentration at t4 by t4. Stenström et al. [35], however, propose an exponential regression to accommodate for the increasing gas production rate between samplings: 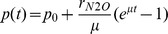 , with p = the amount of gas at time t, p0 = the amount of product at t0, rN2O = the N2O production rate (see below) and µ = the specific growth rate constant. Since the serum vials were flushed with helium, there was no N2O at the start of the incubation and p0 was set to zero. This function was adapted to fit the data for the NO3
- reduction (in the serum vials) to
, with p = the amount of gas at time t, p0 = the amount of product at t0, rN2O = the N2O production rate (see below) and µ = the specific growth rate constant. Since the serum vials were flushed with helium, there was no N2O at the start of the incubation and p0 was set to zero. This function was adapted to fit the data for the NO3
- reduction (in the serum vials) to 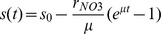 , with s = the amount of substrate (NO3
-) at time t, s0 = the amount of substrate at t0 and rNO3 = the initial NO3
- reduction rate, further referred to as “NO3
- reduction rate”.
, with s = the amount of substrate (NO3
-) at time t, s0 = the amount of substrate at t0 and rNO3 = the initial NO3
- reduction rate, further referred to as “NO3
- reduction rate”.
The N2O production rates (rN2O) obtained from the regression analysis are the production rates of N2O at the start (t0) of the denitrification potential experiment, i.e. after the 7.5 days incubation period (Fig. S1). Likewise, the initial NO3 - reduction rates (rNO3) express the NO3 - reduction rate at the start of the denitrification potential experiment. Thus the rates are corrected for any microbial growth occurring after the NO3 - and glucose addition.
Differences in the obtained N2O production and the NO3 - reduction rates between the different treatments and light conditions were analysed using a permutation-based two-way ANOVA [36] since the data were not normally distributed. Pairwise differences were analyzed using Wilcoxon rank-sum post-hoc test using 95% confidence limits, with Bonferroni correction.
Results
Viability
At the end of the incubation, viability of copepods and diatoms were checked in all treatments. Both microscopic observations (after collecting the cells with the lens-tissue method) and pulse-amplitude modulation (PAM; Maxi-Imaging PAM M-series, Walz) showed healthy and active diatom cells. Visual observations showed active copepods in all microcosms of the copepod and copepod + diatom treatment.
Nutrient levels
Initial nutrient concentrations
Except for the spent medium and the increased NO3 - treatment, the initial nutrient concentrations in the microcosms did not differ among treatments (ANOVA; p<0.05; Table 1). The nitrate concentration in the spent medium was significantly lower than in the other treatments, while in the increased NO3 - treatment, the nitrate concentration was, as expected, significantly higher. Nitrite was significantly lower in the spent medium and in the increased NO3 - treatment. The initial ammonium concentration was highest in the copepod + diatom treatment, but the difference was only significant compared to the increased NO3 - treatment. In contrast, the phosphate concentration in the spent medium treatment was 2.5–4 times higher than in the other treatments.
Table 1. Initial and final nutrient concentrations of the different treatments.
| Nutrients | NO3 -, mean ±SE (µM) | NO2 -, mean ±SE (µM) | NH4 +, mean ±SE (µM) | PO4 3-, mean ±SE (µM) | ||||
| Treatments | Initial*** | Final | Initial*** | Final | Initial* | Final** | Initial*** | Final*** |
| Blank | 125.93±5.00a | 2.24±0.79 | 4.35±0.20a | 0.23±0.10 | 99.67±6.54ab | 360.03±24.42a | 0.85±0.13a | 18.90±1.90 ab |
| Increased NO3- | 1135.29±258.98b | 1.29±0.36 | 3.43±0.21b | 0.25±0.03 | 105.56±5.06a | 400.71±22.01 ab | 0.75±0.11a | 15.30±1.74a |
| Copepod | 424.17±265.21a | 0.83±0.24 | 4.48±0.21a | 0.32±0.06 | 89.13±6.27 ab | 467.19±9.70b | 0.98±0.16a | 23.27±2.36b |
| Diatom | 130.86±4.04a | 0.76±0.21 | 4.17±0.14a | 0.20±0.03 | 88.97±6.75 ab | 372.18±21.35a | 0.94±0.17a | 13.79±1.83a |
| Copepod+diatom | 131.17±2.95a | 1.43±0.58 | 4.39±0.27a | 0.65±0.18 | 78.16±2.25b | 485.69±34.52 ab | 0.66±0.09a | 25.98±2.61b |
| Spent medium | 34.33±10.63c | 0.65±0.31 | 1.42±0.32c | 0.17±0.05 | 94.43±6.97 ab | 425.93±16.24 ab | 2.44±0.30b | 26.29±2.53b |
Significant differences of the ANOVAs indicated with symbols (*** ≤0.001<** ≤0.01<* ≤0.05< ˙ <0.1). The different superscripted letters indicate significant differences (P<0.05; DTK) between the treatments. Light conditions (not shown) did not alter the outcome of treatments (see text).
Final nutrient concentrations
After 7.5 days of incubation, the nutrient concentrations did not differ significantly between light conditions (two-way ANOVA; p>0.05).
At the end of the incubation period, nitrate and nitrate were almost completely depleted in all treatments (on average 1.12±0.03 µM and 0.30±0.01 µM, respectively; Table 1), despite renewal of half of the SW on days 5 and 6. The ammonium concentration more or less quadrupled towards the end of the experiment (average 384.08±3.04 µM). The final ammonium concentration in the copepod treatment was significantly higher than in the diatom and the blank treatment. The final ammonium concentration in the copepod + diatom treatment was the highest of all treatments, although it was not significantly different from the other treatments due to considerable variation between the replicates.
The same pattern was observed in the final phosphate concentrations. The phosphate concentrations strongly increased during the incubation period as the final average phosphate concentration (18.60±0.21 µM) was almost twenty times higher than the initial one.
Potential for nitrate reduction and denitrification
During the measurement of the denitrification potential, nitrate was consumed, while nitrite and nitrous oxide were produced (see Fig. S2 for the average NO3 -, NO2 - and N2O concentrations during the measurement of the denitrification potential). Since the exponential function proposed by Stenström et al. [35] had a significantly better fit than the linear regression proposed by Magalhaes et al. [34], rates were calculated with the exponential function.
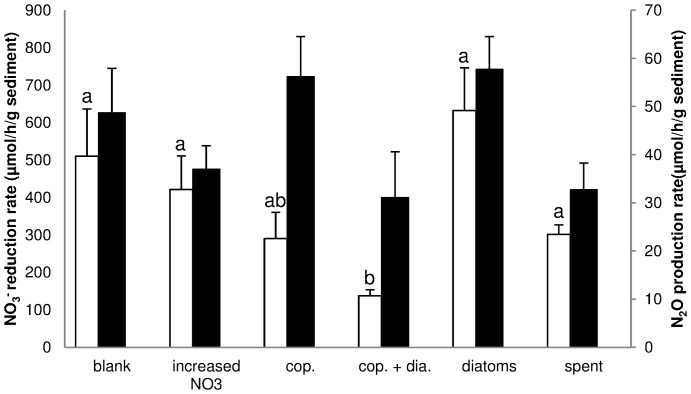
The NO3 - reduction and N2O production rates did not differ between light conditions. In contrast to the NO3 - reduction rate, which did not differ between treatments (Fig 1, black bars), the N2O production rate did significantly differ between the treatments (p<0.01; Fig. 1, white bars). The N2O production rate was significantly lower for the copepod + diatom treatment compared to the blank (p<0.001), diatom (p<0.001), increased NO3 - (p<0.001) and spent medium (p<0.001) treatments.
Figure 1. NO3 - reduction and N2O production rates.
NO3 - reduction rate (black bars; left y-axis) and N2O production rates (white bars; right y-axis) during the measurement of the denitrification potential for the different treatments (mean ± SE; cop. = copepods; cop. + dia. = copepod+ diatom). The different letters above the bar indicate significant differences (P<0.05; DTK) between the treatments. Light conditions (not shown) did not affect the outcome of treatments.
The correlation between N2O production and NO3 - reduction rates was weak but significantly positive (Pearson's r = 0.30, p<0.05).
Discussion
Effect of copepods
The presence of copepods or their spent medium resulted in elevated phosphate and ammonium concentrations in the microcosms. The copepods' outfluxes (including excretion products, moults, remnants of sloppy feeding) therefore proved to be an important source of both N and P which are, in coastal areas, potentially limiting nutrients [37].
Contrary to macrofauna, not much is known about the impact of meiofauna on nitrogen fluxes. Macrofauna seems to have its biggest impact through bioturbation (e.g. [15]). However, the oxygen penetration depth did not increase in the presence of copepods (Text S1), and consequently the effects of copepods are not related to oxygen. Since the N2O production rates of the copepod and their spent medium treatments were low, the copepods appeared to negatively impact denitrification, at least partially via their outfluxes. The outfluxes of the copepods are, apart from being an important nutrient source, a source of organic compounds and hence a substrate for bacterial growth [38]. Since organic matter loading may be one of the most important variables controlling denitrification in aquatic ecosystems [39], the impact of these carbon outfluxes should not be underestimated. The copepod outfluxes contain high amounts of labile carbon [22], which are known to stimulate DNRA over denitrification and anammox (Fig. 2, dashed arrow 1; [9]). Copepods can also indirectly stimulate labile carbon production by mechanically breaking down detrital particles [40], [41]. In addition, more organic matter results in a higher sulphate reduction rate (Fig. 2, dashed arrow 2; [42]). The main product of sulphate reduction is hydrogen sulphide, which inhibits denitrification and nitrification, but not DNRA [7]. These findings are supported by the higher final ammonium concentrations in the treatments with copepods or their spent medium. Furthermore, the NO3 - reduction rate did not differ significantly between the treatments, suggesting that the reduced denitrification activity in the treatments with copepods or their spent medium was compensated by another nitrate reducing process.
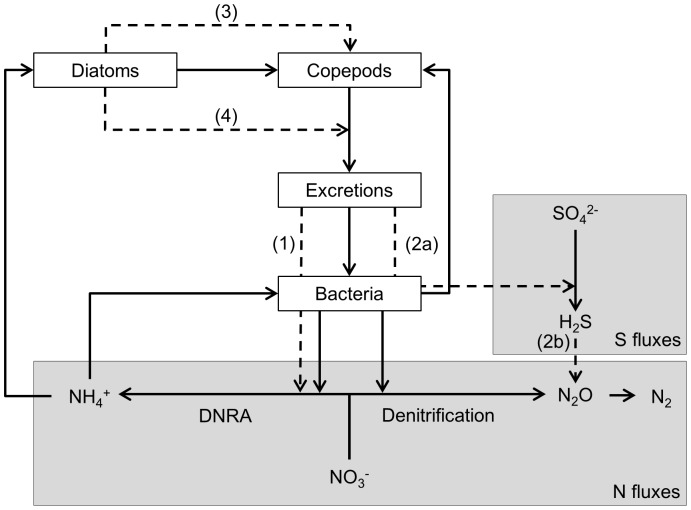
Figure 2. Summary of the assumed interactions to explain the observed differences in N2O production rates.
The assumed interactions which affect denitrification are indicated with dashed arrows. Bacteria mediated relevant reduction reactions of the nitrogen pathway and sulfur pathway are enclosed by grey boxes indicated with respectively ‘N fluxes’ and ‘S fluxes’. Copepods feed on both diatoms and bacteria, and produce excretion producs (excretions). Bacteria feed on the excretion produces and are also responsible for the reduction of SO4 2- to H2S and of NO3 - to NH4 + (DNRA) and N2O+N2 (denitrification) in the microcosm. The produced NH4 + is assimilated by both bacteria and diatoms. Copepods affect the N2O production rate by producing excretion products which provide an extra carbon source, of which mainly the DNRA bacteria can take advantage (1) and also enhances SO4 2- reduction (2a), which results in more H2S. The increased H2S inhibits denitrification (2b). Diatoms have no direct effect on the N2O production rate, but do have an indirect effect by enhancing the survival of the copepods (3) and influencing the quantity and composition of the copepods' excretion products (4).
Effects of diatoms
Since there were no differences between the final nitrate, nitrite and ammonium concentrations of the blank and the diatom treatment, diatoms seemed to have no net effect on the nitrogen fluxes in the microcosm. Likewise, N2O production rates did not differ between the blank and the diatom treatment either. It thus appears that diatoms had no or very little impact on denitrification in the microcosms. This is inconsistent with previous reports, where the presence of benthic microalgae generally had a negative impact on denitrification rates [43] as microalgae outcompeted the bacteria for nitrogen [18]. The opposite has however also been observed (e.g. [44]) were microalgae enhanced denitrification. It is unlikely that the inoculation concentration (2×105 diatoms/cm2) used in this study was too low to have a significant effect on the denitrification rate as it was comparable to the diatom concentrations in other studies were diatoms did have an effect on the nitrogen fluxes (e.g. [18]). It is, however, possible that positive and negative effects of the diatoms cancelled each other out or that the overall effect was too small to be detected. However, one should bear in mind that the used incubation time might be too short to obtain strong effects caused by the diatoms [18], [45].
Effects of diatoms + copepods
The negative effect of the copepods on denitrification was most pronounced and only significant when diatoms were added to the system. This suggests an important interaction effect between diatoms and copepods. Since the diatoms themselves had no effect on denitrification and nitrate reduction, it is unlikely that they were directly responsible for the difference between the copepods + diatoms and the copepods treatments. The diatoms can, however, influence the composition of the copepod outfluxes (Fig. 2, dashed arrow 4), which depends on the food type [22]. As diatoms are thought to be a better food source for copepods than bacteria (e.g. [26], [46]), they might also enhance the survival of the copepods and, accordingly, their activity and the quantity of the excretion products (Fig. 2, dashed arrows 3–4).
Effects of the nitrate addition
The N2O production rate for the increased NO3 - treatment was unexpectedly similar to the blank. In general, the denitrification rate is positively influenced by the nitrate load (e.g. [47], [48]), but such a relation was not observed here. However, a preliminary test in which we used a shorter, three and a half day long incubation period showed that the N2O production rate was almost twice as high for the increased NO3 - treatment compared to the blank (data not shown). This indicates that the supplemented nitrate was depleted within the first few days and that the denitrifying community changed accordingly.
Final considerations
Our findings have potentially important implications for our understanding of nutrient fluxes in marine sediment and the role of meiofauna. It was already known that meiofauna facilitates biomineralization of organic material and enhances nutrient regeneration [40]. This is an indirect process, by stimulating the bacterial community [40] through the production of excretion products [49] and bioturbation [40]. The present study suggests that these processes may also negatively impact denitrification. Consequently, more active nitrogen will be preserved in the ecosystem in the presence of meiofauna. Furthermore, they also increase the freely available phosphorus and carbon (this study, [22]). These elevated nutrient levels will benefit both bacteria and microphytobenthos. Our observations should, however, be interpreted with care as they were obtained from a short-term microcosm experiment. They might therefore not be representative for the highly dynamic estuarine sediments these organisms where obtained from. Our results do, however, prove that the interactions between meiofauna, diatoms and bacteria can potentially impact the nitrogen fluxes and that they should therefore not be neglected in future research. The time-dependent effect of the increased NO3 - treatment clearly illustrates the importance of the temporal scale in this setup. It might therefore prove useful for further research to investigate the effects of the different treatments at different time intervals. Furthermore, additional experiments (for instance relying on the 15N technique; [50]) will be necessary to fully unravel these fine-scale interactions.
Supporting Information
Design of the denitrification potential experiment. Incubation steps are indicated by dotted arrows. Manipulations are indicated by a dashed arrow. Each microcosm (here represented as a rectangle) was incubated for 7.5 days, after which water (blue) and sediment (brown) were transferred from the microcosm to the serum vials (represented as trapezoids). Glucose and potassium nitrate were added to the vial after which it was thoroughly homogenized. The headspace was flushed with helium to remove oxygen, after which acetylene was injected. The vials were sampled four times for N2O determination, every two hours.
(DOC)
NO3-, NO2- and N2O concentrations in the serum vials after the addition of 0.5 mmol KNO3 during the measurement of the denitrification potential (from t0 to t4). Amounts (µmol/h/g sediment) are averaged over all samples (all treatments), as is the time at with the sample was taken (Time; h:min) ± SE. N2O concentration(blue) plotted on the right y axis; NO3- (red) and NO2- (green) concentrations on the left y axis.
(DOC)
Measurement of oxygen penetration depth. To verify the effects of copepods and diatoms on the oxygen pentration depths, the experiment as described in the method section was repeated for the blank, diatom and copepod+diatom treatment. The methodology and results of the additional experiment are shown here.
(DOCX)
Acknowledgments
Evie De Brandt, Bram Vekeman, Sven Hoefman and Dirk Van Gansbeke are acknowledged for their assistance with the lab analyses. Frederik De Laender is acknowledged for his help with the regressions and improving the R-script. The authors thank the 2 reviewers for their comments that helped improve and clarify this manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All data files are available from figshare (http://dx.doi.org/10.6084/m9.figshare.1169927).
Funding Statement
This research was conducted within the frame of research project GOA 01GA1911W of the Special Research Fund at Ghent University (BOF-UGent) on "Understanding biodiversity effects on the functioning of marine benthic ecosystems". M. De Troch is a postdoctoral researcher financed by the same project. Kim Heylen is supported by the Flemish Fund for Scientific Research (FWO11/PDO/084). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Leach AM, Galloway JN, Bleeker A, Erisman JW, Kohn R, et al. (2012) A nitrogen footprint model to help consumers understand their role in nitrogen losses to the environment. Environmental Development 1: 40–66. [Google Scholar]
- 2. Paerl HW (2006) Assessing and managing nutrient-enhanced eutrophication in estuarine and coastal waters: Interactive effects of human and climatic perturbations. Ecological Engineering 26: 40–54. [Google Scholar]
- 3. Pätsch J, Serna A, Dähnke K, Schlarbaum T, Johannsen A, et al. (2010) Nitrogen cycling in the German Bight (SE North Sea)—Clues from modelling stable nitrogen isotopes. Continental Shelf Research 30: 203–213. [Google Scholar]
- 4. Howarth RW, Marino R (2006) Nitrogen as the limiting nutrient for eutrophication in coastal marine ecosystems: evolving views over three decades. Limnology and Oceanography 51: 364–376. [Google Scholar]
- 5. Koop-Jakobsen K, Giblin AE (2010) The effect of increased nitrate loading on nitrate reduction via denitrification and DNRA in salt marsh sediments. Limnology and Oceanography 55: 789. [Google Scholar]
- 6. Schreiber F, Wunderlin P, Udert KM, Wells GF (2012) Nitric oxide and nitrous oxide turnover in natural and engineered microbial communities: biological pathways, chemical reactions, and novel technologies. Front Microbiol 3: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. An S, Gardner WS (2002) Dissimilatory nitrate reduction to ammonium (DNRA) as a nitrogen link, versus denitrification as a sink in a shallow estuary (Laguna Madre/Baffin Bay, Texas). Marine Ecology Progress Series 237. [Google Scholar]
- 8. Herbert R (1999) Nitrogen cycling in coastal marine ecosystems. FEMS microbiology reviews 23: 563–590. [DOI] [PubMed] [Google Scholar]
- 9. Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Frontiers in Ecology and the Environment 5: 89–96. [Google Scholar]
- 10. Strohm TO, Griffin B, Zumft WG, Schink B (2007) Growth yields in bacterial denitrification and nitrate ammonification. Applied and environmental microbiology 73: 1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jetten MS, Wagner M, Fuerst J, van Loosdrecht M, Kuenen G, et al. (2001) Microbiology and application of the anaerobic ammonium oxidation (‘anammox’) process. Current opinion in biotechnology 12: 283–288. [DOI] [PubMed] [Google Scholar]
- 12. Teixeira C, Magalhaes C, Joye SB, Bordalo AA (2012) Potential rates and environmental controls of anaerobic ammonium oxidation in estuarine sediments. Aquatic Microbial Ecology 66: 23–32. [Google Scholar]
- 13. Risgaard-Petersen N (2003) Coupled nitrification-denitrification in autotrophic and heterotrophic estuarine sediments: On the influence of benthic microalgae. Limnology and Oceanography 48: 93–105. [Google Scholar]
- 14. Ferguson A, Eyre B (2013) Interaction of benthic microalgae and macrofauna in the control of benthic metabolism, nutrient fluxes and denitrification in a shallow sub-tropical coastal embayment (western Moreton Bay, Australia). Biogeochemistry 112: 423–440. [Google Scholar]
- 15. Braeckman U, Provoost P, Gribsholt B, Van Gansbeke D, Middelburg JJ, et al. (2009) Role of macrofauna functional traits and density in biogeochemical fluxes and bioturbation. Marine Ecology Progress Series 399: 173. [Google Scholar]
- 16. Binnerup SJ, Jensen K, Revsbech NP, Jensen MH, Sørensen J (1992) Denitrification, dissimilatory reduction of nitrate to ammonium, and nitrification in a bioturbated estuarine sediment as measured with 15N and microsensor techniques. Applied and environmental microbiology 58: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Christensen PB, Nielsen LP, Sørensen J, Revsbech NP (1990) Denitrification in nitrate-rich streams: diurnal and seasonal variation related to benthic oxygen metabolism. Limnology and Oceanography 35: 640–651. [Google Scholar]
- 18. Risgaard-Petersen N, Nicolaisen MH, Revsbech NP, Lomstein BA (2004) Competition between ammonia-oxidizing bacteria and benthic microalgae. Appl Environ Microbiol 70: 5528–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parent S, Morin A (1999) Role of copepod-dominated meiofauna in the nitrification process of a cold marine mesocosm. Canadian Journal of Fisheries and Aquatic Sciences 56: 1639–1648. [Google Scholar]
- 20. Martin P, Boes X, Goddeeris B, Fagel N (2005) A qualitative assessment of the influence of bioturbation in Lake Baikal sediments. Global and Planetary Change 46: 87–99. [Google Scholar]
- 21. Montagna PA (1984) In situ measurement of meiobenthic grazing rates on sediment bacteria and edaphic diatoms. Marine ecology progress series Oldendorf 18: 119–130. [Google Scholar]
- 22. Frangoulis C, Christou ED, Hecq JH (2005) Comparison of marine copepod outfluxes: nature, rate, fate and role in the carbon and nitrogen cycles. Adv Mar Biol 47: 253–309. [DOI] [PubMed] [Google Scholar]
- 23.Cnudde C, De Troch M. unpubl. data.
- 24. De Troch M, Houthoofd L, Chepurnov V, Vanreusel A (2006) Does sediment grain size affect diatom grazing by harpacticoid copepods? Mar Environ Res 61: 265–277. [DOI] [PubMed] [Google Scholar]
- 25. De Troch M, Vergaerde I, Cnudde C, Vanormelingen P, Vyverman W, et al. (2012) The taste of diatoms: the role of diatom growth phase characteristics and associated bacteria for benthic copepod grazing. Aquatic Microbial Ecology 67: 47–58. [Google Scholar]
- 26. Cnudde C, Moens T, Hoste B, Willems A, De Troch M (2013) Limited feeding on bacteria by two intertidal benthic copepod species as revealed by trophic biomarkers. Environ Microbiol Rep 5: 301–309. [DOI] [PubMed] [Google Scholar]
- 27. Sørensen J (1978) Capacity for denitrification and reduction of nitrate to ammonia in a coastal marine sediment. Applied and Environmental Microbiology 35: 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Groffman PM, Altabet MA, Böhlke J, Butterbach-Bahl K, David MB, et al. (2006) Methods for measuring denitrification: diverse approaches to a difficult problem. Ecological Applications 16: 2091–2122. [DOI] [PubMed] [Google Scholar]
- 29. Beyst B, Hostens K, Mees J (2001) Factors influencing fish and macrocrustacean communities in the surf zone of sandy beaches in Belgium: temporal variation. Journal of Sea Research 46: 281–294. [Google Scholar]
- 30. Cataldo D, Maroon M, Schrader L, Youngs V (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid 1. Communications in Soil Science & Plant Analysis 6: 71–80. [Google Scholar]
- 31. Griess P (1879) Bemerkungen zu der Abhandlung der HH. Weselsky und Benedikt „Ueber einige Azoverbindungen” . Berichte der deutschen chemischen Gesellschaft 12: 426–428. [Google Scholar]
- 33. Lau M (2009) DTK: Dunnett–Tukey–Kramer pairwise multiple comparison test adjusted for unequal variances and unequal sample sizes. R package version 2 [Google Scholar]
- 34. Magalhaes CM, Machado A, Matos P, Bordalo AA (2011) Impact of copper on the diversity, abundance and transcription of nitrite and nitrous oxide reductase genes in an urban European estuary. FEMS Microbiol Ecol 77: 274–284. [DOI] [PubMed] [Google Scholar]
- 35. Stenström J, Hansen A, Svensson B (1991) Kinetics of microbial growth-associated product formation. Swedish Journal of Agricultural Research (Sweden) [Google Scholar]
- 36.Wheeler B (2010) lmPerm: Permutation tests for linear models. R package version 1.1–2.
- 37. Vadstein O, Andersen T, Reinertsen HR, Olsen Y (2012) Carbon, nitrogen and phosphorus resource supply and utilisation for coastal planktonic heterotrophic bacteria in a gradient of nutrient loading. Mar Ecol Prog Ser 447: 55–75. [Google Scholar]
- 38. De Troch M, Cnudde C, Willems A, Moens T, Vanreusel A (2010) Bacterial colonization on fecal pellets of harpacticoid copepods and on their diatom food. Microbial ecology 60: 581–591. [DOI] [PubMed] [Google Scholar]
- 39. Cornwell JC, Kemp WM, Kana TM (1999) Denitrification in coastal ecosystems: methods, environmental controls, and ecosystem level controls, a review. Aquatic Ecology 33: 41–54. [Google Scholar]
- 40. Coull BC (1999) Role of meiofauna in estuarine soft-bottom habitats*. Australian Journal of Ecology 24: 327–343. [Google Scholar]
- 41. Nascimento FJ, Näslund J, Elmgren R (2012) Meiofauna enhances organic matter mineralization in soft sediment ecosystems. Limnology and Oceanography 57: 338. [Google Scholar]
- 42. Berner RA, Westrich JT (1985) Bioturbation and the early diagenesis of carbon and sulfur. American Journal of Science 285: 193–206. [Google Scholar]
- 43. Sundbäck K, Miles A, Linares F (2006) Nitrogen dynamics in nontidal littoral sediments: Role of microphytobenthos and denitrification. Estuaries and coasts 29: 1196–1211. [Google Scholar]
- 44. An S, Joye SB (2001) Enhancement of coupled nitrification-denitrification by benthic photosynthesis in shallow estuarine sediments. Limnology and Oceanography 46: 62–74. [Google Scholar]
- 45. Nilsson P, Jonsson B, Swanberg IL, Sundback K (1991) Response of a marine shallow-water sediment system to an increased load of inorganic nutrients. Marine Ecology Progress Series MESEDT 71 [Google Scholar]
- 46. Sundbäck K, Nilsson P, Nilsson C, Jönsson B (1996) Balance between autotrophic and heterotrophic components and processes in microbenthic communities of sandy sediments: a field study. Estuarine, Coastal and Shelf Science 43: 689–706. [Google Scholar]
- 47. Bartoli M, Castaldelli G, Nizzoli D, Viaroli P (2012) Benthic primary production and bacterial denitrification in a Mediterranean eutrophic coastal lagoon. Journal of Experimental Marine Biology and Ecology 438: 41–51. [Google Scholar]
- 48. Koch M, Maltby E, Oliver G, Bakker S (1992) Factors controlling denitrification rates of tidal mudflats and fringing salt marshes in south-west England. Estuarine, Coastal and Shelf Science 34: 471–485. [Google Scholar]
- 49. De Troch M, Steinarsdóttir MB, Chepurnov V, Ólafsson E (2005) Grazing on diatoms by harpacticoid copepods: species-specific density-dependent uptake and microbial gardening. Aquatic microbial ecology 39: 135–144. [Google Scholar]
- 50.Giblin AE, Tobias CR, Song B, Weston N, Banta GT, et al. (2013) The importance of dissimilatory nitrate reduction to ammonium (DNRA) in the nitrogen cycle of coastal ecosystems.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Design of the denitrification potential experiment. Incubation steps are indicated by dotted arrows. Manipulations are indicated by a dashed arrow. Each microcosm (here represented as a rectangle) was incubated for 7.5 days, after which water (blue) and sediment (brown) were transferred from the microcosm to the serum vials (represented as trapezoids). Glucose and potassium nitrate were added to the vial after which it was thoroughly homogenized. The headspace was flushed with helium to remove oxygen, after which acetylene was injected. The vials were sampled four times for N2O determination, every two hours.
(DOC)
NO3-, NO2- and N2O concentrations in the serum vials after the addition of 0.5 mmol KNO3 during the measurement of the denitrification potential (from t0 to t4). Amounts (µmol/h/g sediment) are averaged over all samples (all treatments), as is the time at with the sample was taken (Time; h:min) ± SE. N2O concentration(blue) plotted on the right y axis; NO3- (red) and NO2- (green) concentrations on the left y axis.
(DOC)
Measurement of oxygen penetration depth. To verify the effects of copepods and diatoms on the oxygen pentration depths, the experiment as described in the method section was repeated for the blank, diatom and copepod+diatom treatment. The methodology and results of the additional experiment are shown here.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All data files are available from figshare (http://dx.doi.org/10.6084/m9.figshare.1169927).