Abstract
Primary progressive aphasia is a neurodegenerative syndrome characterized by progressive language dysfunction. The majority of primary progressive aphasia cases can be classified into three subtypes: non-fluent/agrammatic, semantic, and logopenic variants of primary progressive aphasia. Each variant presents with unique clinical features, and is associated with distinctive underlying pathology and neuroimaging findings. Unlike primary progressive aphasia, apraxia of speech is a disorder that involves inaccurate production of sounds secondary to impaired planning or programming of speech movements. Primary progressive apraxia of speech is a neurodegenerative form of apraxia of speech, and it should be distinguished from primary progressive aphasia given its discrete clinicopathological presentation. Recently, there have been substantial advances in our understanding of these speech and language disorders. Here, we review clinical, neuroimaging, and histopathological features of primary progressive aphasia and apraxia of speech. The distinctions among these disorders will be crucial since accurate diagnosis will be important from a prognostic and therapeutic standpoint.
Keywords: Dementia, Primary progressive aphasia, Apraxia of speech
1. Introduction
Primary progressive aphasia (PPA) is a neurodegenerative condition that predominantly affects language. Pick introduced cases of focal language impairments that presented as the first signs of neurodegenerative illnesses over a century ago.1 However, the term PPA was coined by Mesulam in 1982 when he described six patients with slowly progressing aphasia without other cognitive or behavioral dysfunction.2 The language deficits in these patients included non-fluent halting speech and anomia with intact comprehension.2 In an effort to more clearly define and classify PPA, consensus diagnostic criteria for the disorder were proposed in 2011.3 For the diagnosis of PPA, language impairments need to be the primary deficits in the first two years or more, and the disease must be progressive.4 Once the initial diagnosis of PPA is made, patients often can be divided into one of three subtypes: non-fluent/agrammatic variant PPA (naPPA), semantic variant PPA (svPPA), and logopenic variant PPA (lvPPA).3
Unlike aphasia, apraxia of speech (AOS) is a motor speech disorder. It involves impaired planning or programming of movements that prevents accurate production of sounds and syllables across words or within multisyllabic words.5 AOS most often results from left hemisphere stroke, which is associated with static or improving symptoms over time.6 However, AOS can be a sign of an insidiously progressive illness. When AOS presents as the only or predominant symptom of a neurodegenerative condition, it is termed primary progressive AOS (PPAOS).7
Primary progressive aphasia variants and PPAOS have distinguishable clinical features, and are associated with distinctive cortical atrophy patterns and underlying pathology. As we advance our knowledge, careful differentiation among these disorders will be important since accurate diagnosis will likely guide appropriate medical and behavioral therapeutic measures in the future. In this review, we will provide an overview of clinical, neuroimaging, and histopathological findings associated with PPA and PPAOS.
2. Clinical Features of PPA and PPAOS
The clinical features of PPA and PPAOS are summarized in Table 1. The first variant of PPA, naPPA, is characterized by slow effortful speech, grammatic or syntactic errors, reduced sentence complexity, and sound errors.3 Individuals with naPPA may simplify grammatical forms with short phrases and decreased use of passive sentences (e.g., a cat was chased by a dog). They may omit grammatical morphemes (e.g., the, a/an, -ed, un-, re-, and, of), use inappropriate inflections, or arrange words in a grammatically incorrect order. The average rate of speech produced by patients with naPPA is about 45 words per minute in comparison to 140 words per minute generated by healthy individuals.8 The effortful speech observed in naPPA is thought to reflect effects of grammatical errors and apraxia of speech (AOS).9–11
Table 1.
Clinical characteristics of PPA and PPAOS
| naPPA | svPPA | lvPPA | PPAOS | ||
|---|---|---|---|---|---|
| Clinical features | Agrammatism | + | − | − | − |
| Apraxia of speech | + | − | − | + | |
| Impaired comprehension | + (syntactically complex sentences) | + (single words) | +/− | − | |
| Impaired naming | − | + | +/− | − | |
| Impaired object knowledge | − | + | − | − | |
| Paraphasia | + | +/− | + | − | |
| Speech rate | Significantly reduced | Normal | Moderately reduced | Significantly reduced | |
| Impaired repetition | + (single words) | − | + (sentences and phrases) | + (single words) | |
| Other | Surface dyslexia or dysgraphia |
Abbreviations: naPPA=non-fluent/agrammatic variant of primary progressive aphasia; svPPA = semantic variant of primary progressive aphasia; lvPPA = logopenic variant of primary progressive aphasia; PPAOS = primary progressive apraxia of speech
Speech of individuals with AOS is characterized by slow speaking rate, distorted articulation, sound substitutions, articulatory groping, false starts and restarts, segmentation of syllables, and increased difficulty with increasing utterance length.5 Progressive AOS is present in the majority of individuals with naPPA.12, 13 In fact, the 2011 PPA consensus criteria define AOS as a core feature of naPPA.3 However, recent studies suggest that progressive AOS may occur in the absence ofaphasia.5, 13–15 When that is the case, AOS should be considered a primary neurodegenerative disorder (i.e., PPAOS), one distinct from naPPA.
The hallmark of svPPA is progressive difficulty with naming and single word comprehension, especially for low-frequency words.3 Impairments in lexical retrieval are thought to be attributable to increased use of closed class words (e.g., a/an, the, you, she, them, of, in) and high frequency nouns.16 Individuals with svPPA may also develop surface dyslexia and/or dysgraphia for irregularly spelled words (e.g., yacht, comb, ache) with relatively spared ability to read and write non-real words. Unlike patients with naPPA, these individuals have fluent speech with normal rate and exhibit minimal speech or syntactic errors.3 AOS is not a feature of svPPA.
lvPPA is the most recently described PPA subtype.11 It is characterized by hesitant speech, sentence repetition deficits, and word-finding difficulty with preserved grammar and motor speech.3 The rate of speech in lvPPA falls between naPPA and svPPA.16 Phonological paraphasias are common.17 Although both naPPA and lvPPA involve difficulty with repetition, the deficit in lvPPA is most often attributable to phonological short-term memory loss resulting in impaired storage of incoming verbal information or phonological planning deficits, rather than motor speech errors or agrammatism.17 Impaired phonological memory also contributes to frequent word-finding pauses that result from errors in phonological retrieval. In comparison to svPPA, lexical retrieval deficits are less common in lvPPA.16 Single word comprehension is relatively intact in individuals with lvPPA.
Because PPA predominantly presents as a language disorder, affected individuals often maintain their functional status relatively well. However, recent studies demonstrate that patients with PPA can develop impairments in other cognitive domains. Individuals with naPPA may develop working memory and executive control deficits.9 lvPPA can be accompanied by relatively rapid global cognitive deterioration. Memory, attention, orientation, and visuospatial functions can be compromised in lvPPA.18 In comparison to lvPPA, patients with svPPA show relatively preserved cognitive function with the exception of verbally mediated measures.18 However, individuals with svPPA can exhibit early behavioral changes, such as compulsions and loss of empathy.3, 19
3. Pathological Features of PPA and PPAOS
Primary progressive aphasia and PPAOS display distinctive pathological associations with some overlapping features (Table 2). naPPA is predominantly associated with tau pathology when AOS is present.14, 20, 21 Similarly, PPAOS is also related to tauopathy.14, 22 Therefore, it is not surprising that naPPA and PPAOS sometimes share clinical features of progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD), which are tauopathies.14, 23 However, tar-DNA binding protein 43 (TDP-43) or Alzheimer’s disease pathology has been reported by some groups as the underlying histopathology in naPPA.23–27 svPPA is predominantly associated with TDP-43, but some studies have reported that tau and AD related pathological changes can occur.20, 24, 28,29 lvPPA is most commonly associated with Alzheimer’s disease (AD) pathology.21, 30,31 Recent studies demonstrate that cortical β-amyloid deposition, a key feature of AD, is also seen in lvPPA.31–33 Amyloid deposition in lvPPA is similar to the pattern observed in AD.33 In addition, a high CSF tau/Aβ ratio is seen in this subtype.30 Interestingly, microhemorrhage that is typically observed in AD is also seen in lvPPA.34 In addition, neurofibrillary tangles (NFT), an important pathological feature of AD, are also present in lvPPA. In comparison to AD, pathological samples of lvPPA display significantly increased NFT density in the left temporoparietal region, which is the area mostly affected in this subtype.35 Despite the strong association with AD pathology, some cases of lvPPA have been reported with tau or TDP-43 pathology.21, 27
Table 2.
Pathological and anatomical correlations of PPA and PPAOS
| naPPA | svPPA | lvPPA | PPAOS | |
|---|---|---|---|---|
| Pathology | Tau (52%) AD (25%) TDP-43 (19%) |
TDP-43 (69%) AD (25%) Tau (6%) |
AD (50%) TDP-43 (38%) Tau (12%) |
Tau (100%) |
| Cortical atrophy or hypometabolism | Left posterior fronto-insular | Anterior temporal | Left temporo-parietal | Superior premotor, supplementary motor |
Abbreviations: naPPA=non-fluent/agrammatic variant of primary progressive aphasia; svPPA = semantic variant of primary progressive aphasia; lvPPA = logopenic variant of primary progressive aphasia; PPAOS = primary progressive apraxia of speech; AD = Alzheimer’s disease; TDP-43 =tar-DNA binding protein 43
4. Neuroimaging Findings of PPA and PPAOS
Advanced imaging techniques have been useful for identifying the brain regions that are affected by PPA and PPAOS (Table 2). MRI of naPPA patients demonstrates atrophy in the left inferior frontal regions, including the frontal operculum and the anterior insula.11, 14, 36 The structural abnormalities may also extend to involve the left prefrontal and superior temporal lobes.10, 37 Reduced fluency is associated with damage to regions dorsal to Broca’s area, and grammatical errors are associated with damage to the inferior frontal and supramarginal gyri in naPPA.16, 38 These associations are supported by FDG-PET findings that show hypometabolism in the posterior fronto-insular region as well as the superior temporal lobe (Figure 1).15, 39
Figure 1.
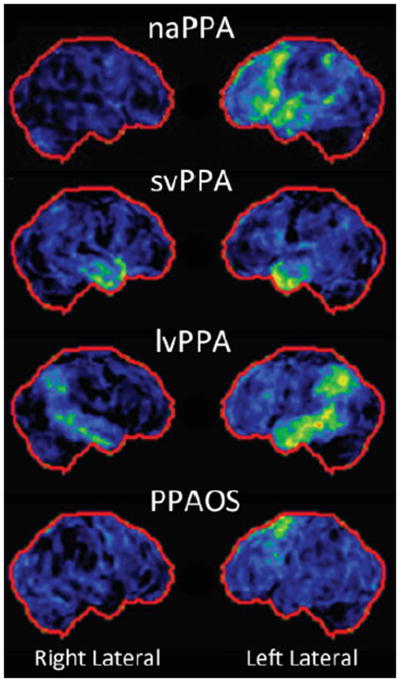
Fluorodeoxyglucose positron emission tomography demonstrates hypometabolism in the left posterior frontoinsular region in the nonfluent/agrammatic variant of primary progressive aphasia, bilateral anterior temporal regions in the semantic variant of primary progressive aphasia, left temporoparietal region in the logopenic variant of primary progressive aphasia, and premotor/supplementary motor areas in primary progressive apraxia of speech.
Interestingly, PPAOS displays unique cortical involvement that is different from the areas affected by naPPA with damage in the superior lateral premotor and supplementary motor areas in the left hemisphere (Figure 1).7, 14 In a recent FDG-PET study, Whitwell and colleagues demonstrated that hypometabolism in the left lateral superior premotor cortex was strongly associated with AOS rating scale performance in patients with PPAOS.40 However, Western Aphasia Battery (WAB)41, a global measure of aphasia severity, and Token Test 42, a measure of grammatic/syntactic comprehension, were correlated with hypometabolism in the areas that were previously shown to be affected by naPPA, such as the left inferior triangularis, pars opercularis, middle frontal gyrus, superior temporal gyrus and inferior parietal lobe. The unique regions of FDG hypometabolism in PPAOS may enable anatomic differentiation of PPAOS from naPPA.
In svPPA, the most prominent MRI and PET abnormalities are seen in the bilateral temporal lobes, although there is evidence of asymmetric involvement favoring the left (Figure 1).11, 31, 43 Disruptions in semantic processing in svPPA are correlated with the anterior temporal pole atrophy, whereas lexical retrieval errors are associated with the anterior and inferior temporal atrophy.16 As the disease progresses, the damage extends along the middle and inferior temporal gyri without involving Wernicke’s area.43
Structural and functional abnormalities in lvPPA are concentrated in the left temporoparietal junction involving the posterior superior temporal, medial temporal, and inferior parietal regions as well as the posterior cingulate (Figure 1).44–46 Impaired phonological memory in lvPPA is linked to the left posterior temporoparietal atrophy.17, 38 lvPPA shows increased atrophy and decreased hypometabolism in the left inferior, middle, and superior lateral temporal regions in comparison to AD, which is consistent with the asymmetrical pathologic involvement observed in a previous study.35, 44
As PPA advances, the clinical features that differentiate one PPA subtype from another become less distinct. Despite the presence of asymmetrical involvement of the brain, anatomical progression resulting in wider areas of cortical atrophy becomes noticeable in advanced stages of the disease. Furthermore, the progressing patterns of atrophy become more closely related to specific language functions, rather than the individual PPA subtypes. A recent study by Rogalski and colleagues demonstrates significant clinical and pathological progression in PPA patients over a 2-year period.47 In these subjects, unique clinico-anatomical features of each subtype became less distinctive with disease progression. Cortical involvement extended beyond the initial areas of atrophy to include all major language networks, such as the inferior frontal, temporoparietal, and lateral temporal regions. Impairments in naming and single-word comprehension were noted in individuals with lvPPA because of the new involvement of the lateral and anterior frontotemporal lobe as well as worsening of the preexisting atrophy in the left temporoparietal region. Similarly, the spread of cortical atrophy to the inferior frontal gyrus lead to the development of impaired grammatical processing in svPPA.47
5. Genetics of PPA and PPAOS
Although most cases of PPA and PPAOS are sporadic, familial forms of the disorders exist. The degree of heritability varies among the PPA subtypes. About 35% of naPPA cases are hereditary, and 7% of them are inherited in an autosomal dominant fashion.48 On the other hand, 17% of svPPA cases are familial with 2% being autosomal dominant.48 PPAOS has not been reported to be familial.7, 13 Several genetic mutations have been linked to familial cases of PPA. Microtubule associated protein tau (MAPT) on chromosome 17q21.32 is associated with tau pathology.49 Progranulin (PGRN) on chromosome 17q21.31 and chromosome 9 open reading frame 72 (C9ORF72) on chromosome 9p21.2 are associated with TDP-43 pathology.24, 50–53 APOE ε4 haplotype, which is linked to AD pathology, is also associated with these disorders.11 In familial cases, language and speech deficits may lack specific characteristics of PPA subtypes.50 Heterogeneity in phenotypes may exist within the same family, as well.54
6. Management
The management of PPA and PPAOS is primarily symptomatic at this time, and typically provided by speech-language pathologists. It is generally accepted that established behavioral strategies for managing aphasia and AOS due to stroke are applicable to neurodegenerative etiologies when general criteria are met for recommending therapy,55 and there are an accumulating number of single case reports suggesting that focused treatment may maintain or improve word retrieval and speech production or improve communication using alternative strategies (e.g., text-to-speech device) in well-selected individuals with PPA or progressive AOS 56–58. Counseling about the nature of the communication difficulties and their likely course, the capacity to work and work accommodations, and the need for and possible benefits to be derived from formal therapy are essential. Management efforts that focus on compensatory strategies, including augmentative and alternative communication, are often best staged with periodic reassessment and updated recommendations about methods to facilitate communication during everyday activities. Support groups and dedicated web sites (www.ppaconnection.org; www.aphasia.org) that serve as a registry and/or information resources can be very beneficial.
7. Conclusion and Future Directions
Primary progressive aphasia and PPAOS are neurodegenerative disorders that have predominant effects on language and speech. Recently, significant advances have been made in our understanding of these illnesses, permitting identification of their unique clinical, histopathological, and neuroimaging characteristics. Recognizing these features is an essential first step towards early detection and diagnosis as well as therapeutic interventions. Further refinement of the clinical diagnostic criteria may help minimize diagnostic uncertainty. Longitudinal studies that include molecular and pathological approaches will be also be informative for identifying biomarkers that may be targeted by disease-modifying agents in the future.
Acknowledgments
The authors were funded by R01-DC010367.
References
- 1.Pick A. Uber die Beziehungen der senilen Hirnatrophie zue Aphasie. Prager Medicinische Wochenschrift. 1892;17:165–167. [Google Scholar]
- 2.Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–598. doi: 10.1002/ana.410110607. [DOI] [PubMed] [Google Scholar]
- 3.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–432. [PubMed] [Google Scholar]
- 5.Duffy J. Apraxia of speech in degenrative neurologic disease. Aphasiology. 2006;20:511–527. [Google Scholar]
- 6.Ogar J, Slama H, Dronkers N, Amici S, Gorno-Tempini ML. Apraxia of speech: an overview. Neurocase. 2005;11:427–432. doi: 10.1080/13554790500263529. [DOI] [PubMed] [Google Scholar]
- 7.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, Lowe VJ, Jack CR, Jr, Whitwell JL. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain. 2012;135:1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman M, Powers J, Ash S, McMilan C, Burkholder L, Irwin D, Trojanowski JQ. Disruption of large scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain Lang. 2012 doi: 10.1016/j.bandl.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 2012;11:545–555. doi: 10.1016/S1474-4422(12)70099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunawardena D, Ash S, McMillan C, Avants B, Gee J, Grossman M. Why are patients with progressive nonfluent aphasia nonfluent? Neurology. 2010;17:588–594. doi: 10.1212/WNL.0b013e3181ed9c7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, Johnson JK, Weiner MW, Miller BL. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogar JM, Dronkers NF, Brambati SM, Miller BL, Gorno-Tempini ML. Progressive nonfluent aphasia and its characteristic motor speech deficits. Alzheimer Dis Assoc Disord. 2007;21:S23–30. doi: 10.1097/WAD.0b013e31815d19fe. [DOI] [PubMed] [Google Scholar]
- 13.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, Jack CR, Jr, Whitwell JL. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013;81:337–345. doi: 10.1212/WNL.0b013e31829c5ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, Hauser MF, Witte RJ, Boeve BF, Knopman DS, Dickson DW, Jack CR, Jr, Petersen RC. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josephs KA, Duffy JR, Fossett TR, Strand EA, Claassen DO, Whitwell JL, Peller PJ. Fluorodeoxyglucose F18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Arch Neurol. 2010;67:596–605. doi: 10.1001/archneurol.2010.78. [DOI] [PubMed] [Google Scholar]
- 16.Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, Miller BL, Gorno-Tempini ML. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133:2069–2088. doi: 10.1093/brain/awq129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, Perani D, Garibotto V, Cappa SF, Miller BL. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. doi: 10.1212/01.wnl.0000320506.79811.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leyton CE, Hsieh S, Mioshi E, Hodges JR. Cognitive decline in logopenic aphasia: More than losing words. Neurology. 2013;80:897–903. doi: 10.1212/WNL.0b013e318285c15b. [DOI] [PubMed] [Google Scholar]
- 19.Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, Rosen HJ. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64:1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodges JR, Davies RR, Xuereb JH, Casey B, Broe M, Bak TH, Kril JJ, Halliday GM. Clinicopathological correlates in frontotemporal dementia. Ann Neurol. 2004;56:399–406. doi: 10.1002/ana.20203. [DOI] [PubMed] [Google Scholar]
- 21.Mesulam M, Wicklund A, Johnson N, Rogalski E, Léger GC, Rademaker A, Weintraub S, Bigio EH. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, Kerdraon O, Buée L, Maurage CA, Pasquier F. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74:42–49. doi: 10.1212/WNL.0b013e3181c7198e. [DOI] [PubMed] [Google Scholar]
- 23.Kertesz A, Martinez-Lage P, Davidson W, Munoz DG. The corticobasal degeneration syndrome overlaps progressive aphasia and frontotemporal dementia. Neurology. 2000;55:1368–1375. doi: 10.1212/wnl.55.9.1368. [DOI] [PubMed] [Google Scholar]
- 24.Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007;114:31–38. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- 25.Josephs KA, Stroh A, Dugger B, Dickson DW. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol. 2009;118:349–358. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snowden JS, Thompson JC, Stopford CL, Richardson AM, Gerhard A, Neary D, Mann DM. The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain. 2011;134:2478–2492. doi: 10.1093/brain/awr189. [DOI] [PubMed] [Google Scholar]
- 27.Grossman M, Xie SX, Libon DJ, Wang X, Massimo L, Moore P, Vesely L, Berkowitz R, Chatterjee A, Coslett HB, Hurtig HI, Forman MS, Lee VM, Trojanowski JQ. Longitudinal decline in autopsy-defined frontotemporal lobar degeneration. Neurology. 2008;70:2036–2045. doi: 10.1212/01.wnl.0000303816.25065.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–165. doi: 10.1002/ana.20700. [DOI] [PubMed] [Google Scholar]
- 29.Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, Xuereb JH. The pathological basis of semantic dementia. Brain. 2005;128:1984–1995. doi: 10.1093/brain/awh582. [DOI] [PubMed] [Google Scholar]
- 30.Rohrer JD, Rossor MN, Warren JD. Alzheimer’s pathology in primary progressive aphasia. Neurobiol Aging. 2012;33:744–752. doi: 10.1016/j.neurobiolaging.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, O’Neil JP, Lal RA, Dronkers NF, Miller BL, Gorno-Tempini ML. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leyton CE, Villemagne VL, Savage S, Pike KE, Ballard KJ, Piguet O, Burrell JR, Rowe CC, Hodges JR. Subtypes of progressive aphasia: application of the International Consensus Criteria and validation using β-amyloid imaging. Brain. 2011:3030–3043. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- 33.Lehmann M, Ghosh PM, Madison C, Laforce R, Jr, Corbetta-Rastelli C, Weiner MW, Greicius MD, Seeley WW, Gorno-Tempini ML, Rosen HJ, Miller BL, Jagust WJ, Rabinovici GD. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer’s disease. Brain. 2013;136:844–858. doi: 10.1093/brain/aws327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitwell JL, Jack CR, Jr, Duffy JR, Strand EA, Gunter JL, Senjem ML, Murphy MC, Kantarci K, Machulda MM, Lowe VJ, Josephs KA. Microbleeds in the logopenic variant of primary progressive aphasia. Alzheimers Dement. 2013 doi: 10.1016/j.jalz.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Josephs KA, Dickson DW, Murray ME, Senjem ML, Parisi JE, Petersen RC, Jack CR, Jr, Whitwell JL. Quantitative neurofibrillary tangle density and brain volumetric MRI analyses in Alzheimer’s disease presenting as logopenic progressive aphasia. Brain Lang. 2013 doi: 10.1016/j.bandl.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peelle JE, Troiani V, Gee J, Moore P, McMillan C, Vesely L, Grossman M. Sentence comprehension and voxel-based morphometry in progressive nonfluent aphasia, semantic dementia, and nonaphasic frontotemporal dementia. J Neurolinguistics. 2008;5:418–432. doi: 10.1016/j.jneuroling.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohrer JD, Warren JD, Modat M, Ridgway GR, Douiri A, Rossor MN, Ourselin S, Fox NC. Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology. 2009;72:1562–1569. doi: 10.1212/WNL.0b013e3181a4124e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rogalski E, Cobia D, Harrison TM, Wieneke C, Thompson CK, Weintraub S, Mesulam MM. Anatomy of language impairments in primary progressive aphasia. J Neurosci. 2011;31:3344–3350. doi: 10.1523/JNEUROSCI.5544-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nestor PJ, Graham NL, Fryer TD, Williams GB, Patterson K, Hodges JR. Progressive non-fluent aphasia is associated with hypometabolism centred on the left anterior insula. Brain. 2003;126:2406–2418. doi: 10.1093/brain/awg240. [DOI] [PubMed] [Google Scholar]
- 40.Whitwell JL, Duffy JR, Strand EA, Xia R, Mandrekar J, Machulda MM, Senjem ML, Lowe VJ, Jack CR, Jr, Josephs KA. Distinct regional anatomic and functional correlates of neurodegenerative apraxia of speech and aphasia: an MRI and FDG-PET study. Brain Lang. 2013;125:245–252. doi: 10.1016/j.bandl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shewan CM, Kertesz A. Reliability and validity characteristics of the Western Aphasia Battery (WAB) J Speech Hear Disord. 1980;45:308–324. doi: 10.1044/jshd.4503.308. [DOI] [PubMed] [Google Scholar]
- 42.De Renzi E, Vignolo LA. The token test: A sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- 43.Mummery CJ, Patterson K, Price CJ, Ashburner J, Frackowiak RS, Hodges JR. A voxel-based morphometry study of semantic dementia: relationship between temporal lobe atrophy and semantic memory. Ann Neurol. 2000;47:36–45. [PubMed] [Google Scholar]
- 44.Madhavan A, Whitwell JL, Weigand SD, Duffy JR, Strand EA, Machulda MM, Tosakulwong N, Senjem ML, Gunter JL, Lowe VJ, Petersen RC, Jack CR, Jr, Josephs KA. FDG PET and MRI in Logopenic Primary Progressive Aphasia versus Dementia of the Alzheimer’s Type. PLoS One. 2013;8:e62471. doi: 10.1371/journal.pone.0062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rohrer JD, Clarkson MJ, Kittus R, Rossor MN, Ourselin S, Warren JD, Fox NC. Rates of hemispheric and lobar atrophy in the language variants of frontotemporal lobar degeneration. J Alzheimers Dis. 2012;30:407–411. doi: 10.3233/JAD-2012-111556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Josephs KA, Whitwell JL, Duffy JR, Vanvoorst WA, Strand EA, Hu WT, Boeve BF, Graff-Radford NR, Parisi JE, Knopman DS, Dickson DW, Jack CR, Jr, Petersen RC. Progressive aphasia secondary to Alzheimer disease vs FTLD pathology. Neurology. 2008;70:25–34. doi: 10.1212/01.wnl.0000287073.12737.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogalski E, Cobia D, Harrison TM, Wieneke C, Weintraub S, Mesulam MM. Progression of language decline and cortical atrophy in subtypes of primary progressive aphasia. Neurology. 2011;76:1804–1810. doi: 10.1212/WNL.0b013e31821ccd3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldman JS, Farmer JM, Wood EM, Johnson JK, Boxer A, Neuhaus J, Lomen-Hoerth C, Wilhelmsen KC, Lee VM, Grossman M, Miller BL. Comparison of family histories in FTLD subtypes and related tauopathies. Neurology. 2005;65:1817–1819. doi: 10.1212/01.wnl.0000187068.92184.63. [DOI] [PubMed] [Google Scholar]
- 49.van Swieten J, Spillantini MG. Hereditary frontotemporal dementia caused by tau gene mutations. Brain Pathol. 2007;17:63–73. doi: 10.1111/j.1750-3639.2007.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mesulam M, Johnson N, Krefft TA, Gass JM, Cannon AD, Adamson JL, Bigio EH, Weintraub S, Dickson DW, Hutton ML, Graff-Radford NR. Progranulin mutations in primary progressive aphasia: the PPA1 and PPA3 families. Arch Neurol. 2007;64:43–47. doi: 10.1001/archneur.64.1.43. [DOI] [PubMed] [Google Scholar]
- 51.Josephs KA, Ahmed Z, Katsuse O, Parisi JF, Boeve BF, Knopman DS, Petersen RC, Davies P, Duara R, Graff-Radford NR, Uitti RJ, Rademakers R, Adamson J, Baker M, Hutton ML, Dickson DW. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol. 2007;66:142–151. doi: 10.1097/nen.0b013e31803020cf. [DOI] [PubMed] [Google Scholar]
- 52.Snowden JS, Rrollinson S, Thompson JC, Harris JM, Stopford CL, Richardson AM, Jones M, Gerhard A, Davidson YS, Robinson A, Gibbons L, Hu Q, DuPlessis D, Neary D, Mann DM, Pickering-Brown SM. Distinct clinical and pathological characteristics of frontotemporal dementia associated with C9ORF72 mutations. Brain. 2012;135:693–708. doi: 10.1093/brain/awr355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, Yeatman T, Warrington EK, Schott JM, Fox NC, Rossor MN, Hardy J, Collinge J, Revesz T, Mead S, Warren JD. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135:736–750. doi: 10.1093/brain/awr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Zee J, Rademakers R, Engelborghs S, Gijselinck I, Bogaerts V, Vandenberghe R, Santens P, Caekebeke J, De Pooter T, Peeters K, Lübke U, Van den Broeck M, Martin JJ, Cruts M, De Deyn PP, Van Broeckhoven C, Dermaut B. A Belgian ancestral haplotype harbours a highly prevalent mutation for 17q21-linked tau-negative FTLD. Brain. 2006;129:841–852. doi: 10.1093/brain/awl029. [DOI] [PubMed] [Google Scholar]
- 55.Duffy JR, McNeil MR. Primary progressive aphasia and apraxia of speech. In: Chapey R, editor. Language intervention strategies in aphasia and related neurogenic communication disorders. 5. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 543–564. [Google Scholar]
- 56.Beeson PM, King RM, Bonakdarpour B, Henry ML, Cho H, Rapcsak SZ. Positive effects of language treatment for the logopenic variant of primary progressive aphasia. J Mol Neurosci. 2011;45:724–736. doi: 10.1007/s12031-011-9579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henry ML, Meese MV, Truong S, Babiak MC, Miller BL, Gorno-Tempini ML. Treatment for apraxia of speech in nonfluent variant primary progressive aphasia. Behav Neurol. 2013;26:77–88. doi: 10.3233/BEN-2012-120260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pattee C, Von Berg S, Ghezzi P. Effects of alternative communication on the communicative effectiveness of an individual with a progressive language disorder. Int J Rehabil Res. 2006;29:151–153. doi: 10.1097/01.mrr.0000210046.02044.4d. [DOI] [PubMed] [Google Scholar]


