Preface
During the final stage of cell division, the future daughter cells are physically separated in a process called abscission. This process requires coordination of a number of molecular machines that mediate a complex series of events to culminate in the final separation of daughter cells. Abscission is coordinated with other cellular processes (for example, nuclear pore reassembly) through mitotic kinases that act as master regulators to ensure proper progression of abscission.
Introduction
The goal of cell division is to segregate chromosomes with high fidelity and partition cytoplasm into the two future daughter cells. In the cytokinetic stage of cell division in most cells, the site of furrow ingression is chosen and the actomyosin ring is assembled between the two reforming nuclei in the midzone region where anti-parallel overlapping microtubules from anaphase are found1. With time, the midzone microtubules are compacted and integrated with amorphous electron-dense material to form the midbody, which lies within the intercellular bridge connecting the nascent daughter cells. Midbodies appear to serve as a staging area for abscission based on the localization of numerous abscission proteins to this site2. As cells approach abscission, the bridge narrows and microtubules decrease in the midbody region3, 4, presumably due to intercellular bridge remodeling, microtubule severing and microtubule depolymerization. Following abscission, a number of short microtubules are retained by the post-mitotic midbody4–6, suggesting that complete microtubule elimination is not a prerequisite for abscission.
In the final step of cell division, the intercellular bridge is abscised adjacent to the midbody giving birth to two nascent daughter cells (Fig. 1). Although much has been learned about the events leading up to abscission, little is known about the mechanisms and molecules that mediate the individual processes that contribute to the final severing of the bridge. Some insights into these issues have come from the use of small molecule inhibitors, RNA interference and long-term live-imaging of fluorescent-tagged proteins. Recent studies have identified several major events that contribute to abscission. These include polarized vesicle transport and fusion within the intercellular bridge7, 8; recruitment of endosomal sorting complex required for transport (ESCRT) machinery to the site of bridge severing4, 5, 9, 10; and signaling cascades that coordinate abscission with cell cycle progression11, 12 and other cellular processes (for example, chromatin clearance from the bridge and nuclear pore reassembly)13–15. These events occur at or adjacent to the midbody, further implicating this organelle in the coordination of abscission.
Fig 1. The major stages from cytokinesis to abscission.
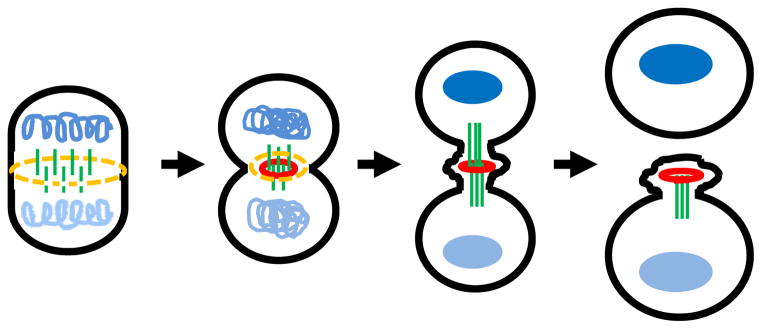
Schematic representation of cytokinesis progression from its entry to abscission is shown from left to right. Left: Upon cytokinetic entry, the actinomysin ring (yellow) and anti-parallel midzone microtubules (green) emerge between the segregated chromosomes (light and dark blue) that start to decondense. Middle left: In early cytokinesis, midzone microtubules gradually compact by the cross-linking of associated midzone proteins (red) while narrowing of the cleavage furrow. Middle right: After furrowing, the intercellular bridge with the compacted microtubules connects the two daughter cells. The midbody within the intercellular bridge contains the overlapping microtubules, midbody ring derived from cross-linked midzone proteins (red) and amorphous electron-dense material (not shown). Right: Abscission occurs adjacent to the midbody after multiple pathways orchestrate to sever the intercellular bride (for detail, Fig. 2). In certain cell types, a second bridge-severing event at the other side of the bridge has been documented.
In this article, we review the current understanding of abscission with a focus on the molecular mechanisms of bridge severing and the surveillance networks that ensure abscission completion. We propose a model that includes the coordinated action and integration of several events and pathways that culminate in abscission.
Membrane trafficking during abscission
Work over the last few years has shown that abscission requires selective transport of secretory and endocytic vesicles to the midbody (Fig. 2)6, 16, 17. It was first noted that disruption of post-Golgi apparatus secretion in C. elegans led to abscission failure18. Other studies on live cells later demonstrated that Golgi complex-derived vesicles were targeted to the midbody during abscission, where they appeared to dock and/or fuse with the intercellular bridge membrane6, 16, 17. In addition, proteins involved in secretory vesicle tethering (for example, exocyst) and fusion (for example, SNAREs) were enriched at the midbody during abscission6, 19, 20, and were dependent on the midbody proteins, centriolin and Cep55, for their localizaiton6, 20. Loss of these molecules from the midbody caused defects in abscission that ultimately prevented cell separation and led to binucleated cells or multiple cells interconnected by intercellular bridges6, 17, 19–21. This highlights the importance of Golgi-derived vesicle delivery and fusion in the abscission process. It is interesting to note that the actual bridge cutting event occurred at least 10 minutes after vesicle fusion within the intercellular bridge suggesting additional steps in the final stages of the process. The reason for this lag is unclear. It is possible that secretory vesicles act as scaffolds for transporting and anchoring proteins for membrane remodeling, deformation and possibly scission in the bridge (for example, ESCRT machinery; see below) or that vesicle fusion directly contributes to bridge severing. It is important to note that none of the major events that occur during abscission including vesicle delivery, vesicle fusion, bridge deformation, ESCRT delivery and helical filament formation, cause immediate bridge severing. There is always a lag after these events and before abscission suggesting that some additional regulation that we have yet to uncover takes place.
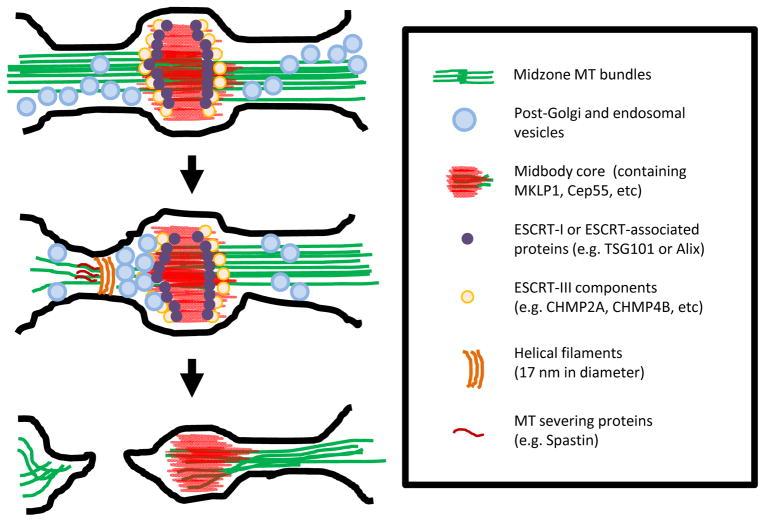
Fig 2. Multiple pathways are required for accomplishing abscission.
Multiple pathways that contribute to abscission are represented schematically from top to bottom. Top: As the cell approaches abscission, ESCRT-I (purple ovals), ESCRT-associated protein (purple ovals) and later ESCRT-III (pale yellow ovals) are recruited to the midbody (red). Meanwhile, the intercellular bridge continues to remodel, the bridge microtubules (green) gradually compact, and different types of vesicles (light blue ovals) selectively traffic to the midbody. Middle: About 10–20 minutes before abscission, vesicles are increasingly recruited, presumably facilitating remodeling of the bridge membrane in vicinity of the midbody. Concordantly, 17-nm helical filaments (dark brown stripes) whose presence depends on ESCRT-III components appear at the ultimate bridge scission site; these filaments may be spirally grown from the midbody or directly formed at this site. Spastin (dark red stripes) is also concentrated at the same site to selectively sever microtubules. These aforementioned events appear to all occur 10–20 minutes prior to the final physical separation of daughter cells, suggesting that they either orchestrate abscission or set up stage for further downstream events. Bottom: After the first bridge-severing event, abscission is accomplished and the two daughter cells are physically separated but some cells have been documented to have a second severing event.
Like Golgi complex-derived vesicles, endocytic membrane transport to the cytokinetic bridge is required for abscission17, 22, 23. Recent evidence demonstrates that endosomes targeted to the midbody are bound by the Rab GTPase Rab11 or Rab35. Both Rabs are involved in endocytic recycling during interphase but localize to discrete endocytic compartments. Depletion of either Rab causes binucleated cell formation resulting from cytokinesis failure possibly through two separate mechanisms17, 22, 23. For example, inhibition of Rab35 activity prevents midbody targeting of septin, a cytoskeleton component crucial for cytokinesis22, providing an explanation for the mechanism of abscission failure under this condition. In contrast, the polarized transport of Rab11 endosomes to the midbody requires Rab11 and its effectors, a family of Rab11-interacting proteins (FIPs; for example, FIP3 and FIP4)17. FIP3 and FIP4 also interact with and require ADP ribosylation factor 6 (Arf6) and exocyst subunit Exo70 for localization to the intercellular bridge and the midbody24. Depletion of either FIP3 or Rab11 disrupts abscission17. However, the precise contribution of Rab11 membranes to abscission is unknown. One intriguing model for Rabs in abscission is that cross-regulation between the Rab35- and Rab11-mediated endocytic pathways occurs during cytokinesis via Arf6 due to a recent study by Chesneau et al. showing that an Arf6 effector, EPI64B, is a GTPase activating protein (GAP) for Rab35 (Echard’s Current Bio paper).
A potentially similar cross-regulating system may be operating between the secretory and endocytic pathways. For example, the exocyst subunit, Sec15, binds both centriolin, the apparent anchor for Golgi-derived secretory vesicles at the midbody, and active Rab11, an interaction implicated in endocytic recycling6, 25. Moreover, exocyst disruption seems to impair both secretory vesicle fusion at the midbody and localization of Rab11-containing endosomes to the intercellular bridge6, 24. This suggests that the exocyst co-functions in secretory and endosomal pathways and in this capacity may co-regulate and coordinate these pathways during abscission. Furthermore, secretory or endocytic vesicles decorated with Rab8 are also delivered to the cytokinetic bridge. Surprisingly, Guizetti et al. illustrated that blocking Rab8-decorated vesicle delivery to the bridge had no effect on cytokinesis (REFERENCE). This could suggest one of two things that abscission is not sensitive to secretory or endocytic vesicle contribution in all cell types, or that there is a distinct role for specific vesicle trafficking pathways at the midbody
Targeted tethering of secretory and endocytic vesicles by the Exocyst complex to the intercellular bridge is similar to exocyst mediated-vesicle tethering to the leading edge in migrating cells and vesicle tethering in cells forming apical and basolateral domains 26. Based on this similarity, we propose that polarized vesicle transport contributes to polarity formation as early as cytokinesis when new epithelial cells are created (further discussed in Hehnly and Doxsey8). In support of this idea is the observation that Rab11 is required for both polarized transport of endosomes to cell division sites17, and proper targeting of the polarity protein Crumbs 3a (Crb3a) to the intercellular bridge7. While Rab11 is required for cytokinesis and Crb3a delivery to the bridge, a role for Crb3a in cytokinesis has not yet been established. A better understanding of the role of Crb3a could link cell polarity to abscission. A further link may be found to the polarity pathway involving the PAR complex, Cdc42 and Par-6. This pathway has been linked to endocytic recycling in mammalian cells and C. elegans coelomocytes27, suggesting a model in which polarized membrane traffic and polarity protein recruitment to the division site contribute synergistically to abscission perhaps through molecular coordination and co-regulation.
ESCRTs and the bridge cutting
The role of ESCRT in multivesicular body (MVB) biogenesis and virus budding is widely accepted28, 29. The topological similarity between the neck of a budding virus and the intercellular bridge between two nascent daughter cells, suggests an additional role for ESCRTs in abscission. This was, in fact, recently demonstrated in a series of studies (reviewed in Caballe and Martin-Serrano (2011)30; Fig. 2). ESCRT is comprised of four distinct subcomplexes (ESCRT-0, ESCRT-I, ESCRT-II and ESCRT-III) that normally function in order28, 29, and several associated proteins. A subset of ESCRTs and associated proteins are directed to the midbody by Cep559, 31, 32 and/or may be dependent on the midbody ubiquitination33. With the aid of three-dimensional structured illumination microscopy (SIM) and EM tomography, fluorescent protein-tagged or immunostained components of ESCRT-III (for example, CHMP4B and Vps4B) are found to initially concentrate at the midbody and at a later time point at a separate site where the helical filaments reside (~1 μm away from the MB; Fig. 2, middle)4, 5. It is thus proposed that ESCRT-III polymerizes into helical filaments appearing at the bridge cutting site and causes the deformation of intercellular bridge membrane adjacent to the MB4, 5, 30. Interestingly, ESCRT-III components and ESCRT-associated protein also appear to recruit the AAA+ ATPase spastin to the bridge cutting site, suggesting a role in coordinating microtubule severing adjacent to the midbody4, 10, 34.
These studies suggest bridge severing occurs adjacent to, rather than at the midbody. However, it is unclear how ESCRT-III components and spastin are recruited to the severing site. One possibility is that midbody-localized ESCRT-III components are ‘activated’ by other midbody components (for example, kinases, ubiqutin ligases and de-ubiquitylating enzymes), allowing ESCRT-III components to grow spirally towards or translocate to the scission site. Alternatively, the ESCRT-III components and spastin localized at the severing site may be recruited from a cytoplasmic pool. Further work is needed to define the mechanism of ESCRT-III recruitment to the scission site, and its contribution to abscission. For instance, the diameter of the intercellular bridge is significantly larger than that modified by ESCRT-III during MVB biogenesis or viral budding28, 29. Additional studies are required to determine how ESCRT-III resolves this large tubular intercellular bridge.
It is easy to comprehend how helical filaments could form in the intercellular bridge and how ESCRT could contribute to abscission. However, the molecular composition of the helical filaments has not been thoroughly investigated and the mechanism of membrane deformation by these filaments has not yet been demonstrated. In fact, a more recent study from Martin-Serrano and colleagues showed that CHMP4C, a paralog of CHMP4B, was recruited to the midbody much earlier suggesting an earlier role for the protein and a different regulation of abscission, rather than acting at the final stage of abscission15. This work also raises the possibility that the helical filaments linked to bridge cutting may be different from the analogous filamentous structures observed in viral budding28, 29 and may contain only a subset of ESCRT-III components (for example, CHMP2A ad CHMP4B). Further studies are required to more precisely determine how ESCRT-III contributes to abscission. In any case, helical filament assembly, deformation of the intercellular bridge and microtubule severing prior to abscission, appears to draw the cell closer to abscission. However, it is rather surprising that abscission occurs 10–20 minutes after these events5. This delay is similar in timing to vesicle recruitment to and/or fusion in the intercellular bridge6, 16. The similar timing of these events may reflect a coordinated effort that contributes to abscission or a preparatory step, perhaps membrane remodeling, for a separate downstream event that mediates the final severing of the bridge such as ESCRT deformation of the bridge.
Kinases ensure faithful abscission
It is reasonable to envision master regulators of the complex abscission process that ensure abscission location, timing and coordination with other cellular events. It is thus not surprising that the well-known mitotic kinases, Polo-like kinase 1 (Plk1) and Aurora B, have important roles in abscission. Aurora B also coordinates abscission with other cellular processes such as nuclear pore reassembly.
Mitotic kinases regulate midbody assembly and abscission timing
It has been known for some time that Plk1 and Aurora B are required for proper progression through mitosis and cytokinesis35, 36, but only recently were their roles in abscission uncovered11, 12, 37. The gradual decline of Plk1 activity after anaphase is crucial for cytokinesis entry38. The midzone appears during anaphase and is transformed into the midbody during cytokinesis in a sequence of events1. Bastos and Barr (2010) recently showed that the recruitment of Cep55 to the midbody depends on the decline of Plk1 activity after anaphase11. More specifically, Plk1 phosphorylates Cep55 and prevents its association with the midzone until cytokinesis entry (Fig. 3)11. Only after Plk1 activity decreased did Cep55 translocate to and integrate into the midbody (Fig. 3)11. Inhibition of Plk1 caused Cep55 to translocate to the midbody prematurely and resulted in abscission failure, presumably due to aberrant midbody architecture and the inability to properly target ESCRT-III components to the midbody11. Thus, Plk1 appears to regulate cytokinesis progression and faithful abscission through its ability to recruit midbody components in an orderly manner through phosphorylation of substrates.
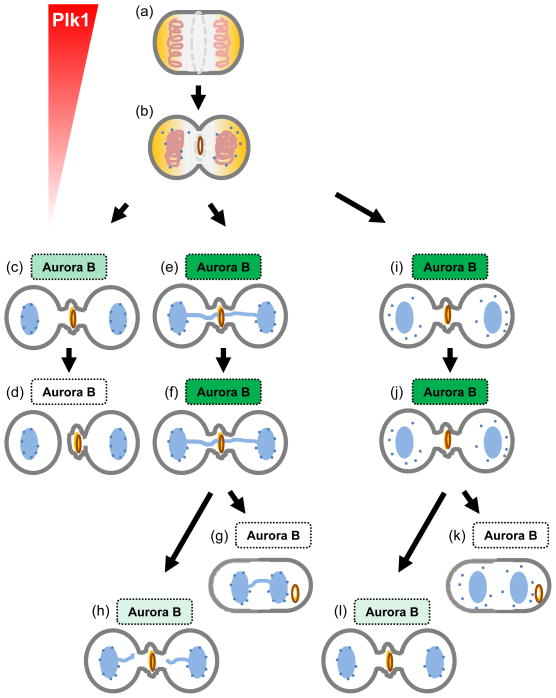
Fig 3. Mitotic Kinases regulate cytokinesis progression and coordination.
Illustration shows how mitotic kinases Plk1 and Aurora B regulate cytokinesis progression and abscission timing. Top: Active Plk1 (red) phosphorylates Cep55 (yellow) to keep it away from the midzone. Middle and bottom (a): Plk1 activity decreases since anaphase, eventually allowing the late translocation of Cep55 to the midzone to integrate into the future midbody (yellow circle). This ordered assembly of midbody is required for proper cytokinesis progression and faithful abscission. Bottom (a): Normally, Aurora B phosphorylates Mklp1 (dark brown circle) to maintain the midbody integrity. Its activity decreases slowly throughout cytokinesis (light green box); Aurora B eventually becomes inactivated (white box) and Mklp1 is no longer phosphorylated upon abscission. Bottom (b,c): When chromatin is trapped in the intercellular bridge (light blue line; b) or nuclear pore complexes are improperly reassembled (dark blue dots; c), Aurora B remains active, keeps Mklp1 phosphorylated thus delaying abscission. When chromatin is eventually untangled and cleared from the bridge (b) or the improper assembly of nuclear pore complexes is fixed (c), Aurora B activity is inactivated, allowing abscission. This Aurora B-dependent abscission checkpoint is proposed to protect cells from premature or uncoordinated abscission.
Similar to Plk1, the mitotic kinase, Aurora B, phosphorylates the core midbody component, Mklp112, which appears to stabilize Mklp1 integration at the midbody and thus maintain midbody integrity. Aurora B has a gradual decline in activity during cytokinesis36, and becomes inactivated at the midbody upon abscission. If Aurora B is prematurely inactivated, it promotes abscission12, suggesting a mechanism for controlling abscission timing and progression. When chromatin is trapped within the intercellular bridge, the connected daughter cells delay abscission so the chormatin can be cleared12. This requires sustained activation of Aurora B and Mklp1 phosphorylation12. This Aurora B-mediated phosphorylation pathway is thought to act as an abscission checkpoint, protecting cells from inappropriate transfer or damage of genetic material (Fig. 3)12.
Coordination between nuclear pore reassembly and cytokinesis
Upon entry into cytokinesis, cells decondense segregated chromosomes and reassemble nuclear pore complexes (NPCs)39. NPCs are made up of three elements: cytoplasmic filaments, transmembrane scaffolds and nuclear baskets39. NPC proteins have been long known to function in early cell division (for example, during kinetochore and spindle assembly); however, their roles in cytokinesis are only now becoming clear.
One subunit of the NPC scaffold Nup107-160 complex, Seh1, is not only required for NPC reassembly, but when depleted induces the formation of multi-nucleated cells14, suggesting a cytokinetic role. Strikingly, Seh1 participates in the translocation of Aurora B to the midzone14, an event required for midzone organization36. Proper midzone organization, in turn, contributes to the progression of cytokinesis and the organization of the midbody for abscission1. Although the detailed mechanisms of these events have not been revealed, the Seh1-regulated Aurora B translocation provides clues to how NPC reassembly is coordinated with cytokinesis to ensure faithful division.
Another link between NPC reassembly and Aurora B is through the nuclear basket proteins, Nup50 and Nup15313. Depletion of Nup50 or Nup153 not only mislocalizes multiple NPC components from the nuclear envelope, showing defective NPC reassembly, but also delays cells in abscission (Fig. 3)13. The mislocalized NPC components in the cytoplasm appear to trap active Aurora B and disturb Aurora B targeting to the midbody during cytokinesis13. Inhibition of Aurora B activity restores abscission13, suggesting that the delay is caused by prolonged Aurora B activation in response to improper NPC assembly. This Aurora B-dependent surveillance system for NPC reassembly is similar to its response to chromatin trapped in the bridge12. However, it is unclear if Aurora B modulates common or distinct molecular targets in these scenarios. Future work is required to test if nuclear basket proteins (for example, Nup153) or other NPC components interact with and modulate Aurora B activity. Nevertheless, these new findings reveal exciting new roles for NPCs in cytokinesis, and indicate that Aurora B is important for coordinating chromatin clearance and nuclear pore reassembly with abscission.
Conclusions and perspectives
Our understanding of abscission has evolved significantly over the last few years, with many unexpected discoveries driven by new technologies and new perspectives. Some of these findings are consistently observed while others diverge. Despite this, one can construct a generalized model of abscission based on the findings to date (Fig. 2). For many years, the mechanism of cell separation included the widespread idea that cells simply broke the intercellular bridge by crawling apart from one another. The best example was the slime mold, Dictyostelium discoideum. The term used for cell separation in Dictyolstelium was cytofission, the process of crawling apart and breaking the interconnection between the two cells40. However, recent evidence shows that even cytofission is controlled by a set of genes and that disruption of these genes blocks abscission. Proteomic analysis of mammalian midbodies from cytokinetic cells2 reinforces the idea that abscission requires a diversity of cellular activities that operate with precise temporal resolution and coordination to achieve a common goal, the final cut that generates two daughter cells.
Vesicle trafficking is a key feature of abscission and is represented by a diverse number of membrane pathways. Vesicle trafficking is even more important in plant cell division as plants do not employ an actomyosin ring and have a non-pliant cell wall. Instead, plants use vesicles to build the cell plate, a flattened membranous compartment that continues to grow centripetally through vesicle delivery and fusion, to ultimately join the plasma membrane and complete cell separation41.
Conceptualization of the idea that ESCRT could contribute to abscission and the experimental demonstration that it indeed does, was a remarkable and seminal discovery. The requirement for ESCRT in abscission suggested a plausible mechanism for abscission through persistent pinching/constricting of the plasma membrane until the intercellular bridge was severed, as in viral budding. There remain some issues related to the divergence of results on ESCRT function and the mechanism by which the large caliber of the intercellular bridge is resolved. These must be addressed in order to obtain a robust understanding of the role of ESCRT in abscission.
Microtubule severing and depolymerization is required for resolving the intercellular bridge and proteins such as spastin have emerged as major players. It seems that not all microtubules are severed and depolymerized in the bridge, as a subset persists transiently in post-mitotic midbodies. Bridge microtubules are dynamic42 but may be stabilized by regulatory post-translational modification, such as acetylation or detyrosination43, from early cytokinesis to abscission. Whether and how these modifications can affect abscission progression also require further investigations.
Plk1, Aurora B and most likely other regulatory molecules modify midbody proteins to regulate midbody assembly and integrity throughout cytokinesis (Fig. 2,3). These kinases operate as a surveillance system to ensure the progression and fidelity of mitotic processes such as chromatin segregation, nuclear pore assembly, proper execution of abscission and prevention of genomic instability (Fig. 3). In most cells, abscission takes longer than all the other stages of mitosis combined. Perhaps this is to confirm that chromatin within the intercellular bridge is completely and effectively moved to the two daughter cells12, 15. The discovery of an Aurora B-mediated abscission checkpoint activated by chromatin was an important step forward in understanding how abscission is regulated12.
When comparing studies within the field, it appears that (late) cytokinesis defects are defined differently among studies. Thus, a live super-resolution imaging-based analysis with a comparable starting or end point is indispensible and will more accurately resolve the spatiotemporal dynamics of midbody proteins during cytokinesis and perhaps facilitate imaging of structural elements observed by electron microscopy. This will provide insight into where and when different cellular pathways are recruited, and how they function coordinately. There is still much to be learned about the underpinnings of abscission as well as cell polarity, given the polar nature of abscission.
Acknowledgments
We apologize to colleagues whose work is not discussed or cited due to space constraints. We are particularly grateful to Alison Bright and Sambra Redick (Doxsey lab, University of Massachusetts Medical School) for critical reading of the manuscript and thoughtful discussions. Work in the Doxsey laboratory is supported by The National Institutes of Health, the Ellison Medical Foundation, the W. M. Keck Foundation and the Department of Defense.
Key references
- 1.Eggert US, Mitchison TJ, Field CM. Animal cytokinesis: from parts list to mechanisms. Annu Rev Biochem. 2006;75:543–66. doi: 10.1146/annurev.biochem.74.082803.133425. [DOI] [PubMed] [Google Scholar]
- 2.Skop AR, Liu H, Yates J, 3rd, Meyer BJ, Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–6. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connell JW, Lindon C, Luzio JP, Reid E. Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic. 2009;10:42–56. doi: 10.1111/j.1600-0854.2008.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guizetti J, et al. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science. 331:1616–20. doi: 10.1126/science.1201847. [DOI] [PubMed] [Google Scholar]
- 5.Elia N, Sougrat R, Spurlin TA, Hurley JH, Lippincott-Schwartz J. Dynamics of endosomal sorting complex required for transport (ESCRT) machinery during cytokinesis and its role in abscission. Proc Natl Acad Sci U S A. 108:4846–51. doi: 10.1073/pnas.1102714108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gromley A, et al. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 7.Schluter MA, et al. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell. 2009;20:4652–63. doi: 10.1091/mbc.E09-02-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hehnly H, Doxsey S. Polarity sets the stage for cytokinesis. Mol Biol Cell. 23:7–11. doi: 10.1091/mbc.E11-06-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlton JG, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science. 2007;316:1908–12. doi: 10.1126/science.1143422. [DOI] [PubMed] [Google Scholar]
- 10.Yang D, et al. Structural basis for midbody targeting of spastin by the ESCRT-III protein CHMP1B. Nat Struct Mol Biol. 2008;15:1278–86. doi: 10.1038/nsmb.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastos RN, Barr FA. Plk1 negatively regulates Cep55 recruitment to the midbody to ensure orderly abscission. J Cell Biol. 191:751–60. doi: 10.1083/jcb.201008108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steigemann P, et al. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell. 2009;136:473–84. doi: 10.1016/j.cell.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Mackay DR, Elgort SW, Ullman KS. The nucleoporin Nup153 has separable roles in both early mitotic progression and the resolution of mitosis. Mol Biol Cell. 2009;20:1652–60. doi: 10.1091/mbc.E08-08-0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Platani M, et al. The Nup107-160 nucleoporin complex promotes mitotic events via control of the localization state of the chromosome passenger complex. Mol Biol Cell. 2009;20:5260–75. doi: 10.1091/mbc.E09-05-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carlton JG, Caballe A, Agromayor M, Kloc M, Martin-Serrano J. ESCRT-III governs the Aurora B-mediated abscission checkpoint through CHMP4C. Science. 336:220–5. doi: 10.1126/science.1217180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goss JW, Toomre DK. Both daughter cells traffic and exocytose membrane at the cleavage furrow during mammalian cytokinesis. J Cell Biol. 2008;181:1047–54. doi: 10.1083/jcb.200712137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson GM, et al. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol Biol Cell. 2005;16:849–60. doi: 10.1091/mbc.E04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skop AR, Bergmann D, Mohler WA, White JG. Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr Biol. 2001;11:735–46. doi: 10.1016/s0960-9822(01)00231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cascone I, et al. Distinct roles of RalA and RalB in the progression of cytokinesis are supported by distinct RalGEFs. EMBO J. 2008;27:2375–87. doi: 10.1038/emboj.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao WM, Seki A, Fang G. Cep55, a microtubule-bundling protein, associates with centralspindlin to control the midbody integrity and cell abscission during cytokinesis. Mol Biol Cell. 2006;17:3881–96. doi: 10.1091/mbc.E06-01-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Low SH, et al. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev Cell. 2003;4:753–9. doi: 10.1016/s1534-5807(03)00122-9. [DOI] [PubMed] [Google Scholar]
- 22.Kouranti I, Sachse M, Arouche N, Goud B, Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr Biol. 2006;16:1719–25. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 23.Chesneau L, et al. An ARF6/Rab35 GTPase Cascade for Endocytic Recycling and Successful Cytokinesis. Curr Biol. 22:147–53. doi: 10.1016/j.cub.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 24.Fielding AB, et al. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–99. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu S, Mehta SQ, Pichaud F, Bellen HJ, Quiocho FA. Sec15 interacts with Rab11 via a novel domain and affects Rab11 localization in vivo. Nat Struct Mol Biol. 2005;12:879–85. doi: 10.1038/nsmb987. [DOI] [PubMed] [Google Scholar]
- 26.Bryant DM, et al. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 12:1035–45. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balklava Z, Pant S, Fares H, Grant BD. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat Cell Biol. 2007;9:1066–73. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- 28.Wollert T, et al. The ESCRT machinery at a glance. J Cell Sci. 2009;122:2163–6. doi: 10.1242/jcs.029884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henne WM, Buchkovich NJ, Emr SD. The ESCRT pathway. Dev Cell. 21:77–91. doi: 10.1016/j.devcel.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Caballe A, Martin-Serrano J. ESCRT machinery and cytokinesis: the road to daughter cell separation. Traffic. 12:1318–26. doi: 10.1111/j.1600-0854.2011.01244.x. [DOI] [PubMed] [Google Scholar]
- 31.Morita E, et al. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007;26:4215–27. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HH, Elia N, Ghirlando R, Lippincott-Schwartz J, Hurley JH. Midbody targeting of the ESCRT machinery by a noncanonical coiled coil in CEP55. Science. 2008;322:576–80. doi: 10.1126/science.1162042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pohl C, Jentsch S. Final stages of cytokinesis and midbody ring formation are controlled by BRUCE. Cell. 2008;132:832–45. doi: 10.1016/j.cell.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Agromayor M, et al. Essential role of hIST1 in cytokinesis. Mol Biol Cell. 2009;20:1374–87. doi: 10.1091/mbc.E08-05-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petronczki M, Lenart P, Peters JM. Polo on the Rise-from Mitotic Entry to Cytokinesis with Plk1. Dev Cell. 2008;14:646–59. doi: 10.1016/j.devcel.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Carmena M, Ruchaud S, Earnshaw WC. Making the Auroras glow: regulation of Aurora A and B kinase function by interacting proteins. Curr Opin Cell Biol. 2009;21:796–805. doi: 10.1016/j.ceb.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fabbro M, et al. Cdk1/Erk2- and Plk1-dependent phosphorylation of a centrosome protein, Cep55, is required for its recruitment to midbody and cytokinesis. Dev Cell. 2005;9:477–88. doi: 10.1016/j.devcel.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Lindon C, Pines J. Ordered proteolysis in anaphase inactivates Plk1 to contribute to proper mitotic exit in human cells. J Cell Biol. 2004;164:233–41. doi: 10.1083/jcb.200309035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.D’Angelo MA, Hetzer MW. Structure, dynamics and function of nuclear pore complexes. Trends Cell Biol. 2008;18:456–66. doi: 10.1016/j.tcb.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson DN, Girard KD, Octtaviani E, Reichl EM. Dictyostelium cytokinesis: from molecules to mechanics. J Muscle Res Cell Motil. 2002;23:719–27. doi: 10.1023/a:1024419510314. [DOI] [PubMed] [Google Scholar]
- 41.Jurgens G. Membrane trafficking in plants. Annu Rev Cell Dev Biol. 2004;20:481–504. doi: 10.1146/annurev.cellbio.20.082503.103057. [DOI] [PubMed] [Google Scholar]
- 42.Rosa J, Canovas P, Islam A, Altieri DC, Doxsey SJ. Survivin modulates microtubule dynamics and nucleation throughout the cell cycle. Mol Biol Cell. 2006;17:1483–93. doi: 10.1091/mbc.E05-08-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janke C, Bulinski JC. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat Rev Mol Cell Biol. 12:773–86. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]