Abstract
With consideration of multifactorial etiology of diabetic peripheral neuropathy, an ideal drug or drug combination should target at least several key pathogenetic mechanisms. The flavonoid baicalein (5,6,7-trihydroxyflavone) has been reported to counteract sorbitol accumulation, activation of 12/15-lipoxygenase, oxidative-nitrosative stress, inflammation, and impaired signaling in models of chronic disease. This study evaluated baicalein on diabetic peripheral neuropathy. Control and streptozotocin-diabetic C57Bl6/J mice were maintained with or without baicalein treatment (30 mgkg−1d−1, i.p., for 4 weeks after 12 weeks without treatment). Neuropathy was evaluated by sciatic motor and hind-limb digital sensory nerve conduction velocities, thermal algesia (Hargreaves test), tactile response threshold (flexible von Frey filament test), and intraepidermal nerve fiber density (fluorescent immunohistochemistry with confocal microscopy). Sciatic nerve and spinal cord 12/15-lipoxygenase and total and phosphorylated p38 mitogen-activated protein kinase expression and nitrated protein levels were evaluated by Western blot analysis, 12(S)hydroxyeicosatetraenoic acid concentration (a measure of 12/15-lipoxygenase activity) by ELISA, and glucose and sorbitol pathway intermediate concentrations by enzymatic spectrofluorometric assays. Baicalein did not affect diabetic hyperglycemia, and alleviated nerve conduction deficit and small sensory nerve fiber dysfunction, but not intraepidermal nerve fiber loss. It counteracted diabetes-associated p38 mitogen-activated protein kinase phosphorylation, oxidative-nitrosative stress, and 12/15-lipoxygenase overexpression and activation, but not glucose or sorbitol pathway intermediate accumulation. In conclusion, baicalein targets several mechanisms implicated in diabetic peripheral neuropathy. The findings provide rationale for studying hydroxyflavones with improved pharmacological profile as potential treatments for diabetic neuropathy and other diabetic complications.
Keywords: baicalein, diabetic peripheral neuropathy, 12/15-lipoxygenase, oxidative-nitrosative stress, p38 mitogen-activated protein kinase, sorbitol pathway of glucose metabolism
INTRODUCTION
Diabetic peripheral neuropathy (DPN)-related research during the last 20–30 years led to identification of multiple pathogenetic mechanisms including, but not limited to, increased aldose reductase (AR) activity (Ho et al., 2006; Obrosova et al., 2002; Yagihashi et al., 2001), non-enzymatic glycation/glycooxidation (Bierhaus et al., 2004; Cameron et al., 2005; Toth et al., 2008), activation of protein kinase C (Cameron et al., 1999; Nakamura et al., 1999) and mitogen-activated protein kinases [MAPKs (Du et al., 2010; Purves et al., 2001)], oxidative-nitrosative stress (Cameron et al., 2001; Coppey et al., 2001b; Nagamatsu et al., 1995; Obrosova et al., 2005; Schmeichel et al, 2003), C-peptide deficiency (Sima et al.,2008; Stevens et al., 2004) and impaired neurotrophic support (Chattopadhyay et al., 2005; Christianson et al., 2007; Francis et al., 2009; Goss et al., 2002; Nakae et al., 2006; Wu et al., 2011), which contribute to this devastating complication of diabetes mellitus. Unfortunately, all monotherapies for DPN studied so far, including AR and protein kinase C inhibitors, acetyl carnitine, nerve growth factor, and the antioxidant α–lipoic acid, showed modest efficacy in clinical trials, or have been abandoned due to adverse side effects (Tesfaye et al, 2010). The continued drug discovery for DPN is currently focused on 1) invention and development of new inhibitors of previously characterized pathogenetic mechanisms e.g., new AR (Bril et al, 2009) and non-enzymatic glycation (Wada et al., 2001) inhibitors, as well as potent superoxide dismutase mimetics (Coppey et al., 2001a) and peroxynitrite decomposition catalysts (Drel et al., 2007a; Obrosova et al., 2007a) to combat oxidative-nitrosative stress; 2) identification of new pathogenetic mechanisms and drug targets e.g., poly(ADP-ribose) polymerase (Drel et al., 2010; Obrosova et al., 2004; Obrosova et al., 2008; Szabo et al, 2004), low-grade inflammation (Dopius et al., 2009; Wang et al., 2006; Wang et al., 2008) likely to be associated with cyclooxygenase-2 (Kellogg et al., 2007), 12/15-lipoxygenase (Obrosova et al., 2010; Stavniichuk et al., 2010), and nuclear factor-κB (Cameron and Cotter, 2009) activation, disrupted neuregulin/caveolin-1 signaling (McGuire et al., 2009) and molecular chaperone activity (Urban et al., 2010), and activation of neutral endopeptidase, a protease that degrades vaso- and neuro-active peptides (Coppey et al., 2010); 3) studies of combination therapies (Cotter et al., 2001; Li et al., 2005) and mono-drugs targeting several pathogenetic mechanisms (Kumar and Sharma, 2010).
Flavonoids and polyphenols counteract oxidative-nitrosative stress and many chronic diseases (Gohil and Packer, 2002; Schroeter et al., 2002; Sies, 2010). Baicalein (5,6,7-trihydroxyflavone), a flavonoid originally isolated from the roots of Scutellaria baicalensis, has been employed for many centuries in traditional Chinese herbal medicine as antibacterial and antiviral remedy (Huang et al., 2005). In addition to its antioxidant properties, baicalein has been described to inhibit xanthine oxidase, 12/15-lipoxygenase, p38 MAPK, cytosolic phospholipase A2, inflammatory response, as well as sorbitol accumulation in animal and cell culture models of chronic disease (Huang et al., 2005; Cui et al., 2010; Zhou and Zhang 1989). Reports suggest good efficacy of baicalein against experimental renin-dependent hypertension (Huang et al., 2005), endothelial dysfunction associated with cardiovascular diseases (Huang et al., 2005), cerebral ischemia (Pallast et al., 2010), cancer (Androutsopoulos et al., 2010), Parkinson disease (Hong et al., 2008), dermatitis (Yun et al., 2010), liver fibrosis (Sun et al., 2010), as well as pain of inflammatory origin (Yoo et al., 2009). Efficacy of baicalein or other hydroxyflavones against diabetes and diabetic complications has not sufficiently been explored although two experimental studies suggest that baicalein counteracts cytokine-induced beta cell dysfunction, a key phenomenon in the development of autoimmune diabetes (Chen et al., 2005), as well as glial activation, pro-inflammatory response, ganglion cell loss, and increased vascular permeability characteristic for early diabetic retinopathy (Yang et al., 2009).
The present study evaluated baicalein on motor and sensory nerve conduction velocity (MNCV and SNCV) deficits, small sensory nerve fiber dysfunction, intraepidermal nerve fiber loss, and biochemical changes in the peripheral nerve and spinal cord characteristic for DPN. We employed C57Bl6/J mice with the duration of streptozotocin-diabetes of 12–16 weeks i.e., a model that develops both functional and structural changes associated with DPN in human subjects with diabetes mellitus (Christianson et al., 2007; Ho et al., 2006; Obrosova et al., 2007a; Obrosova et al., 2010; Stavniichuk et al., 2010; Yagihashi et al., 2001).
METHODS
A. Reagents
Unless otherwise stated, all chemicals were of reagent-grade quality, and were purchased from Sigma Chemical Co., St. Louis, MO, USA. Baicalein was obtained from Cayman Chemical, Ann Abor, MI. Mouse monoclonal (clone 1A6) anti-nitrotyrosine (NT) antibody for Western blot analysis of nitrated proteins was purchased from Millipore, Billerica, MA, USA. Rabbit polyclonal (clone H-100) anti-12-LO antibody and rabbit polyclonal antibody to total p38 MAPK were obtained from Santa Cruz Biotechnology, Santa Cruz, CA, USA. Rabbit polyclonal antibody to phosphorylated p38 MAPK was purchased from Cell Signaling, Danvers, MA. For assessment of intraepidermal nerve fiber density (INFD), rabbit polyclonal anti-protein gene product 9.5 (PGP 9.5) antiserum was obtained from UltraClone, Isle of Wight, UK; Alexa Fluor 488 goat anti-rabbit highly cross-adsorbed IgG (H+L) from Invitrogen, Eugene, OR, USA; SuperBlock blocking buffer from Thermo Scientific, Rockford, IL, USA; and the optimum cutting temperature (O.C.T.) compound from Sakura Finetek USA, Torrance, CA, USA. VECTASHIELD Mounting Medium was obtained from Vector Laboratories, Burlingame, CA, USA. Other reagents for immunohistochemistry were purchased from Dako Laboratories, Inc., Santa Barbara, CA, USA.
B. Animals
The experiments were performed in accordance with regulations specified by The Guide for the Care and Handling of Laboratory Animals (NIH Publication No. 85-23) and Pennington Biomedical Research Center Protocol for Animal Studies. Mature C57Bl6/J mice were purchased from Jackson Laboratories. All the mice were fed standard mouse chow (PMI Nutrition International, Brentwood, MO, USA) and had ad libitum access to water. Diabetes was induced by STZ as we described previously (Obrosova et al., 2007a). Blood samples for glucose measurements were taken from the tail vein three days after STZ injection and the day before the animals were killed. The mice with blood glucose ≥13.8 mM were considered diabetic. The control and diabetic mice were maintained for 12 weeks and then divided into three subgroups. One subgroup was euthanized for tissue harvest. Two other subgroups were maintained with or without treatment with baicalein, 30 mg kg−1d−1 i.p., for another 4 weeks. Non-fasting blood glucose measurements were performed at induction of diabetes and at the end of the study. Physiological and behavioral measurements were taken at three time-points i.e., at the beginning of the study and before and after baicalein treatment. MNCV and SNCV were measured in mice anaesthetized with a mixture of ketamine and xylazine (45 mgkg−1 body weight and 15 mgkg−1 body weight, respectively, i.p.).
C. Anesthesia, euthanasia and tissue sampling
The animals were sedated by CO2, and immediately sacrificed by cervical dislocation. Sciatic nerves and spinal cords were rapidly dissected and frozen in liquid nitrogen for subsequent assessment of glucose, sorbitol, fructose, and 12(S)HETE concentrations, total and phosphorylated p38 MAPK and LO expression, and nitrated protein level (a measure of oxidative-nitrosative stress). Footpads were sampled for assessment of INFD.
D. Specific Methods
D.1. Physiology and behavioral tests
Sciatic MNCV, hind-limb digital SNCV, thermal algesia (plantar test by Hargreaves method), and tactile response thresholds (flexible von Frey filament test) were measured as described previously (Obrosova et al., 2004; Obrosova et al., 2007b).
D.2. INFD
Footpads were fixed in ice-cold Zamboni’s fixative for 3 hr, washed in 100 mM phosphate-buffered saline (PBS) overnight, and then in PBS containing increasing concentrations of sucrose i.e., 10%, 15%, and 20%, 3 hr in each solution. After washing, the samples were snap-frozen in O.C.T. compound and stored at −80°C. Three longitudinal 50 μm-thick footpad sections from each mouse were cut on Leica CM1950 cryostat (Leica Microsystems, Nussloch, Germany). Non-specific binding was blocked by 3% goat serum containing 0.5% porcine gelatin and 0.5% Triton X-100 in SuperBlock blocking buffer at room temperature, for 2 hr. The sections were incubated overnight with PGP 9.5 antiserum in 1:400 dilution, at 4°C, after which secondary Alexa Fluor 488 IgG (H+L) in 1:1000 was applied at room temperature, for 1 h. Sections were then coverslipped with VECTASHIELD mounting medium. Intraepidermal nerve fiber profiles were counted blindly by three independent investigators under Axioplan 2 microscope (Zeiss) at 40X magnification, and the average values were used. The length of epidermis was assessed on the microphotographs of stained sections taken at 5X magnification with a 3I Everest imaging system (Intelligent Imaging Innovations, Inc., Denver, CO, USA) equipped with Axioplan 2 microscope (Zeiss), using the NIH ImageJ software (version 1.42q). An average of 2.8 ± 0.3 mm of the sample length was investigated to calculate a number of nerve fiber profiles per mm of epidermis. Representative images of intraepidermal nerve fibers were obtained by confocal laser scanning microscopy at 40X magnification, using Leica TCS SP5 confocal system (Leica Microsystems, Mannheim, Germany).
D.3. Biochemical studies
D.3.1. Western blot analysis of total and phosphorylated p38 MAPK, LO, and nitrated proteins
Sciatic nerve and spinal cord materials (~ 10–20 mg) were placed on ice in 200 μl of RIPA buffer containing 50 mmol/l Tris-HCl, pH 7.2; 150 mmol/l NaCl; 0.1% sodium dodecyl sulfate; 1% NP-40; 5 mmol/l EDTA; 1 mmol/l EGTA; 1% sodium deoxycholate and the protease/phosphatase inhibitors leupeptin (10 μg/ml), pepstatin (1 μg/ml), aprotinin (20 μg/ml), benzamidine (10 mM), phenylmethylsulfonyl fluoride (1 mM), sodium orthovanadate (1 mmol/l), and homogenized on ice. The homogenates were sonicated (4 × 10 s) and centrifuged at 14,000 g for 20 min. All the afore-mentioned steps were performed at 4 °C. The lysates (20 and 40 μg protein for sciatic nerve and spinal cord, respectively) were mixed with equal volumes of 2x sample-loading buffer containing 62.5 mmol/l Tris-HCl, pH 6.8; 2% sodium dodecyl sulfate; 5% β-mercaptoethanol; 10% glycerol and 0.025% bromophenol blue, and fractionated in 5–20% gradient (nitrated proteins), 10 % (total and phosphorylated p38 MAPK) or 7.5% (LO) SDS-PAGE in an electrophoresis cell (Mini-Protean III; Bio-Rad Laboratories, Richmond, CA). Electrophoresis was conducted at 15 mA constant current for stacking, and at 25 mA for protein separation. Gel contents were electrotransferred (80 V, 2 hr) to nitrocellulose membranes using Mini Trans-Blot cell (Bio-Rad Laboratories, Richmond, CA) and western transfer buffer [25 mmol/l Tris-HCl, pH 8.3; 192 mmol/l glycine; and 20% (v/v) methanol]. Free binding sites were blocked in 2% (w/v) BSA (nitrated proteins) and 5% (w/v) BSA (LO and total and phosphorylated p38MAPK) in 20 mmol/l Tris-HCl buffer, pH 7.5, containing 150 mmol/l NaCl and 0.05% Tween 20, for 1 h, after which the corresponding primary antibodies (see Reagents) were applied overnight. The horseradish peroxidase-conjugated secondary antibodies were then applied for 1 h. In Western blot analysis of phosphorylated p38MAPK, the signal enhancer HIKARI (Nacalai USA, San Diego, CA) was applied with both primary and secondary antibodies according to the manufacturer instructions. After extensive washing, protein bands detected by the antibodies were visualized with the Amersham ECL™ Western Blotting Detection Reagent (Little Chalfont, Buckinghamshire, UK). Membranes were then stripped in the 25 mmol/l glycine-HCl, pH 2.5 buffer containing 2% SDS, and reprobed with β-actin antibody to confirm equal protein loading.
D.3.2. 12(S)HETE measurements
For assessment of 12(S)HETE, sciatic nerve and spinal cord samples were homogenized on ice in 15 mM Tris-HCI buffer (1:100 w/v) containing 140 mM NaCl, pH 7.6, and centrifuged. 12(S)HETE was measured in supernatants with the 12(S)-hydroxyeicosa-tetraenoic acid [12(S)HETE] Enzyme Immuno Assay kit (Assay Designs, Ann Arbor, MI).
D.3.3. Glucose and sorbitol pathway intermediates
Sciatic nerve and spinal cord glucose, sorbitol, and fructose concentrations were assessed by enzymatic spectrofluorometric methods with hexokinase/glucose 6-phosphate dehydro-genase, sorbitol dehydrogenase, and fructose dehydrogenase as we described in detail (Obrosova et al., 1999). Measurements were taken at LS 55 Luminescence Spectrometer (Perkin Elmer, MA).
E. Statistical analysis
The results are expressed as Mean ± SEM. Data were subjected to equality of variance F test, and then to log transformation, if necessary, before one-way analysis of variance. Where overall significance (p<0.05) was attained, individual between group comparisons for multiple groups were made using the Student-Newman-Keuls multiple range test. When between-group variance differences could not be normalized by log transformation (datasets for body weights and plasma glucose), the data were analyzed by the nonparametric Kruskal-Wallis one-way analysis of variance, followed by the Bonferroni/Dunn test for multiple comparisons. Individual pair-wise comparisons (INFD before treatment) were made using the unpaired two-tailed Student’s t-test. Significance was defined at p ≤ 0.05.
RESULTS
The initial (prior to STZ administration) body weights were similar in all experimental groups (Table 1). Weight gain during a 16-wk study was 78% lower in untreated diabetic group compared with non-diabetic controls. Baicalein treatment reduced weight gain in both non-diabetic (by 31%) and diabetic (by 79%) mice. Non-diabetic and diabetic groups displayed mortalities of 8% and 16%, respectively, during a 30-d baicalein treatment.
Table 1.
Initial and final body weights and blood glucose concentrations in control and diabetic mice maintained with and without baicalein treatment
| Variable Group | Body weight (g) | Blood glucose (mmol/l) | ||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| Control | 25.0 ± 0.5 | 38.4 ± 1.0 | 9.0 ± 0.6 | 9.5 ± 0.4 |
| Control + baicalein | 25.1 ± 0.4 | 34.3 ± 0.8** | 8.6 ± 0.3 | 9.0 ± 0.3 |
| Diabetic | 25.2 ± 0.5 | 28.1 ± 0.9** | 16.8 ± 1.0** | 28.7 ± 1.3** |
| Diabetic + baicalein | 23.9 ± 0.5 | 24.5 ± 0.6 **# | 16.2 ± 1.5** | 27.5 ± 0.9** |
Data are expressed as Means ± SEM, n = 24 per group.
p < 0.01 vs controls;
p < 0.05 vs untreated diabetic group.
The initial (after STZ administration) non-fasting blood glucose concentrations were 87% and 80% higher in untreated and baicalein-treated diabetic mice than in the non-diabetic group. Progression of hyperglycemia with the prolongation of diabetes resulted in ~3-fold differences between final blood glucose concentrations in both diabetic groups and non-diabetic controls. Baicalein treatment did not affect non-fasting glycemia in either non-diabetic or diabetic mice.
Mice with both 12-wk and 16-wk durations of diabetes exhibited MNCV and SNCV deficits (Table 2). MNCV and SNCV were similar in non-diabetic mice at the beginning of the study and at the 12-wk time point which suggests that diabetes-induced nerve conduction slowing did not develop due to affected peripheral nerve growth and maturation. Baicalein alleviated, but did not completely correct, MNCV and SNCV deficits in diabetic mice, without affecting those variables in non-diabetic controls.
Table 2.
Motor and sensory nerve conduction velocities, thermal response latencies, and tactile response thresholds in control and diabetic mice maintained with and without baicalein treatment
| Groups | ||||
|---|---|---|---|---|
| Variables | Control | Control + baicalein | Diabetic | Diabetic + baicalein |
| Baseline (prior to induction of STZ-diabetes) | ||||
| MNCV, ms−1 | 54.8 ± 1.1 | |||
| SNCV, ms−1 | 37.4 ± 0.4 | |||
| Thermal response latencies, s | 9.0 ± 0.6 | |||
| Tactile response thresholds, g | 2.1 ± 0.16 | |||
|
| ||||
| 12-wk time point (prior to interventions)
| ||||
| MNCV, ms−1 | 55.5 ± 1.2 | 41.2 ± 0.8** | ||
| SNCV, ms−1 | 38.0 ± 0.5 | 32.4 ± 0.8** | ||
| Thermal response latencies, s | 8.9 ± 0.7 | 17.9 ± 0.7** | ||
| Tactile response thresholds, g | 2.4 ± 0.2 | 0.8 ± 0.15** | ||
|
| ||||
| 14-wk time point (final measurements) | ||||
| MNCV, ms−1 | 57.2 ± 1.1 | 56.8 ± 1.6 | 41.3 ± 1.2** | 47.8 ± 1.5**# |
| SNCV, ms−1 | 38.4 ± 0.6 | 37.5 ± 0.7 | 30.8 ± 0.3** | 34.1 ± 0.4**## |
| Thermal response latencies, s | 10.1 ± 0.6 | 11.5 ± 0.3 | 18.5 ± 0.8** | 15.7 ± 0.6**## |
| Tactile response thresholds, g | 2.6 ± 0.2 | 2.3 ± 0.1 | 1.3 ± 0.1** | 1.7 ± 0.1**, # |
Data are expressed as Mean ± SEM, n = 10–12 per group.
p < 0.01 vs controls;
p < 0.05 and < 0.01 vs untreated diabetic group
Diabetic mice manifested thermal hypoalgesia and tactile allodynia at both 12-wk and 16- wk time points after induction of diabetes. Baicalein treatment ameliorated both sensory disorders in diabetic mice, without affecting either thermal response latencies or tactile response thresholds in non-diabetic controls.
Diabetic mice displayed a 23% reduction in INFD at the 12-wk time point (Fig. 1, A and B). This reduction progressed to 44% at the 16-wk time point (Fig. 1, C and D). Baicalein treatment did not affect INFD in non-diabetic mice, and did not induce intraepidermal nerve fiber regeneration or slowed down their degeneration in diabetic mice.
Fig. 1.
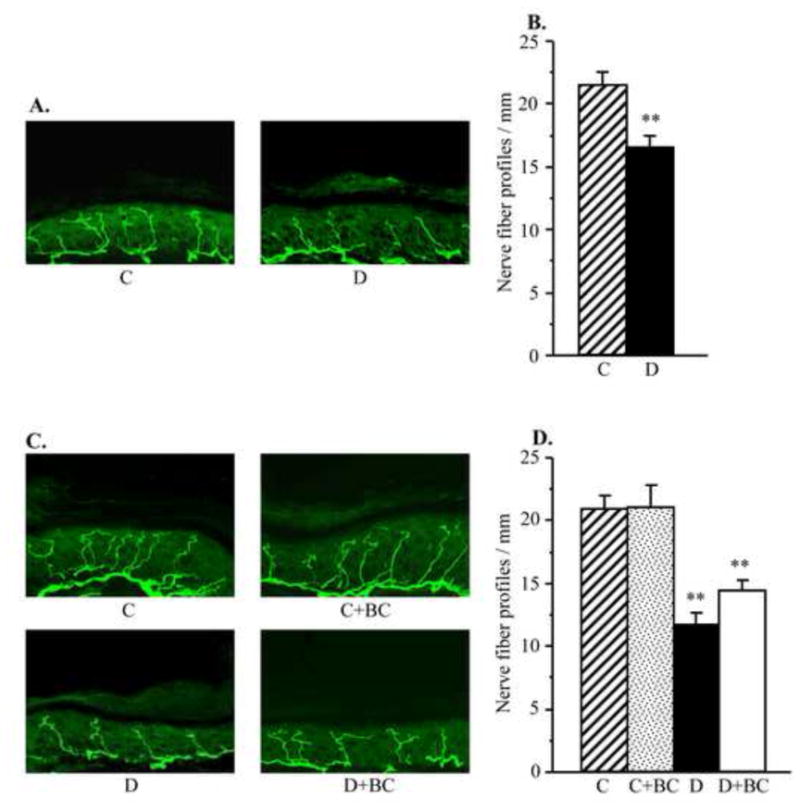
Intraepidermal nerve fiber profiles in control and diabetic mice at the 12-wk time point (prior to CDC intervention, A and B) and control and diabetic mice maintained with or without baicalein treatments (a 16-wk time point, C and D). A,C. Representative images of intraepidermal nerve fiber profiles, magnification x 40; B,D. Skin fiber density. Mean ± SEM, n = 10–12 per group. C – control mice, D – diabetic mice, ** – p < 0.01 vs non-diabetic control group.
As we reported earlier (Stavniichuk et al., 2010), C57Bl6/J mice with a 12-wk duration of diabetes demonstrate oxidative-nitrosative stress, and LO overexpression and activation. They also display p38 MAPK activation, and accumulation of glucose and sorbitol pathway intermediates in sciatic nerve and spinal cord (not shown). In the present study in the same animal model, increase in nitrated protein level (Fig. 2), LO expression (Fig. 3), and 12(S)HETE concentrations (Fig. 4) as well as glucose and sorbitol pathway intermediate accumulation (Table 3) were clearly manifest in sciatic nerve and spinal cord of diabetic mice with 16-wk duration of diabetes. Sciatic nerve p38 MAPK expression was increased by 142%, whereas total p38 MAPK expression was unchanged compared with non-diabetic control (Fig. 5). Spinal cord phosphorylated p38 MAPK expression was below the limit of detection in all experimental groups, and, for this reason, measurements of total p38 MAPK in this tissue were not performed. Treatment of diabetic mice with baicalein decreased diabetes-associated nitrated protein accumulation in the sciatic nerve and essentially normalized this variable in the spinal cord. It did not affect sciatic nerve and spinal cord LO overexpression, but significantly reduced 12(S)HETE concentrations, indicative of a reduction of LO activity in both tissues. Sciatic nerve and spinal cord glucose and sorbitol concentrations were similarly elevated in untreated and baicalein-treated diabetic mice compared with non-diabetic controls. Sciatic nerve fructose concentration was slightly (by 10%), but significantly, lower in baicalein-treated diabetic mice compared with the untreated diabetic group whereas no statistically significant differences between the two groups were observed for spinal cord fructose concentrations. Baicalein treatment normalized sciatic nerve phosphorylated p38 MAPK expression without affecting total p38 MAPK expression. Baicalein did not affect any afore-mentioned biochemical variables in either sciatic nerve or spinal cord of non-diabetic mice.
Fig. 2.
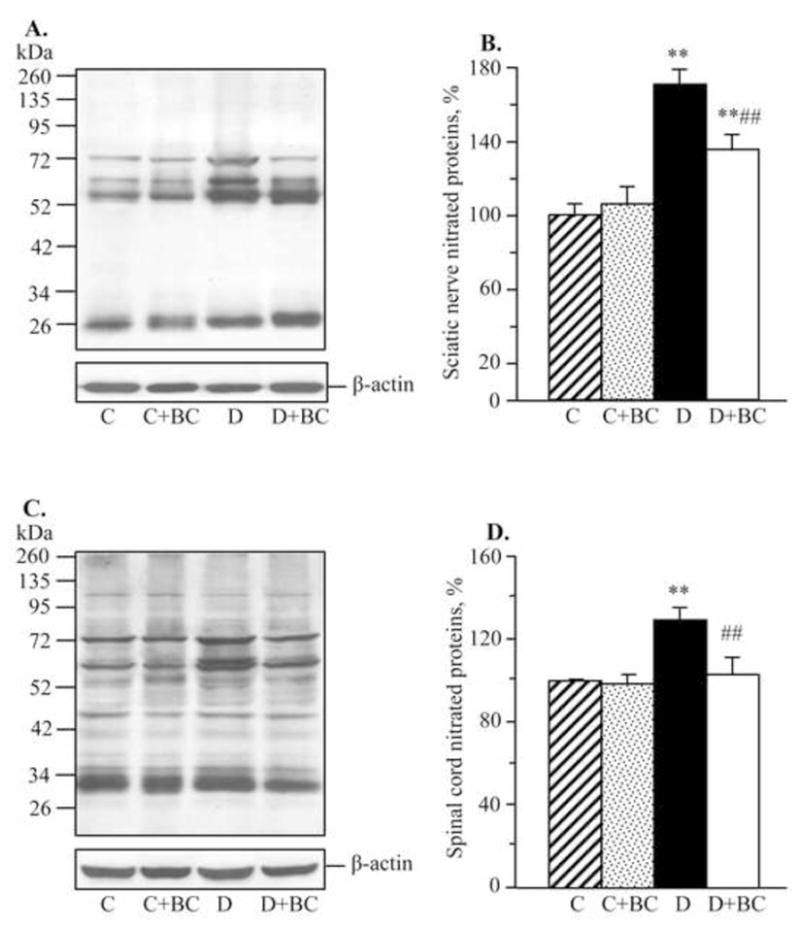
Representative Western blot analyses of nitrated proteins (A,C) and nitrated protein contents (densitometry, %, B,D) in the sciatic nerve and spinal cord of control and diabetic mice maintained with or without baicalein treatments. C – control, D – diabetic. Mean ± SEM, n = 6–10 per group. ** p <0.01 vs non-diabetic control group. ## p < 0.01 vs untreated diabetic group.
Fig. 3.
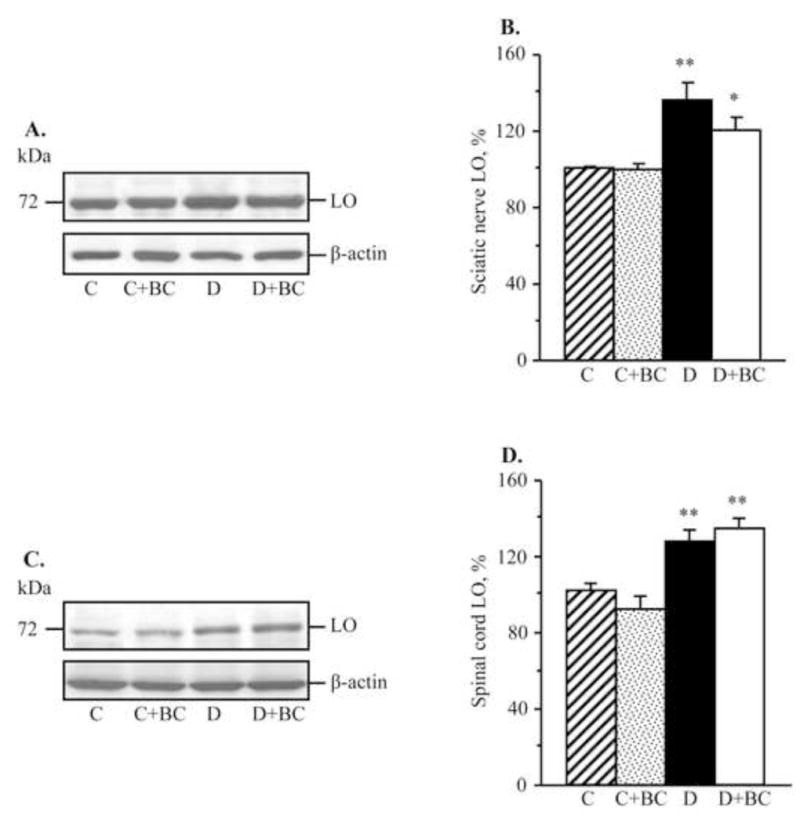
Representative Western blot analyses of 12/15-lipoxygenase expression (A,C) and 12/15-lipoxygenase protein contents (densitometry, %, B,D) in the sciatic nerve and spinal cord of control and diabetic mice maintained with or without baicalein treatments. C – control, D – diabetic. LO – 12/15-lipoxygenase. Mean ± SEM, n = 6–10 per group. *,** p <0.05 and < 0.01 vs non-diabetic control group.
Fig. 4.
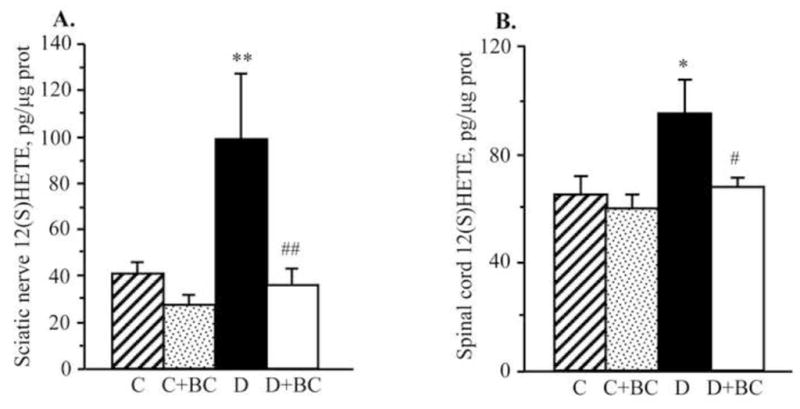
12(S) hydroxyeicosatetraenoic acid concentrations in the sciatic nerve (A) and spinal cord (B) of control and diabetic mice maintained with or without baicalein treatments. C – control; D – diabetic. 12(S)HETE – 12(S) hydroxyeicosatetraenoic acid. Mean ± SEM, n = 5–12 per group. *,** p < 0.05 and < 0.01 vs non-diabetic control group. #,## p < 0.05 and < 0.01 vs untreated diabetic group.
Table 3.
Sciatic nerve and spinal cord glucose, sorbitol, and fructose concentrations (nmol mg−1 protein) in control and diabetic mice maintained with and without baicalein treatment
| Groups | ||||
|---|---|---|---|---|
| Variables | Control | Control + baicalein | Diabetic | Diabetic + baicalein |
| Sciatic nerve | ||||
| Glucose | 12.6 ± 1.4 | 18.6 ± 2.2 | 110 ± 1.4 | 119 ± 14 |
| Sorbitol | 0.96 ± 0.06 | 0.79 ± 0.06 | 2.1 ± 0.21** | 1.9 ± 0.17** |
| Fructose | 2.2 ± 0.21 | 2.0 ± 0.13 | 10.5 ± 0.23** | 8.5 ± 0.21**## |
| Spinal cord
| ||||
| Glucose | 0.29 ± 0.2 | 0.35 ± 0.05 | 7.1 ± 1.6** | 8.8 ± 1.0** |
| Sorbitol | 0.86 ± 0.12 | 0.78 ± 0.09 | 1.23 ± 0.25* | 1.33 ± 0.11* |
| Fructose | 0.35 ± 0.08 | 0.36 ± 0.11 | 6.2 ± 1.1** | 8.1 ± 0.79** |
Data are expressed as Mean ± SEM, n = 7–8 per group.
p < 0.05 and < 0.01 vs controls;
p < 0.01 vs untreated diabetic group
Fig. 5.
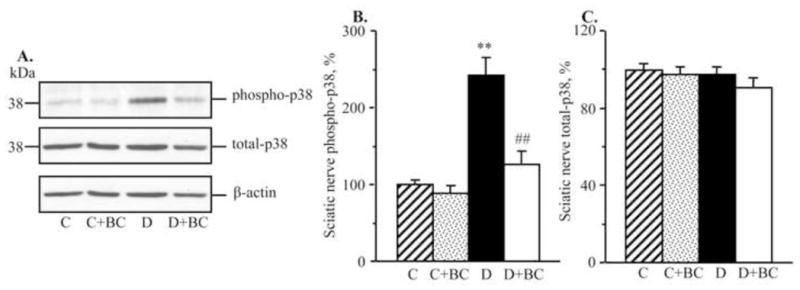
Representative Western blot analyses of total and phosphorylated mitogen-activated protein kinase p38 (A,C) and total and phosphorylated mitogen-activated protein kinase p38 protein contents (densitometry, %, B,D) in the sciatic nerve and spinal cord of control and diabetic mice maintained with or without baicalein treatments. C – control, D – diabetic. MAPK - mitogen-activated protein kinase. Mean ± SEM, n = 8 per group. ** p < 0.01 vs non-diabetic control group. ## p < 0.01 vs untreated diabetic group.
DISCUSSION AND CONCLUSIONS
The findings reported herein suggest that the flavonoid baicalein alleviates MNCV and SNCV deficits, thermal hypoalgesia, and tactile allodynia characteristic for DPN, without slowing down diabetes-associated loss of intraepidermal nerve fibers and promoting their regeneration. The study has a number of important implications for understanding interactions among individual pathogenetic mechanisms of PDN.
First, baicalein efficacy against large and small nerve fiber dysfunction is likely related to inhibition of three mechanisms previously implicated in DPN i.e., oxidative-nitrosative stress (Cameron et al., 2001; Coppey et al., 2001; Nagamatsu et al., 1995; Obrosova et al., 2005), 12/15-lipoxygenase activation (Obrosova et al., 2010; Stavniichuk et al., 2010), and p38 MAPK activation (Du et al., 2010; Purves et al., 2001). These mechanisms are interrelated creating “vicious cycles”. Diabetes-induced oxidative-nitrosative stress promotes p38 MAPK activation (Purves et al., 2001), whereas p38 MAPK activation contributes to oxidative injury via GSH depletion and changes in the glutathione redox state (Price et al., 2006). 12/15-LO overexpression and activation contribute to both oxidative-nitrosative stress and p38 MAPK activation (Kang et al., 2003; Reddy et al., 2002; Stavniichuk et al., 2010) whereas the latter two phenomena lead to an increase in 12/15-LO expression and activity via NF-κB activation and cytosolic Ca++ (Conrad and Lu 2000; Natarajan et al 1996). Thus, our findings identify baicalein as a multi-target drug acting on several mechanisms implicated in diabetes-induced nerve conduction slowing and small sensory nerve fiber dysfunction.
Interestingly and surprisingly, despite its multi-target action, baicalein completely failed to induce intraepidermal nerve fiber regeneration. It tended to slow down, but did not completely arrest, a progressive reduction in intraepidermal nerve fiber density with the prolongation of diabetes. This suggests that neither oxidative-nitrosative stress, nor 12/15-lipoxygenase or p38 MAPK activations, play a major role in intraepidermal nerve fiber loss, a phenomenon indicative of small sensory nerve fiber degeneration, described in human subjects with diabetes mellitus and impaired glucose tolerance (Quattrini et al., 2007; Sumner et al., 2003) and animal models of both Type 1 and Type 2 diabetes (Brussee et al., 2008; Christianson et al., 2007; Drel et al., 2006a; Francis et al., 2009; Kellogg et al., 2007; Obrosova et al., 2007a; Obrosova et al., 2008; Obrosova et al., 2010; Stavniichuk et al., 2010; Toth et al., 2008). Note, that intraepidermal nerve fiber regeneration in STZ-diabetic rats was observed with low-dose insulin and insulin-like growth factor-1 administered intrathecally (Toth et al, 2006). This approach is unlikely to be employed in clinical practice.
The present data also indicate that a reasonable amelioration of small sensory nerve fiber dysfunction associated with DPN can be achieved in the absence of small sensory nerve fiber regeneration. This conclusion is in agreement with several other studies (Brussee et al., 2008; Drel et al., 2007b; Obrosova et al., 2010; Stavniichuk et al., 2010) showing that peripheral nerve function and intraepidermal nerve loss do not necessarily correlate in diabetic rodents. It is possible that there is “an excess” of intraepidermal nerve fibers in diabetic rodents, and their loss to a certain threshold is not necessarily accompanied by worsening of peripheral nerve function. Thus, according to the results of experimental studies, the lack of effect on intraepidermal nerve fiber density in diabetic animal models should not necessarily lead to discontinuation of further development of a drug candidate. This conclusion should, however, be verified in human subjects with diabetic neuropathy. Intraepidermal nerve fiber density is a relatively new test, which still needs to be validated in clinical trials of new therapeutics. Also note, that development of thermal hypoalgesia is a complex process which occurs with participation of dorsal root ganglion neurons, and, potentially, spinal cord, in addition to small sensory nerve fibers per se. As we have shown in this study, baicalein corrects several diabetes-induced biochemical changes in the spinal cord. It is also likely to act on DRG neurons, although it has not been studied.
Numerous findings in diabetic animal models (Brussee et al., 2008; Drel et al., 2007b; Obrosova et al., 2002; Yagihashi et al., 2001) and several clinical trials (Greene et al., 1999; Hotta et al., 2001) suggest that increased AR activity manifest by sorbitol and fructose accumulation in peripheral nerve and spinal cord is a key mechanism in DPN. AR has been implicated in other biochemical changes in tissue-sites for diabetic complications including formation of advanced glycation end-products, oxidative-nitrosative stress, activation of poly(ADP-ribose)polymerase, cyclooxygenase-2, p38 MAPK, NF-κB and activator protein-1, and accumulation of cytosolic Ca++[reviewed in (Obrosova, 2009)]. Because diabetes-induced 12/15-LO overexpression is driven by the interleukin-4/STAT-6 pathway and requires NF-κB and activator protein-1 for interleukin-4 promoter activity (Conrad and Lu, 2000), and cytosolic Ca++ is required for 12/15-LO catalytic activity (Natarajan et al., 1996), it is reasonable to suggest that sorbitol pathway activation also plays an important role in 12/15-LO overexpression and activation in DPN. The present study, however, demonstrates that inhibition of diabetes-induced oxidative-nitrosative stress and 12/15-LO and p38 MAPK activation in tissue-sites for DPN can be achieved by a pharmacological intervention that displayed a weak sorbitol pathway inhibiting activity in the sciatic nerve, and did not suppress this pathway at all in the spinal cord.
It has also been hypothesized (Brrownlee, 2001) that oxidative stress precedes and underlies sorbitol pathway activation in tissue-sites for diabetic complications. This premise is not supported by a number of findings demonstrating that whereas diabetes-induced increase in AR activity contributes to oxidative stress in peripheral nerve (Ho et al., 2006; Yagihashi et al., 2001), retina (Gerhardinger 2009; Obrosova et al., 2003), kidney (Drel et al., 2006b), and endothelial cells (El-Remessy et al., 2003), antioxidants do not suppress sorbitol pathway intermediate accumulation (Sagara et al., 1996; Stevens et al., 1993; Stevens et al., 1996). In the present study, baicalein significantly reduced (sciatic nerve) or completely blunted (spinal cord) oxidative-nitrosative stress, whereas the effect on sorbitol pathway activity was either minor or totally absent.
In conclusion, the present findings identify baicalein as a multi-target drug alleviating multiple manifestations of DPN. They provide the rationale for screening and preclinical studies of hydroxyflavones, especially those with improved pharmacological profile (oral availability, low toxicity, acceptable pharmaceutical properties), as potential therapeutics for diabetic neuropathy and, potentially, other diabetic complications.
RESEARCH HIGHLIGHTS.
Baicalein alleviates nerve conduction deficit and sensory neuropathy in diabetes
Baicalein had no effect on diabetes-induced intraepidermal nerve fiber loss
Baicalein inhibits oxidative stress and p38 MAPK activation in diabetic neuropathy
Baicalein had no effect on diabetes-induced sorbitol pathway metabolite accumulation
Hydroxyflavone potential in diabetic neuropathy treatment deserves further studies
Acknowledgments
The study was supported by the National Institutes of Health Grants RO1DK074517, RO1DK077141, and RO1 DK074517 and the American Diabetes Association Grant 7-08-RA-102 (all to I.G.O). The Cell Biology and Bioimaging Core utilized in this work is supported in part by COBRE (NIH P20 RR021945) and CNRU (NIH 1P30-DK072476) center grants from the National Institutes of Health. The authors thank Drs. Douglas E. Wright from the University of Kansas Medical Center, Kansas City, KS, USA, and Gary L. Pittenger from Eastern Virginia Medical School, Norfolk, VA, USA, for valuable recommendations regarding intraepidermal nerve fiber density measurements.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Androutsopoulos VP, Ruparelia K, Arroo RR, Tsatsakis AM, Spandidos DA. CYP1-mediated antiproliferative activity of dietary flavonoids in MDA-MB-468 breast cancer cells. Toxicology. 2010;264:162–170. doi: 10.1016/j.tox.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Haslbeck KM, Humpert PM, Liliensiek B, Dehmer T, Morcos M, Sayed AA, Andrassy M, Schiekofer S, Schneider JG, Schulz JB, Heuss D, Neundörfer B, Dierl S, Huber J, Tritschler H, Schmidt AM, Schwaninger M, Haering HU, Schleicher E, Kasper M, Stern DM, Arnold B, Nawroth PP. Loss of pain perception in diabetes is dependent on a receptor of the immunoglobulin superfamily. J Clin Invest. 2004;114:1741–1751. doi: 10.1172/JCI18058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bril V, Hirose T, Tomioka S, Buchanan R. Ranirestat Study Group. Ranirestat for the management of diabetic sensorimotor polyneuropathy. Diabetes Care. 2009;32:1256–1260. doi: 10.2337/dc08-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- Brussee V, Guo G, Dong Y, Cheng C, Martinez JA, Smith D, Glazner GW, Fernyhough P, Zochodne DW. Distal degenerative sensory neuropathy in a long-term type 2 diabetes rat model. Diabetes. 2008;57:1664–1673. doi: 10.2337/db07-1737. [DOI] [PubMed] [Google Scholar]
- Cameron NE, Cotter MA. Pro-inflammatory mechanisms in diabetic neuropathy: focus on the nuclear factor kappa B pathway. Curr Drug Targets. 2008;9:60–67. doi: 10.2174/138945008783431718. [DOI] [PubMed] [Google Scholar]
- Cameron NE, Cotter MA, Jack AM, Basso MD, Hohman TC. Protein kinase C effects on nerve function, perfusion, Na(+), K(+)-ATPase activity and glutathione content in diabetic rats. Diabetologia. 1999;42:1120–1130. doi: 10.1007/s001250051280. [DOI] [PubMed] [Google Scholar]
- Cameron NE, Gibson TM, Nangle MR, Cotter MA. Inhibitors of advanced glycation end product formation and neurovascular dysfunction in experimental diabetes. Ann N Y Acad Sci. 2005;1043:784–792. doi: 10.1196/annals.1333.091. [DOI] [PubMed] [Google Scholar]
- Cameron NE, Tuck Z, McCabe L, Cotter MA. Effect of the hydroxyl radical scavenger, dimethylthiourea, on peripheral nerve tissue perfusion, conduction velocity and nociception in experimental diabetes. Diabetologia. 2001;44:1161–1169. doi: 10.1007/s001250100626. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Krisky D, Wolfe D, Glorioso JC, Mata M, Fink DJ. HSV-mediated gene transfer of vascular endothelial growth factor to dorsal root ganglia prevents diabetic neuropathy. Gene Ther. 2005;12:1377–1384. doi: 10.1038/sj.gt.3302533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Yang ZD, Smith KM, Carter JD, Nadler JL. Activation of 12-lipoxygenase in proinflammatory cytokine-mediated beta cell toxicity. Diabetologia. 2005;48:486–495. doi: 10.1007/s00125-005-1673-y. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Ryals JM, Johnson MS, Dobrowsky RT, Wright DE. Neurotrophic modulation of myelinated cutaneous innervation and mechanical sensory loss in diabetic mice. Neuroscience. 2007;145:303–313. doi: 10.1016/j.neuroscience.2006.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad DJ, Lu M. Regulation of human 12/15-lipoxygenase by Stat6-dependent transcription. Am J Respir Cell Mol Biol. 2000;22:226–234. doi: 10.1165/ajrcmb.22.2.3786. [DOI] [PubMed] [Google Scholar]
- Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Salvemini D, Yorek MA. Effect of M40403 treatment of diabetic rats on endoneurial blood flow, motor nerve conduction velocity and vascular function of epineurial arterioles of the sciatic nerve. Br J Pharmacol. 2001;134:21–29. doi: 10.1038/sj.bjp.0704216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppey LJ, Gellett JS, Davidson EP, Dunlap JA, Lund DD, Yorek MA. Effect of antioxidant treatment of streptozotocin-induced diabetic rats on endoneurial blood flow, motor nerve conduction velocity, and vascular reactivity of epineurial arterioles of the sciatic nerve. Diabetes. 2001;50:1927–1937. doi: 10.2337/diabetes.50.8.1927. [DOI] [PubMed] [Google Scholar]
- Coppey L, Davidson E, Lu B, Gerard C, Yorek M. Vasopeptidase inhibitor ilepatril (AVE7688) prevents obesity- and diabetes-induced neuropathy in C57Bl/6J mice. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.09.008. [Epub in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter MA, Mirrlees DJ, Cameron NE. Neurovascular interactions between aldose reductase and angiotensin-converting enzyme inhibition in diabetic rats. Eur J Pharmacol. 2001;417:223–230. doi: 10.1016/s0014-2999(01)00909-8. [DOI] [PubMed] [Google Scholar]
- Cui L, Zhang X, Yang R, Liu L, Wang L, Li M, Du W. Baicalein is neuroprotective in rat MCAO model: Role of 12/15-lipoxygenase, mitogen-activated protein kinase and cytosolic phospholipase A2. Pharmacol Biochem Behav. 2010;96:469–475. doi: 10.1016/j.pbb.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab. 2009;94:2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drel VR, Lupachyk S, Shevalye H, Vareniuk I, Xu W, Zhang J, Delamere NA, Shahidullah M, Slusher B, Obrosova IG. New therapeutic and biomarker discovery for peripheral diabetic neuropathy: PARP inhibitor, nitrotyrosine, and tumor necrosis factor-{alpha} Endocrinology. 2010;151:2547–2555. doi: 10.1210/en.2009-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, Lyzogubov VV, Obrosova IG. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335–3343. doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- Drel VR, Pacher P, Stevens MJ, Obrosova IG. Aldose reductase inhibition counteracts nitrosative stress and poly(ADP-ribose) polymerase activation in diabetic rat kidney and high-glucose-exposed human mesangial cells. Free Radic Biol Med. 2006;40:1454–1465. doi: 10.1016/j.freeradbiomed.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drel VR, Pacher P, Vareniuk I, Pavlov IA, Ilnytska O, Lyzogubov VV, Bell SR, Groves JT, Obrosova IG. Evaluation of the peroxynitrite decomposition catalyst Fe(III) tetra-mesitylporphyrin octasulfonate on peripheral neuropathy in a mouse model of type 1 diabetes. Int J Mol Med. 2007;20:783–792. [PMC free article] [PubMed] [Google Scholar]
- Drel VR, Pacher P, Vareniuk I, Pavlov I, Ilnytska O, Lyzogubov VV, Tibrewala J, Groves JT, Obrosova IG. A peroxynitrite decomposition catalyst counteracts sensory neuropathy in streptozotocin-diabetic mice. Eur J Pharmacol. 2007;569:48–58. doi: 10.1016/j.ejphar.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Tang J, Li G, Berti-Mattera L, Lee CA, Bartkowski D, Gale D, Monahan J, Niesman MR, Alton G, Kern TS. Effects of p38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Invest Ophthalmol Vis Sci. 2010;51:2158–2164. doi: 10.1167/iovs.09-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Remessy AB, Abou-Mohamed G, Caldwell RW, Caldwell RB. High glucose-induced tyrosine nitration in endothelial cells: role of eNOS uncoupling and aldose reductase activation. Invest Ophthalmol Vis Sci. 2003;44:3135–3143. doi: 10.1167/iovs.02-1022. [DOI] [PubMed] [Google Scholar]
- Francis G, Martinez J, Liu W, Nguyen T, Ayer A, Fine J, Zochodne D, Hanson LR, Frey WH, 2nd, Toth C. Intranasal insulin ameliorates experimental diabetic neuropathy. Diabetes. 2009;58:934–945. doi: 10.2337/db08-1287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gerhardinger C, Dagher Z, Sebastiani P, Park YS, Lorenzi M. The transforming growth factor-beta pathway is a common target of drugs that prevent experimental diabetic retinopathy. Diabetes. 2009;58:1659–1667. doi: 10.2337/db08-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohil K, Packer L. Bioflavonoid-rich botanical extracts show antioxidant and gene regulatory activity. Ann N Y Acad Sci. 2002;957:70–77. doi: 10.1111/j.1749-6632.2002.tb02906.x. [DOI] [PubMed] [Google Scholar]
- Goss JR, Goins WF, Lacomis D, Mata M, Glorioso JC, Fink DJ. Herpes simplex-mediated gene transfer of nerve growth factor protects against peripheral neuropathy in streptozotocin-induced diabetes in the mouse. Diabetes. 2002;51:2227–2232. doi: 10.2337/diabetes.51.7.2227. [DOI] [PubMed] [Google Scholar]
- Greene DA, Arezzo JC, Brown MB. Effect of aldose reductase inhibition on nerve conduction and morphometry in diabetic neuropathy. Zenarestat Study Group. Neurology. 1999;53:580–591. doi: 10.1212/wnl.53.3.580. [DOI] [PubMed] [Google Scholar]
- Ho EC, Lam KS, Chen YS, Yip JC, Arvindakshan M, Yamagishi S, Yagihashi S, Oates PJ, Ellery CA, Chung SS, Chung SK. Aldose reductase-deficient mice are protected from delayed motor nerve conduction velocity, increased c-Jun NH2-terminal kinase activation, depletion of reduced glutathione, increased superoxide accumulation, and DNA damage. Diabetes. 2006;55:1946–1953. doi: 10.2337/db05-1497. [DOI] [PubMed] [Google Scholar]
- Hong DP, Fink AL, Uversky VN. Structural characteristics of alpha-synuclein oligomers stabilized by the flavonoid baicalein. J Mol Biol. 2008;383:214–223. doi: 10.1016/j.jmb.2008.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta N, Toyota T, Matsuoka K, Shigeta Y, Kikkawa R, Kaneko T, Takahashi A, Sugimura K, Koike Y, Ishii J, Sakamoto N SNK-860 Diabetic Neuropathy Study Group. Clinical efficacy of fidarestat, a novel aldose reductase inhibitor, for diabetic peripheral neuropathy: a 52-week multicenter placebo-controlled double-blind parallel group study. Diabetes Care. 2001;24:1776–1782. doi: 10.2337/diacare.24.10.1776. [DOI] [PubMed] [Google Scholar]
- Huang Y, Tsang SY, Yao X, Chen ZY. Biological properties of baicalein in cardiovascular system. Curr Drug Targets Cardiovasc Haematol Disord. 2005;5:177–184. doi: 10.2174/1568006043586206. [DOI] [PubMed] [Google Scholar]
- Kang SW, Natarajan R, Shahed A, Nast CC, LaPage J, Mundel P, Kashtan C, Adler SG. Role of 12-lipoxygenase in the stimulation of p38 mitogen-activated protein kinase and collagen alpha5(IV) in experimental diabetic nephropathy and in glucose-stimulated podocytes. J Am Soc Nephrol. 2003;14:3178–3187. doi: 10.1097/01.asn.0000099702.16315.de. [DOI] [PubMed] [Google Scholar]
- Kellogg AP, Wiggin TD, Larkin DD, Hayes JM, Stevens MJ, Pop-Busui R. Protective effects of cyclooxygenase-2 gene inactivation against peripheral nerve dysfunction and intraepidermal nerve fiber loss in experimental diabetes. Diabetes. 2007;56:2997–3005. doi: 10.2337/db07-0740. [DOI] [PubMed] [Google Scholar]
- Kumar A, Sharma SS. NF-kappaB inhibitory action of resveratrol: a probable mechanism of neuroprotection in experimental diabetic neuropathy. Biochem Biophys Res Commun. 2010;394:360–365. doi: 10.1016/j.bbrc.2010.03.014. [DOI] [PubMed] [Google Scholar]
- Li F, Drel VR, Szabó C, Stevens MJ, Obrosova IG. Low-dose poly(ADP-ribose) polymerase inhibitor-containing combination therapies reverse early peripheral diabetic neuropathy. Diabetes. 2005;54:1514–1522. doi: 10.2337/diabetes.54.5.1514. [DOI] [PubMed] [Google Scholar]
- McGuire JF, Rouen S, Siegfreid E, Wright DE, Dobrowsky RT. Caveolin-1 and altered neuregulin signaling contribute to the pathophysiological progression of diabetic peripheral neuropathy. Diabetes. 2009;58:2677–2686. doi: 10.2337/db09-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu M, Nickander KK, Schmelzer JD, Raya A, Wittrock DA, Tritschler H, Low PA. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care. 1995;18:1160–1167. doi: 10.2337/diacare.18.8.1160. [DOI] [PubMed] [Google Scholar]
- Nakae M, Kamiya H, Naruse K, Horio N, Ito Y, Mizubayashi R, Hamada Y, Nakashima E, Akiyama N, Kobayashi Y, Watarai A, Kimura N, Horiguchi M, Tabata Y, Oiso Y, Nakamura J. Effects of basic fibroblast growth factor on experimental diabetic neuropathy in rats. Diabetes. 2006;55:1470–1477. doi: 10.2337/db05-1160. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Kato K, Hamada Y, Nakayama M, Chaya S, Nakashima E, Naruse K, Kasuya Y, Mizubayashi R, Miwa K, Yasuda Y, Kamiya H, Ienaga K, Sakakibara F, Koh N, Hotta N. A protein kinase C-beta-selective inhibitor ameliorates neural dysfunction in streptozotocin-induced diabetic rats. Diabetes. 1999;48:2090–2095. doi: 10.2337/diabetes.48.10.2090. [DOI] [PubMed] [Google Scholar]
- Natarajan R, Bai W, Rangarajan V, Gonzales N, Gu JL, Lanting L, Nadler JL. Platelet-derived growth factor BB mediated regulation of 12-lipoxygenase in porcine aortic smooth muscle cells. J Cell Physiol. 1996;169:391–400. doi: 10.1002/(SICI)1097-4652(199611)169:2<391::AID-JCP19>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Obrosova IG. Diabetes and the peripheral nerve. Biochim Biophys Acta. 2009;1792:931–940. doi: 10.1016/j.bbadis.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Drel VR, Oltman CL, Mashtalir N, Tibrewala J, Groves JT, Yorek MA. Role of nitrosative stress in early neuropathy and vascular dysfunction in streptozotocin-diabetic rats. Am J Physiol Endocrinol Metab. 2007;293:E1645–E1655. doi: 10.1152/ajpendo.00479.2007. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Fathallah L, Lang HJ, Greene DA. Evaluation of a sorbitol dehydrogenase inhibitor on diabetic peripheral nerve metabolism: a prevention study. Diabetologia. 1999;42:1187–1194. doi: 10.1007/s001250051290. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, Drel VR. High-fat diet induced neuropathy of pre-diabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007;56:2598–2608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Li F, Abatan OI, Forsell MA, Komjáti K, Pacher P, Szabó C, Stevens MJ. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Mabley JG, Zsengellér Z, Charniauskaya T, Abatan OI, Groves JT, Szabó C. Role for nitrosative stress in diabetic neuropathy: evidence from studies with a peroxynitrite decomposition catalyst. FASEB J. 2005;19:401–403. doi: 10.1096/fj.04-1913fje. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Minchenko AG, Vasupuram R, White L, Abatan OI, Kumagai AK, Frank RN, Stevens MJ. Aldose reductase inhibitor fidarestat prevents retinal oxidative stress and vascular endothelial growth factor overexpression in streptozotocin-diabetic rats. Diabetes. 2003;52:864–871. doi: 10.2337/diabetes.52.3.864. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Stavniichuk R, Drel VR, Shevalye H, Vareniuk I, Nadler JL, Schmidt RE. Different roles of 12/15-lipoxygenase in diabetic large and small fiber peripheral and autonomic neuropathies. Am J Pathol. 2010;177:1436–1447. doi: 10.2353/ajpath.2010.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrosova IG, Van Huysen C, Fathallah L, Cao XC, Greene DA, Stevens MJ. An aldose reductase inhibitor reverses early diabetes-induced changes in peripheral nerve function, metabolism, and antioxidative defense. FASEB J. 2002;16:123–125. doi: 10.1096/fj.01-0603fje. [DOI] [PubMed] [Google Scholar]
- Obrosova IG, Xu W, Lyzogubov VV, Ilnytska O, Mashtalir N, Vareniuk I, Pavlov IA, Zhang J, Slusher B, Drel VR. PARP inhibition or gene deficiency counteracts intraepidermal nerve fiber loss and neuropathic pain in advanced diabetic neuropathy. Free Radic Biol Med. 2008;44:972–981. doi: 10.1016/j.freeradbiomed.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallast S, Arai K, Pekcec A, Yigitkanli K, Yu Z, Wang X, Lo EH, van Leyen K. Increased nuclear apoptosis-inducing factor after transient focal ischemia: a 12/15-lipoxygenase-dependent organelle damage pathway. J Cereb Blood Flow Metab. 2010;30:1157–1167. doi: 10.1038/jcbfm.2009.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price SA, Gardiner NJ, Duran-Jimenez B, Zeef LA, Obrosova IG, Tomlinson DR. Thioredoxin interacting protein is increased in sensory neurons in experimental diabetes. Brain Res. 2006;1116:206–214. doi: 10.1016/j.brainres.2006.07.109. [DOI] [PubMed] [Google Scholar]
- Purves T, Middlemas A, Agthong S, Jude EB, Boulton AJ, Fernyhough P, Tomlinson DR. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001;15:2508–2514. doi: 10.1096/fj.01-0253hyp. [DOI] [PubMed] [Google Scholar]
- Quattrini C, Tavakoli M, Jeziorska M, Kallinikos P, Tesfaye S, Finnigan J, Marshall A, Boulton AJ, Efron N, Malik RA. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56:2148–2154. doi: 10.2337/db07-0285. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Thimmalapura PR, Lanting L, Nadler JL, Fatima S, Natarajan R. The oxidized lipid and lipoxygenase product 12(S)-hydroxyeicosatetraenoic acid induces hypertrophy and fibronectin transcription in vascular smooth muscle cells via p38 MAPK and cAMP response element-binding protein activation. Mediation of angiotensin II effects. J Biol Chem. 2002;277:9920–9928. doi: 10.1074/jbc.M111305200. [DOI] [PubMed] [Google Scholar]
- Sagara M, Satoh J, Wada R, Yagihashi S, Takahashi K, Fukuzawa M, Muto G, Muto Y, Toyota T. Inhibition of development of peripheral neuropathy in streptozotocin-induced diabetic rats with N-acetylcysteine. Diabetologia. 1996;39:263–269. doi: 10.1007/BF00418340. [DOI] [PubMed] [Google Scholar]
- Schroeter H, Boyd C, Spencer JP, Williams RJ, Cadenas E, Rice-Evans C. MAPK signaling in neurodegeneration: influences of flavonoids and of nitric oxide. Neurobiol Aging. 2002;23:861–880. doi: 10.1016/s0197-4580(02)00075-1. [DOI] [PubMed] [Google Scholar]
- Schmeichel AM, Schmelzer JD, Low PA. Oxidative injury and apoptosis of dorsal root ganglion neurons in chronic experimental diabetic neuropathy. Diabetes. 2003;52:165–171. doi: 10.2337/diabetes.52.1.165. [DOI] [PubMed] [Google Scholar]
- Sies H. Polyphenols and health: update and perspectives. Arch Biochem Biophys. 2010;501:2–5. doi: 10.1016/j.abb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Sima AA, Kamiya H. Is C-peptide replacement the missing link for successful treatment of neurological complications in type 1 diabetes? Curr Drug Targets. 2008;9:37–46. doi: 10.2174/138945008783431745. [DOI] [PubMed] [Google Scholar]
- Stavniichuk R, Drel VR, Shevalye H, Vareniuk I, Stevens MJ, Nadler JL, Obrosova IG. Role of 12/15-lipoxygenase in nitrosative stress and peripheral prediabetic and diabetic neuropathies. Free Radic Biol Med. 2010;49:1036–1045. doi: 10.1016/j.freeradbiomed.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MJ, Lattimer SA, Kamijo M, Van Huysen C, Sima AA, Greene DA. Osmotically-induced nerve taurine depletion and the compatible osmolyte hypothesis in experimental diabetic neuropathy in the rat. Diabetologia. 1993;36:608–614. doi: 10.1007/BF00404069. [DOI] [PubMed] [Google Scholar]
- Stevens MJ, Obrosova I, Cao X, Van Huysen C, Greene DA. Effects of DL-alpha-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism, and oxidative stress in experimental diabetic neuropathy. Diabetes. 2000;49:1006–1015. doi: 10.2337/diabetes.49.6.1006. [DOI] [PubMed] [Google Scholar]
- Stevens MJ, Zhang W, Li F, Sima AA. C-peptide corrects endoneurial blood flow but not oxidative stress in type 1 BB/Wor rats. Am J Physiol Endocrinol Metab. 2004;287:E497–E505. doi: 10.1152/ajpendo.00048.2004. [DOI] [PubMed] [Google Scholar]
- Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–111. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- Sun H, Che QM, Zhao X, Pu XP. Antifibrotic effects of chronic baicalein administration in a CCl4 liver fibrosis model in rats. Eur J Pharmacol. 2010;631:53–60. doi: 10.1016/j.ejphar.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Szabó C, Zanchi A, Komjáti K, Pacher P, Krolewski AS, Quist WC, LoGerfo FW, Horton ES, Veves A. Poly(ADP-Ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation. 2002;106:2680–2686. doi: 10.1161/01.cir.0000038365.78031.9c. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P. Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth C, Brussee V, Zochodne DW. Remote neurotrophic support of epidermal nerve fibres in experimental diabetes. Diabetologia. 2006;49:1081–1088. doi: 10.1007/s00125-006-0169-8. [DOI] [PubMed] [Google Scholar]
- Toth C, Rong LL, Yang C, Martinez J, Song F, Ramji N, Brussee V, Liu W, Durand J, Nguyen MD, Schmidt AM, Zochodne DW. Receptor for advanced glycation end products (RAGEs) and experimental diabetic neuropathy. Diabetes. 2008;57:1002–1017. doi: 10.2337/db07-0339. [DOI] [PubMed] [Google Scholar]
- Urban MJ, Li C, Yu C, Lu Y, Krise JM, McIntosh MP, Rajewski RA, Blagg BS, Dobrowsky RT. Inhibiting heat-shock protein 90 reverses sensory hypoalgesia in diabetic mice. ASN Neuro. 2010;11 doi: 10.1042/AN20100015. pii: e00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada R, Nishizawa Y, Yagihashi N, Takeuchi M, Ishikawa Y, Yasumura K, Nakano M, Yagihashi S. Effects of OPB-9195, anti-glycation agent, on experimental diabetic neuropathy. Eur J Clin Invest. 2001;31:513–520. doi: 10.1046/j.1365-2362.2001.00826.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Schmeichel AM, Iida H, Schmelzer JD, Low PA. Enhanced inflammatory response via activation of NF-kappaB in acute experimental diabetic neuropathy subjected to ischemia-reperfusion injury. J Neurol Sci. 2006;247:47–52. doi: 10.1016/j.jns.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kawamura N, Schmelzer JD, Schmeichel AM, Low PA. Decreased peripheral nerve damage after ischemia-reperfusion injury in mice lacking TNF-alpha. J Neurol Sci. 2008;267:107–111. doi: 10.1016/j.jns.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Mata M, Fink DJ. Prevention of Diabetic Neuropathy by Regulatable Expression of HSV-Mediated Erythropoietin. Mol Ther. 2011;19:310–317. doi: 10.1038/mt.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagihashi S, Yamagishi SI, Wada RiR, Baba M, Hohman TC, Yabe-Nishimura C, Kokai Y. Neuropathy in diabetic mice overexpressing human aldose reductase and effects of aldose reductase inhibitor. Brain. 2001;124:2448–2458. doi: 10.1093/brain/124.12.2448. [DOI] [PubMed] [Google Scholar]
- Yang LP, Sun HL, Wu LM, Guo XJ, Dou HL, Tso MO, Zhao L, Li SM. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:2319–2327. doi: 10.1167/iovs.08-2642. [DOI] [PubMed] [Google Scholar]
- Yoo S, Han S, Park YS, Lee JH, Oh U, Hwang SW. Lipoxygenase inhibitors suppressed carrageenan-induced Fos-expression and inflammatory pain responses in the rat. Mol Cells. 2009;27:417–422. doi: 10.1007/s10059-009-0059-2. [DOI] [PubMed] [Google Scholar]
- Yun MY, Yang JH, Kim DK, Cheong KJ, Song HH, Kim DH, Cheong KJ, Kim YI, Shin SC. Therapeutic effects of Baicalein on atopic dermatitis-like skin lesions of NC/Nga mice induced by dermatophagoides pteronyssinus. Int Immunopharmacol. 2010;10:1142–1148. doi: 10.1016/j.intimp.2010.06.020. [DOI] [PubMed] [Google Scholar]
- Zhou YP, Zhang JQ. Oral baicalin and liquid extract of licorice reduce sorbitol levels in red blood cell of diabetic rats. Chin Med J (Engl) 1989;102:203–206. [PubMed] [Google Scholar]


