Abstract
A versatile strategy to prepare fac-[MI(CO)3]+ and cis-[MI(CO)2]+ (M = Re, 99mTc) complexes was developed using Huisgen click chemistry and monodentate phosphine ligands to readily incorporate biomolecules and tailor the chemical properties.
In diagnostic nuclear medicine, 99mTc remains the most utilized radionuclide due its ideal nuclear properties (t1/2 = 6.0 h, γ = 140 keV (89%)) for single photon emission computed tomography (SPECT), kit chemistry, and the portability of the 99Mo-99mTc generator system.1 A water soluble organometallic complex, Alberto’s reagent fac-[99mTcI(OH2)3(CO)3]+, has proven to be a versatile synthon due to its facile preparation via an Isolink® kit and labile aquo ligands to accommodate a variety of ligand types and denticity.2 Multiple strategies have emerged from tridentate ligands to a combination of mono- and bidentate ligands to saturate the coordination sphere of fac-[99mTcI(CO)3]+.3 2+1 complexes have the flexibility to tailor the chemical nature and in vivo properties by adjusting either the mono- or bidentate ligands.4 This methodology can also be extrapolated to multi-valent or orthogonal targeting molecules using a combinatorial approach, compared to a single targeting molecule.5
In radiopharmaceuticals, the CuI catalyzed azide alkyne cycloaddition (CuAAC) reaction has emerged as an important technique to improve the design and preparation for coupling a chelate or radionuclide to a targeting molecule.6 CuAAC strategies are applied in fac-[MI(CO)3]+ (M= 99mTc, Re) chemistry for ligand design and coupling of radioactive complexes. Pioneered by Schibli and Mindt, “click to chelate” provides a versatile strategy using CuAAC to rapidly assemble chelates on azide- or alkyne-functionalized molecules, while incorporating the triazole donor into the newly formed chelate for subsequent metal complexation.7 “Click to chelate” uses an exchangeable strategy to generate unique chelates for tuning the chemical properties of functionalized targeting molecules.8
In the present report, the versatility of the multi-ligand and “click to chelate” approaches were combined to provide tunable complexes for the fac-[MI(CO)3]+ core. Two NO bidentate ligand systems were explored in conjunction with a monodentate phosphine ligand to demonstrate the feasibility of combining these approaches. A pyridine based NO bidentate ligand, picolinic acid (pic) was utilized as a model with the fac-[MI(CO)3]+ core followed by a “click to chelate” NO bidentate ligand prepared from benzylazide and propiolic acid.9 The CuAAC formed chelate was envisioned to have similar coordination mode and strength as pic, while readily allowing incorporation of azide-functionalized targeting molecules without synthetic modification. Phosphines (PR3) were selected for their coordination potency with low valent 99mTc/Re carbonyl complexes and chemical flexibility of the R substituents. PR3’s also provides an avenue to trans labialize a carbonyl for subsequent PR3 substitution to yield 2+1+1 cis-bicarbonyl-trans-phosphine complexes as previously observed with ReI.10
A stepwise strategy was used to probe the complexation formation of NO and PPh3 (model phosphine) ligands with the fac-[ReI(CO)3]+ core. The initial step involved the formation of the NO-pic precursor fac-[ReI(OH2)(CO)3(pic)], 1, from the addition of pic to fac-[ReI(OH2)3(CO)3](SO3CF3) in the presence of NaHCO3 (Scheme 1).3a One equivalent of PPh3 was added to 1 at 70 °C to form the 2+1 complex, fac-[ReI(CO)3(pic)(PPh3)], 2, in moderate yields (53%). The addition of a second equivalent of PPh3 and increasing the reaction temperature converted the 2+1 product, 2, into the 2+1+1cis-trans-[ReI(CO)2(pic)(PPh3)2], 3, in excellent yield (92%). Characterization of 2 and 3 correlated with the previously reported data using alternative conditions and starting materials.11 Additional analytical data for 2 and 3 and single crystal X-ray diffraction experimental parameters with ORTEP drawings are provided in the ESI.12 The structure of 2 exhibited a distorted octahedral geometry comparable to similar 2+1 fac-[ReI(CO)3(NO)(L)] complexes, but with slightly elongated trans (Re(1)-C(27) 1.947 Å) and (Re(1)-P(1) 2.4975 Å) bonds.13 3 also exhibited similar bonding of pic, but contained nearly equidistant trans Re-P bonds (Re-P 2.413, 2.418 Å) and near linear P(1)-Re(1)-P(2) bond angle (174.39°) correlating with other trans PR3 Re complexes.10b, 10c, 14
Scheme 1.
fac-[ReI(CO)3]+ complexes with picolinic acid. a) PPh3. EtOH, 70 °C, 18 h. b) PPh3, mesitylene, 169 °C, 4 h.
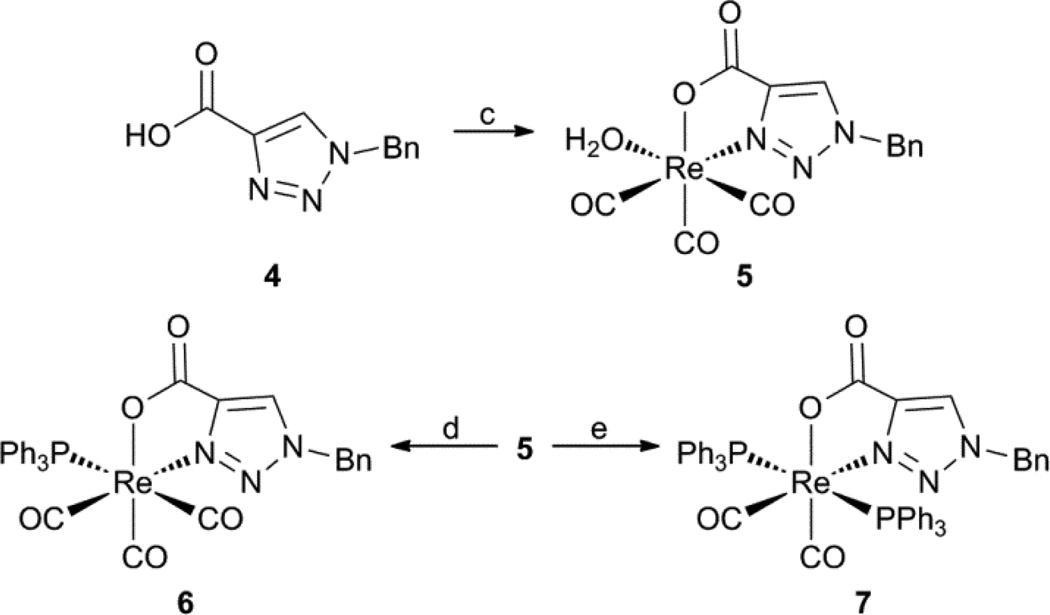
The CuAAC clicked NO bidentate ligand, 1-benzyl-1H-1,2,3-triazole-4-carboxylic acid, 4, was similarly explored in a stepwise approach with the fac-[ReI(CO)3]+ core. Complete synthesis, characterization, and single crystal X-ray experimental details for 5, 6, and 7 can be found in the ESI. Complexation of 4 with fac-[ReI(OH2)3(CO)3](SO3CF3) gave the NO bidentate complex, fac-[ReI(OH2)(CO)3(4)], 5, in moderate yield (49%) (Scheme 2). Interestingly, 1H NMR of 5 revealed two different conformers due to the orientation of the benzyl (Bn) group, either towards or away from the coordinated water. Slight shifts in the triazole proton (8.49, 8.55 ppm) and the CH2 group (5.76, 5.74 ppm) were observed in a 2:1 ratio, respectively. The more favorable Bn conformer is most likely oriented towards the coordinated water as indicated in the X-ray structure. The addition of PPh3 yielded the corresponding 2+1 product, fac-[ReI(CO)3(4)(PPh3)], 6, in excellent yield (91%). Upon PPh3 coordination, 1H NMR indicated the conversion to a single species in 6 with shifts of the triazole (7.61 ppm) and ABq splitting of CH2 (5.42 ppm).
Scheme 2.
fac-[ReI(CO)3]+ complexes with 4. c) fac-[ReI(OH2)3(CO)3](SO3CF3), pH 6, r.t., 18 h. d) PPh3, EtOH, 60 °C, 16 h. d) PPh3, CH2Cl2, mesitylene, 169 °C, 24 h.
Unlike the conversion of 2 to 3, excess PPh3 (5 equiv.) at high temperature for a prolonged period was required to convert 6 into the 2+1+1 complex, cis-trans-[ReI(CO)2(4)(PPh3)2], 7, in good yield (69%). Steric interactions of the Bn group of 4 with the entering PPh3 ligand may have impeded substitution requiring more aggressive conditions. 1H NMR of 7 showed shifts of the triazole singlet (6.64 ppm) and the CH2 group to a singlet (5.06 ppm). 31P NMR exhibited a downfield shift from the 2+1 product 6 (19.76 ppm) to the 2+1+1 product 7 (23.96 ppm). X-ray structures of 5, 6, and 7 displayed similar distorted octahedral geometries of coordinated 4 analogous to complexes 1–3 (Figure 1).12 Notably, 6 also exhibited a lengthening of the Re-P (2.5098 Å) and trans Re-C (1.951 Å) bonds. While the coordination of 4 remained constant throughout the series, Bn interactions within the complex and the entering ligand is clearly evident as Bn oriented towards the water in 5, towards the CO‘s in 6 away from PPh3, and restricted to the equatorial plane by the PPh3‘s in 7.
Figure 1.
Crystal structures of A) 5, B) 6, and C) 7 with thermal ellipsoids at 50% probability. Hydrogens have been omitted to improve clarity.
Radioactive 99mTcI complexes were prepared in a sequential manner analogous to ReI analogs and analyzed by comparative UV/radio-HPLC (Figure 2 (4), Figure S1 (pic)). Complexation of fac-[99mTcI(OH2)3(CO)3]+ with NO bidentate ligands, pic (1×10−3 M) or 4 (5×10−3 M), was achieved by heating at 90 °C or 50 °C for 1 h to give fac-[99mTcI(OH2)(CO)3(L)], L= pic (1a), 4 (5a), in quantitative yields (>98%). Addition of PPh3 (10−3 M) to 1a or 5a at 60 °C for 1 h afforded the 2+1 product fac-[99mTcI(CO)3(PPh3)(L)], L= pic (2a), 4 (6a),, in 93% and 81% yield, respectively. Increasing reaction temperature (>90 °C) for 1 h led to the quantitative formation of the 2+1+1 complex fac-[99mTcI(CO)2(PPh3)2(L)], L= pic (3a), 4 (7a). At intermediate temperatures (60–90 °C), peaks for both bi- and tricarbonyl complexes were observed in the chromatograms. While a mixture of bi- and tricarbonyl complexes is not ideal for radiopharmaceutical applications, temperature control can be utilized for selective complex formation to yield either the 2+1 tricarbonyl complex at low temperatures or the 2+1+1 bicarbonyl complex at high temperatures. Further optimization of reaction conditions ([PR3], reaction time, temperature) can also be used to mitigate mixed complexes in a single sample.
Figure 2.
Normalized and offset UV and radio- HPLC chromatograms of 6 (tR = 22.3 min), 6a (tR = 22.5 min), 7 (tR = 22.9 min) and 7a (tR = 23.2 min).
Log P analysis of the RP-HPLC purified 99mTc complexes (1a–3a, 5a–7a) indicated they were all moderately lipophilic (log P = 0.8–1.4) with slight increases in lipophilicity as each PPh3 ligand was incorporated in the complex (ESI Table S1). Transchelation stability studies were conducted with RP-HPLC purified 2a, 3a, 6a, and 7a in the presence of cysteine or histidine (1 mM) at 37 °C and pH 7.4 (ESI Table S2). At 4 h, all complexes were found to be >99% stable. At 18 h, 2a, 3a, and 7a were >99% stable under both conditions. However, 6a exhibited 95% stability with histidine and nearly complete loss (5% remaining) with cysteine suggesting dissociation or steric interactions may impact the overall stability of 2+1 complexes. Similar results were recently observed with bicarbonyl acetylacetone Re/99mTc complexes.10d
In conclusion, NO bidentate ligands (i.e., pic or CuAAC product, 4) can be used in conjunction with monodentate phosphine ligands to generate 2+1 fac-[MI(CO)3]+ and 2+1+1 cis-[MI(CO)2]+ complexes in macroscale (Re) and radiochemical (99mTc) concentrations. Temperature control was essential to selectively prepare each species, where higher temperatures formed the bicarbonyl complex exclusively. In general, the 99mTc bi- and tricarbonyl complexes displayed excellent in vitro stability towards transchelation. The bicarbonyl 2+1+1 complex with 4 appeared to have increased stability over the 2+1 complex suggesting phosphine ligands contribute to destabilization of the trans metal carbonyl bond. These results indicate the first successful combination of the versatile CuAAC and multi-ligand strategies to generate highly stable, multi-component and customizable complexes from the fac-[MI(CO)3]+ core for radiopharmaceutical applications.
Supplementary Material
Acknowledgments
This worked was funded in part by the DOE, Radiochemistry and Radiochemistry Instrumentation Program (#DE-FG02-08-ER64672) and NIH/NIGMS (Institutional Award T32-GM008336). Isolink® kits were graciously provided by Dr. Mary Dyszlewski at Covidien.
Footnotes
Electronic Supplementary Information (ESI) available: Full experimental details, characterization data and crystallographic tables. See DOI: 10.1039/c000000x/
Contributor Information
Charles L. Barnes, Email: barnesch@missouri.edu.
Paul D. Benny, Email: bennyp@wsu.edu.
Notes and references
- 1.Alberto R. In: Medicinal Organometallic Chemistry. Jaouen G, Metzler-Nolte N, editors. Vol. 32. Berlin Heidelberg: Springer; 2010. pp. 219–246. [Google Scholar]
- 2.a) Alberto R, Schibli R, Egli A, Schubiger AP, Abram U, Kaden TA. J. Am. Chem. Soc. 1998;120:7987–7988. doi: 10.1021/ic980112f. [DOI] [PubMed] [Google Scholar]; b) Alberto R, Schibli R, Waibel R, Abram U, Schubiger AP. Coord. Chem. Rev. 1999;190–192:901–919. [Google Scholar]; c) Morais M, Paulo A, Gano L, Santos I, Correia JDG. J. Organomet. Chem. 2013;744:125–139. [Google Scholar]
- 3.a) Schibli R, La Bella R, Alberto R, Garcia-Garayoa E, Ortner K, Abram U, Schubiger PA. Bioconjugate Chem. 2000;11:345–351. doi: 10.1021/bc990127h. [DOI] [PubMed] [Google Scholar]; b) Alberto R, Kyong Pak J, van Staveren D, Mundwiler S, Benny P. Peptide Science. 2004;76:324–333. doi: 10.1002/bip.20129. [DOI] [PubMed] [Google Scholar]
- 4.a) Alberto R. In: Comprehensive Coordination Chemistry II. McCleverty JA, Meyer TJ, editors. Oxford: Pergamon; 2003. pp. 127–270. [Google Scholar]; b) Tisato F, Porchia M, Bolzati C, Refosco F, Vittadini A. Coord. Chem. Rev. 2006;250:2034–2045. [Google Scholar]
- 5.Ganguly T, Kasten BB, Hayes TR, Benny PD. In: Advances in Chemistry Research. Taylor JC, editor. Vol. 18. New York: Nova Science Publishers, Inc.; 2012. pp. 93–141. [Google Scholar]
- 6.a) Mamat C, Ramenda T, Wuest FR. Mini-Rev. Org. Chem. 2009;6:21–34. [Google Scholar]; b) Moore AL, Bucar D-K, MacGillivray LR, Benny PD. Dalton Trans. 2010;39:1926–1928. doi: 10.1039/b921413e. [DOI] [PubMed] [Google Scholar]
- 7.Mindt TL, Struthers H, Brans L, Anguelov T, Schweinsberg C, Maes V, Tourwe D, Schibli R. J. Am. Chem. Soc. 2006;128:15096–15097. doi: 10.1021/ja066779f. [DOI] [PubMed] [Google Scholar]
- 8.a) Mindt TL, Müller C, Stuker F, Salazar J-F, Hohn A, Mueggler T, Rudin M, Schibli R. Bioconjugate Chem. 2009;20:1940–1949. doi: 10.1021/bc900276b. [DOI] [PubMed] [Google Scholar]; b) Kluba C, Mindt T. Molecules. 2013;18:3206–3226. doi: 10.3390/molecules18033206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maisonial A, Serafin P, Traïkia M, Debiton E, Théry V, Aitken DJ, Lemoine P, Viossat B, Gautier A. Eur. J. Inorg. Chem. 2008;2008:298–305. [Google Scholar]
- 10.a) Doney JJ, Bergman RG, Heathcock CH. J. Am. Chem. Soc. 1985;107:3724–3726. [Google Scholar]; b) Kirillov AM, Haukka M, Guedes da Silva MFC, Pombeiro AJL. Eur. J. Inorg. Chem. 2007;2007:1556–1565. [Google Scholar]; c) Koike K, Tanabe J, Toyama S, Tsubaki H, Sakamoto K, Westwell JR, Johnson FPA, Hori H, Saitoh H, Ishitani O. Inorg. Chem. 2000;39:2777–2783. doi: 10.1021/ic991190l. [DOI] [PubMed] [Google Scholar]; d) Triantis C, Tsotakos T, Tsoukalas C, Sagnou M, Raptopoulou C, Terzis A, Psycharis V, Pelecanou M, Pirmettis I, Papadopoulos M. Inorg. Chem. 2013;52:12995–13003. doi: 10.1021/ic401503b. [DOI] [PubMed] [Google Scholar]
- 11.Dorsett TE, Walton RA. J. Organomet. Chem. 1976;114:127–134. [Google Scholar]
- 12.Complex 2 CCDC 983202, Complex 3 CCDC 983203, Complex 5 CCDC 983204, Complex 6 CCDC 983205, Complex 7 CCDC 983206.
- 13.a) He H, Morely JE, Silva-Lopez E, Bottenus B, Montajano M, Fugate GA, Twamley B, Benny PD. Bioconjugate Chem. 2009;20:78–86. doi: 10.1021/bc8003183. [DOI] [PubMed] [Google Scholar]; b) Alvarez CM, Garcia-Rodriguez R, Miguel D. Dalton Trans. 2007:3546–3554. doi: 10.1039/b702418e. [DOI] [PubMed] [Google Scholar]
- 14.a) Rossi R, Duatti A, Magon L, Casellato U, Graziani R, Toniolo L. Inorg. Chim. Acta. 1983;75:77–83. [Google Scholar]; b) Rossi R, Duatti A, Magon L, Marchi A, Medici A, Fogagnolo M, Casellato U, Graziani R. Transition Met. Chem. 1985;10:413–416. [Google Scholar]; c) Smithback JL, Helms JB, Schutte E, Woessner SM, Sullivan BP. Inorg. Chem. 2006;45:2163–2174. doi: 10.1021/ic050707s. [DOI] [PubMed] [Google Scholar]; d) Machura B, Wolff M, Kruszynski R, Mrozinski J, Kusz J. Polyhedron. 2009;28:2377–2384. [Google Scholar]; e) Rossi R, Marchi A, Duatti A, Magon L, Casellato U, Graziani R. J. Chem. Soc. Dalton Trans. 1987:2299–2303. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.