Abstract
Iron deficiency is a nutritional problem in plants and reduces crop productivity, quality and yield. With the goal of improving the iron (Fe) storage properties of plants, we have investigated the function of three Arabidopsis proteins with homology to Vacuolar Iron Transporter1 (AtVIT1). Heterologous expression of Vacuolar Iron Transporter-Like1 (AtVTL1; At1g21140), AtVTL2 (At1g76800) or AtVTL5 (At3g25190) in the yeast vacuolar Fe transport mutant, Δccc1, restored growth in the presence of 4 mM Fe. Isolated vacuoles from yeast expressing either of the VTL genes in the Δccc1 background had a three- to four-fold increase in Fe concentration compared to vacuoles isolated from the untransformed mutant. Transiently expressed GFP-tagged AtVTL1 was localized exclusively and AtVTL2 was localized primarily to the vacuolar membrane of onion epidermis cells. Seedling root growth of the Arabidopsis nramp3/nramp4 and vit1-1 mutants was decreased compared to the wild type when seedlings were grown under Fe deficiency. When expressed under the 35S promoter in the nramp3/nramp4 or vit1-1 backgrounds, AtVTL1, AtVTL2 or AtVTL5 restored root growth in both mutants. The seed Fe concentration in the nramp3/nramp4 mutant overexpressing AtVTL1, AtVTL2 or AtVTL5 was between 50 and 60% higher than in non-transformed double mutants or wild-type plants. We conclude that the VTL proteins catalyze Fe transport into vacuoles and thus contribute to the regulation of Fe homeostasis in planta.
Introduction
Regulation of the cellular Fe concentration poses the problem of balancing Fe deficiency against toxicity [1]. Equilibrium is maintained by strict regulation of Fe uptake and storage in the cell. Uptake of soil Fe in Arabidopsis is catalyzed by the Fe2+ transporter AtIRT1 [2], [3]. Transcription of AtIRT1 is greatly increased under conditions of Fe deficiency [2], and AtIRT1 is also post-translationally modified to regulate its partitioning between the trans-Golgi network and plasma membranes [4]. Prior to transport, soil Fe3+-chelates are reduced by the plasma membrane ferric reductase, AtFRO2 [5]. The coupling of uptake to reduction is a hallmark of strategy I plants, and an analogous mechanism is also found in Chlamydomonas [6] and Saccharomyces [7] among other organisms. The reduction-based acquisition of Fe is aided by secretion of Fe-binding compounds and H+-ATPase-mediated acidification of the rhizosphere [8], [9], [10], [11].
Once in the cell, Fe2+ can be incorporated into proteins or stored in cellular compartments. In chloroplast and presumably mitochondria, several thousand Fe atoms are stored per ferritin molecule [12]. However, in Arabidopsis only 5% of Fe is stored in ferritin, and vacuoles appear to be the major compartment for seed Fe storage [13]. Vacuolar Fe uptake was shown to be catalyzed by the ferroportin homologue, AtFPN2 [14], [15], and by the CCC1-like protein AtVIT1 (16); however, the primary substrates of AtFPN2 have been reported to be Ni and Co in addition to Fe [14], [15]. AtVIT1 has a specific function in the vacuolar transport of Fe into xylem parenchyma of developing embryos, and the vit1-1 mutant shows misdistribution of Fe in seeds; although, vit1-1 mutant seeds have unchanged Fe concentration compared with the wild type [16]. Efflux of Fe from the vacuole is catalyzed by two NRAMP proteins, NRAMP3 and NRAMP4. The double mutant nramp3/nramp4 shows decreased Fe mobilization from the vacuole in germinating seeds [17].
Two rice orthologs of AtVIT1, OsVIT1 and OsVIT2 [18], and one in tulip, TgVIT1 [19], have been shown to catalyze vacuolar Fe transport. TgVIT1 catalyzes the transport of Fe into the proximal perianth cell vacuole, which was shown to be essential for blue color development in tulips. Both OsVIT1 and OsVIT2 complemented the yeast Δccc1 mutant, and vacuoles that were isolated from complemented cells had increased Fe and Mn concentrations. In addition, both rice genes complemented the Zn transport mutant, Δzrc1, and vacuoles isolated from these cells also had an increased Zn concentration [18]. The transcript abundance of OsVIT2 increased within hours in roots and shoots grown under conditions of excess Fe (4 mM) but decreased in roots and shoots when rice was grown under Fe-deficient conditions. In contrast, OsVIT1 expression responded only weakly to changes in the Fe status. OsVIT1 and OsVIT2 were expressed in flag leaf blades and sheaths, respectively. Consistent with the localization of expression, the osvit1-1 and osvit2-1 T-DNA knockout mutants had decreased Fe and Zn content in flag leaves with no change in Mn. Seeds of osvit1-1 and osvit2-1 had correspondingly increased Fe and Zn content. OsVIT1 and OsVIT2 were shown to regulate the partitioning of Fe and Zn in rice between source and sink tissues [18].
In an analysis of the transcriptional response of Arabidopsis roots to Fe deficiency, we and others have identified three genes whose mRNA abundance decreased in Fe-deficient roots and whose putative amino acid sequences showed significant homology to AtVIT1 and consequently also to yeast Ccc1p [20], [21], [22]. These genes belong to a small, five-membered family that has been annotated as nodulin or nodulin-like in databases. These genes will be subsequently referred to as Vacuolar Iron Transporter-Like (VTL). The VTL family was found both in mono- and dicotyledon plants, as well as Chamydomonas and Physcomitrella. Promoter-β-glucuronidase (GUS) assays showed expression of AtVTL1 in roots, hypocotyls, and cotyledons of seedlings with the greatest activity associated with the vascular bundle and the root stele [21]. The promoter activity was greatly reduced in Fe-deficient seedlings, consistent with the transcriptional analysis. In the present report, we show that AtVTL1 (At1g21140) and AtVTL2 (At1g76800) are localized to the vacuolar membrane in plants, and that AtVTL1, AtVTL2 and AtVTL5 (At3g25190) complement the Δccc1 mutation in Saccharomyces. Over-expression of AtVTL1, AtVTL2 and AtVTL5 also complemented the Fe deficiency-dependent root growth phenotype in the nramp3/nramp4 double mutation and the vit1-1 mutation in Arabidopsis seedlings. These results indicate that the three members of the VTL family are involved in regulation of cellular Fe homeostasis, likely by acting as vacuolar Fe transporters.
Materials and Methods
Arabidopsis Growth Conditions
Arabidopsis seeds were surface-sterilized, vernalized and grown hydroponically as described by Gollhofer et al. [21]. Seeds of the accession Columbia (Col-0) were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus, Ohio). Plants were grown hydroponically according to the method described by Buckhout et al. [20], and the hydroponic nutrient solution was composed of KNO3 (3 mM), MgSO4 (0.5 mM), CaCl2 (1.5 mM), K2SO4 (1.5 mM), NaH2PO4 (1.5 mM), H3BO3 (25 µM), MnCl2 (1 µM), ZnSO4 (0.5 µM), (NH4)6Mo7O24 (0.05 µM) CuSO4 (0.3 µM), Fe-EDTA (40 µM) with the pH adjusted to 6.0 with KOH. For growth on Petri dishes, the method of Santi and Schmidt [11] was used. The medium contained: KNO3 (5 mM), Ca(NO3)2 (2 mM), MgSO4 (2 mM), KH2PO4 (2.5 mM), MnCl2 (14 µM), H3BO3 (70 µM), ZnSO4 (1 µM), CuSO4 (0.5 µM), Na2MoO4 (0.2 µM), CoCl2 (0.01 µM), NaCl (10 µM), and Fe-EDTA (40 µM). Sucrose (44 mM) and 5 mM MES (2-[N-morpholino]ethanesulfonic acid) were included, and the pH was adjusted to 5.5. The medium was solidified with 0.8% agar (Fluka, Taufkirchen, Germany). For determination of root growth seedlings were photographed at the appropriate time, the photographs enlarged and root length measured.
Analysis of the Fe Concentration in Arabidopsis Seeds and in Yeast Vacuoles
The Fe content in seeds was determined by the BPDS method [23]. Dried and powdered samples (4–8 mg) were mixed with glass beads (∼20; ø 425–600 µm) in 2 ml Eppendorf tubes and incubated at 95°C in 75 µl nitric acid (65%) for 6 h to digest the plant material. Fifty µl of H2O2 (30%) was added and the solution incubated at 56°C for 2 h. The volume was adjusted to 200 µl with water. Twenty µl of this solution were diluted in 980 µl of BPDS buffer (1 mM bathophenanthroline disulfonic acid, 0.6 M sodium acetate and 0.48 M hydroxylammonium chloride). The concentration of Fe-BPDS was determined photometrically at 535 nm. A standard calibration curve was prepared by dilution of a stock FeSO4 solution dissolved in 0.1 N HCl. Samples were measured in triplicate and the experiments were conducted at least three times.
Yeast Growth, Complementation and Isolation of Vacuoles
The yeast strain used in this study was Δccc1 (ura3, leu2, his3, ade2, can1, CCC1::HIS3) in DY150. Yeast transformation was carried out by the Li-acetate method [24]. Yeast growth was determined under selection on agar plates with 100–105 cells per dot.
Vacuoles were isolated as described by Li et al. [25]. Briefly, cells harvest at OD600 = 0.4 were suspended in 30 ml of 0.1 M Tris-HCl (pH 9.3) buffer containing 10 mM dithiothreitol and incubated for 10 min at 30°C. The cells were washed once with spheroplast buffer (1.2 M sorbitol, 20 mM K-phosphate, pH 7.4) and incubated with 20 µg/ml lyticase for 30 min at 30°C. Spheroplasts were collected by centrifugation at 3,500 g for 5 min., and the pellet was resuspended in 10 ml of 15% Ficoll buffer (15% Ficoll (Sigma) in 0.2 M sorbitol and 10 mM PIPES-KOH, pH 6.8). Fifty µg/ml DEAE-Dextran (Amersham Pharmacia Biotech) were added to the spheroplasts, and the sample was incubated for 3 min. on ice and for an additional 5 min. at 30°C. The lysate (10 ml) was transferred to SW28 centrifuge tubes (Beckman Instruments) and overlaid with 10 ml of 8% Ficoll, 10 ml of 4% Ficoll, and 10 ml of 0% Ficoll. The tubes were centrifuged at 110,000 g for 3 h. The vacuolar fraction was collected from the 0%/4% interphase. Fe concentration of yeast vacuoles was determined by the BPDS method [23].
Transformation and Transient Expression of GFP::VTL
Arabidopsis wild-type Col-0 was transformed using the floral dip method of Clough and Bent [26]. Transgenic plants were selected for BASTA resistance on potting soil.
Onion epidermal cells were co-bombarded with 2.5 µg of plasmids bearing free GFP or GFP-tagged VTL genes (35S:GFP::AtVTL1 or 35S:GFP::AtVTL2) and the vacuole marker plasmid vac-rk CD3 975 [27]. Plasmids were coated on 1 µm gold particles and delivered into onion epidermal cells at a pressure of 900 psi by a PDS 1000/He particle delivery system (BioRad, U.S.A). After bombardment, onion slices (2 cm2) were placed in a Petri dish containing Murashige and Skoog (MS) salts, 30 g/l sucrose and 1.5% agar (pH = 5.7). Following a minimum of 24 h, the epidermis cells were observed under a confocal laser scanning microscope (Zeiss LSM510 Meta) using a ×63 water objective. Vac-rk CD3 975 images were captured in the 560 to 615 nm range after excitation at 543 nm with a HeNe laser beam. The GFP images were captured in the 505 to 530 nm range after excitation at 488 nm with an argon laser beam. Image overlay was carried out by Z-stack analysis at 0.8 µm intervals and further processed with the projection function under LSM510-Expert Mode software.
qRT-PCR
Total RNA was isolated using TRIsure (Bioline) and treated with DNase using DNAse Kit (Fermentas) as suggested by the manufacturer. cDNA was synthesized using DNA-free RNA with oligo-dT(18) primer and RevertAid reverse transcriptase (Thermo scientific). The cDNA was used as a PCR template in a 10 µl reaction system using the SensiMix SYBR No-ROX Kit (Bioline) with programs recommended by the manufacturer in a CFX96 Realtime system (BioRAD). Three biological replicates were performed for each sample. The ΔΔCT method was used to determine the relative transcript abundance
Results
Complementation of the yeast mutant Δccc1 by AtVTL1, AtVTL2 and AtVTL5
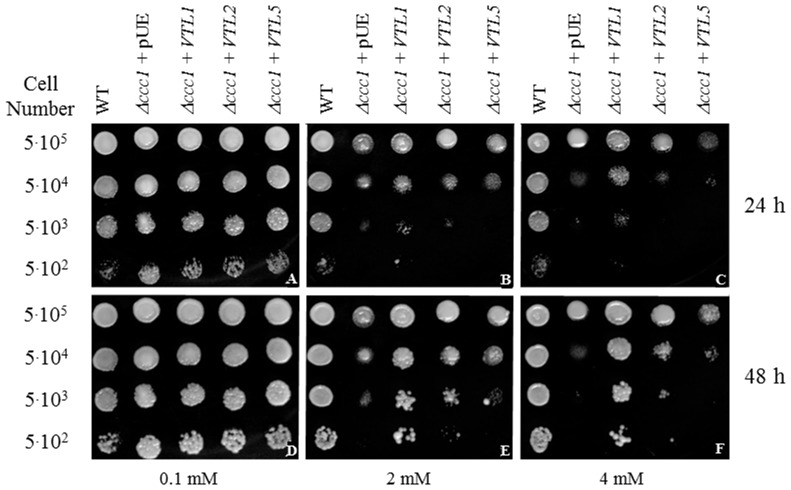
As in Arabidopsis, the yeast vacuole is the major compartment for Fe storage but also protects the cell from Fe toxicity. The yeast gene CCC1 encodes a vacuolar Fe2+/Mn2+ transporter that catalyzes Fe2+ uptake into the vacuole. The Δccc1 mutant is hypersensitive to Fe toxicity, not growing in the presence of greater than ca. 3 mM Fe [25]. Because of the homology of the VTL family member to yeast Ccc1p and Arabidopsis AtVIT1 and because of the transcriptional repression of AtVTL1, AtVTL2 and AtVTL5 under conditions of Fe deficiency, the ability of the three AtVTL genes to complement the Δccc1 yeast mutant was investigated. When expressed in yeast, the VTL proteins were effective in complementing of the Δccc1 phenotype in the order AtVTL1> AtVTL2> AtVTL5 (Fig. 1). These results are consistent with an AtVTL-dependent decreased the cytosolic Fe in the Δccc1 mutant, presumably by transport of Fe into the vacuole.
Figure 1. Complementation of the Δccc1 by heterologous expression of the VTL genes.
Δccc1 (vacuolar Fe2+/Mn2+ transporter) cells were transformed with each of the three VTL genes or the empty vector (pUE) under the control of the PGK promoter and grown on SD medium containing FeSO4 at the concentrations indicated for 24 or 48 h at 30°C. Cells were plated at the densities indicated in the figure.
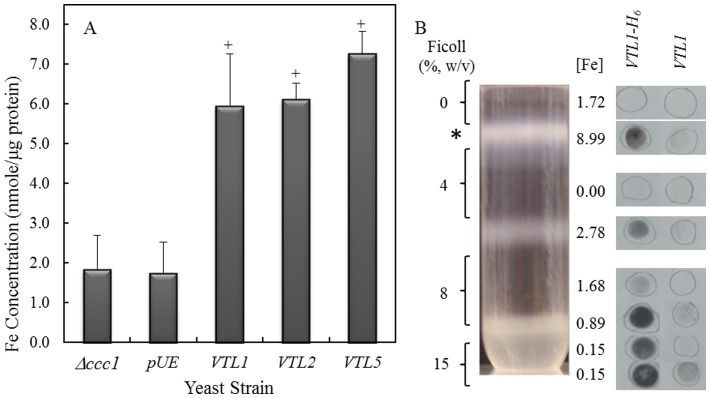
To test this hypothesis, the Fe concentration in isolated yeast vacuoles was investigated. Intact yeast vacuoles were isolated by floatation through a Ficoll step gradient as described by Li et al. [25]. We confirmed the composition of the vacuolar fraction by re-centrifugation the fraction on a continuous sucrose gradient followed by marker enzyme analysis (Fig. S1). As expected, the vacuole marker, bafilomycin-sensitive ATPase, co-localized with membrane proteins and no detectable plasma membrane (vanadate-sensitive ATPase) or endoplasmic reticulum (NADPH cytochrome c reductase) activities were detected in the gradient. Vacuoles, isolated from log-phase Δccc1 cells or from Δccc1 cells transformed with the control vector grown in liquid SD medium containing 1 mM FeSO4, had approximately 2 nmoles Fe per µg protein, whereas Δccc1 cells expressing AtVTL1, AtVTL2 or AtVTL5 had a greater than 3-fold higher Fe content (Fig. 2A).
Figure 2. Determination of the Fe concentration in Δccc1 cells transformed with AtVTL1, AtVTL2 or AtVTL5.
(A) Shown is the Fe concentration in yeast vacuoles isolated from the Δccc1 mutant, Δccc1 transformed with an empty plasmid (pUE), AtVTL1, AtVTL2 or AtVTL5. Error bars indicate the standard error of the mean. Bar graphs labeled with “+” are significantly different for the Δccc1 mutant (p<0.05). (B) Vacuoles were isolated from Δccc1 yeast cells transformed with His-tagged AtVTL1 (VTL1-H6) or with untagged AtVTL1 (VTL1) as a control. AtVTL1-H6 was localized by dot-blots using an anti-His antibody. The positions of the dot-blots are approximate. The Fe concentrations (nmole/µg protein) in the fraction corresponding to the dot-blot are given.
Localization of AtVTL1 was demonstrated by transforming Δccc1 cells with AtVTL1 fused to a 3′ His tag (AtVTL1-H6). Δccc1 cells that were transformed with AtVTL1-H6 complemented the Fe-sensitive phenotype (Fig. S2). Using a His-tag antibody, AtVTL1-H6 protein was localized to the vacuolar fraction at the 0/4% Ficoll interface (Fig. 2B). Iron was also concentrated in this fraction compared to fractions in other regions of the Ficoll gradient. The immunological signals in the pellet and 15%/8% Ficoll interface were most like the result of vacuolar membranes from ruptured vacuoles that that did not float through the gradient. We demonstrated that AtVTL1 was localized on the yeast vacuolar membrane, where it presumably catalyzed transport of Fe into the yeast vacuole.
Localization of AtVTL1 and AtVTL2 in planta
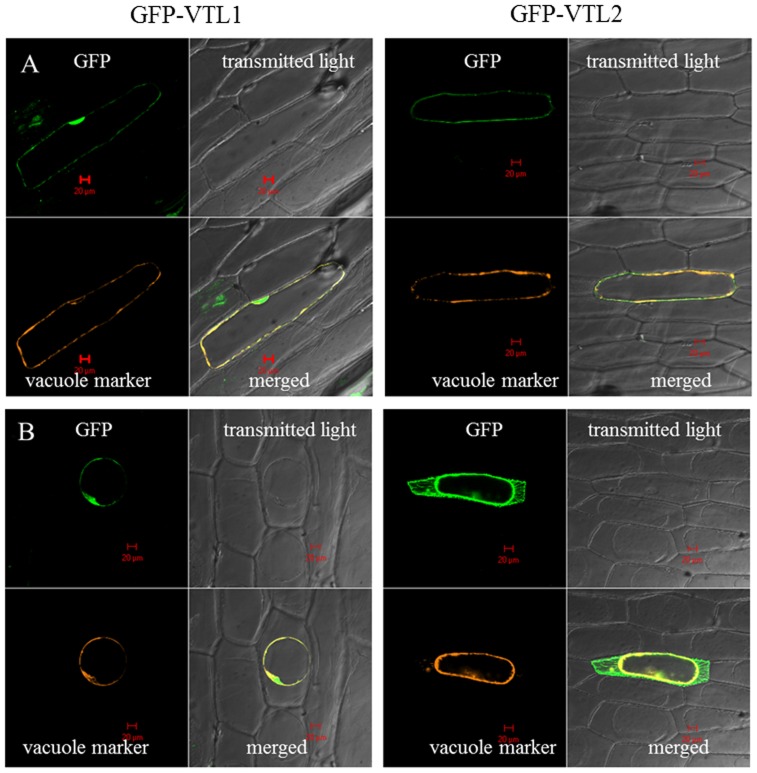
The localization of AtVTL1 and AtVTL2 was investigated in onion epidermis cells. Attempts to localize AtVTL5 in onion or tobacco leaf cells have not been successful. Onion cells were transformed by particle bombardment with 35S:GFP::VTL1 or 35S:GFP::VTL2 reporter gene constructs. In the case of AtVTL1, the GFP fluorescence co-localized with the vacuolar marker vac-rk CD3-975 (Fig. 3A, [27]). When cells were plasmolyzed in 0.8 M mannitol, the GFP signal remained associated with the vacuole (Fig. 3B). The localization of AtVTL2 in epidermis cells was predominately associated with the vacuolar membrane (Fig. 3A); however, GFP fluorescence was also found to be associated with the plasma membrane and the cytoplasm upon plasmolysis (Fig. 3B). The localization of the two AtVTL proteins on the onion vacuolar membrane was consistent with the complementation results in Saccharomyces and with the increased Fe concentration in vacuoles isolated from VTL-expressing yeast cells.
Figure 3. Transient 35S:GFP::VTL1 and 35S:GFP::VTL2 expression in onion epidermis cells.
The GFP-VTL1 fluorescent signal co-localized with the vacuolar marker, vac-rk CD3-975, in turgescent (A) and plasmolyzed (B) cells. The GFP-VTL2 signal was also observed on the plasma membrane and cytoplasma (B).
Complementation of the nramp3/nramp4 and the vit1-1 mutants
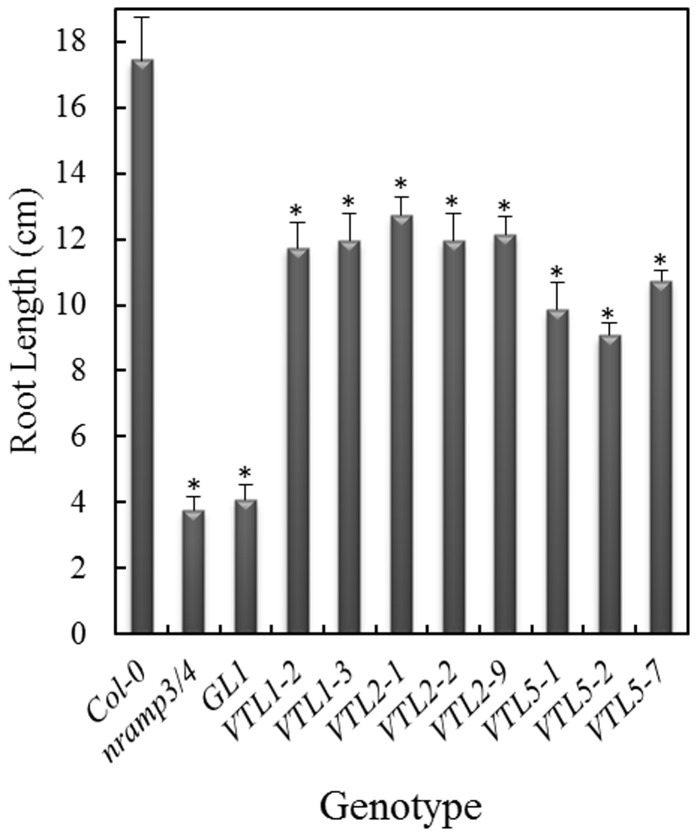
Over-expression of AtVTL1 in Arabidopsis did not greatly alter Fe content of shoots and roots [21], nor were changes observed in the kinetics of the response to Fe deficiency, as determined by the root plasma membrane Fe3+-chelate reductase and chlorophyll content (Figs. S3A and B). In an attempt to characterize their functions, we have ectopically expressed the AtVTL genes in the Arabidopsis nramp3/nramp4 double mutant background (17). Over-expression was confirmed by semi-quantitative RT-PCR (Fig. S4). Both NRAMP3 and NRAMP4 have been shown to be Fe vacuolar efflux carriers. The double mutant had no obvious phenotype when grown on soil; however, growth of mutant seedlings was retarded shortly after germination on an Fe-deficient substrate [17]. The phenotype was not persistent and disappeared after a few days of growth or with supplemental Fe in the media. Under Fe deficiency root growth of the nramp3/nramp4 double mutant and of the double mutant transformed with the empty vector (GL1), was greatly inhibited compared to the wild-type control at 5 d following germination (Fig. 4 and Fig. S5; p<0.001) [17]. Importantly, over-expression of AtVTL1, AtVTL2 or AtVTL5 in nramp3/nramp4 complemented this mutant growth phenotype. The early seedling root length was significantly increased (Fig. 4 and Fig. S5; p<0.001) compared to the double mutant; however, the roots were still significantly shorter than those of the wild type (p<0.001). Thus, over-expression of AtVTL1, AtVTL2 or AtVTL5 partially restored the wild-type phenotype in the nramp3/nramp4 double mutant.
Figure 4. Complementation of the nramp3/nramp4 double mutant by over-expression of AtVTLs.
The three VTL genes were over-expressed in the nramp3/nramp4 double mutant background under the control of the 35S promoter and root length was determined after 5 days of growth. Seedlings were grown without added Fe. Seedlings were grown on agarose Petri plates as described in the Materials and Methods. Asterisks indicate significant differences from the wild-type (p<0.001).
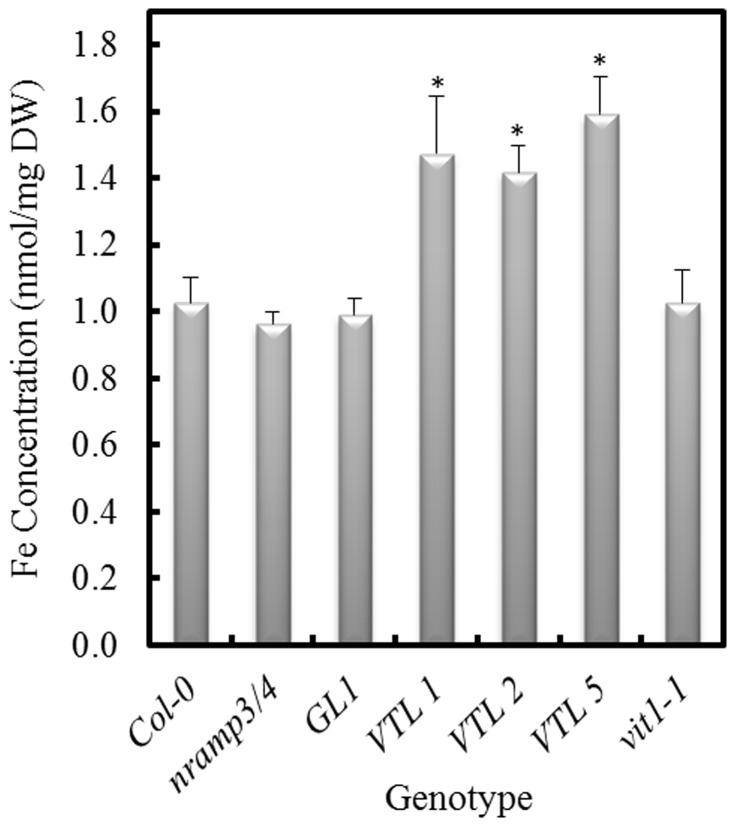
The proposed function of NRAMP3 and NRAMP4 was to mobilize Fe from the vacuole of young seedlings [17]. We hypothesized that over-expression of the AtVTLs might have increased total seed Fe, and thereby increased the Fe supply to the germinating seedling. To test this, we analyzed the Fe concentration in nramp3/nramp4 seeds that over-expressed the AtVTL genes. The results of these analyses demonstrated that the AtVTL1, AtVTL2 or AtVTL5 over-expressing lines had between 40 and 60% increased Fe concentration compared to either the wild type or the nramp3/nramp4 double mutant (Fig. 5; p<0.01). These results supported the concept that an increased content of Fe in the seeds could have supplied the embryo with sufficient Fe even when efflux from the vacuole through the NRAMP transporters was compromised.
Figure 5. Fe concentration of Arabidopsis seeds.
Fe was determined in the wild-type (Col-0), the nramp3/nramp4 double mutant, the vector control (GL1), the vit1-1 mutant and in seeds from the double mutant expressing one of the three VTL genes under the control of the 35S promoter. Asterisks indicate significant differences from the wild-type (p<0.01).
As mentioned above, the CCC1-like protein, AtVIT1, is localized on the vacuolar membrane and functions in the transport of Fe into the parenchyma cell of the provascular strands in developing embryos. The vit1-1 mutant showed misdistribution of Fe; although, vit1-1 mutant seeds had unchanged Fe content compared to the wild type (Fig. 5) [16]. vit1-1 had a severely chlorotic phenotype when grown on alkaline soil [16]. In addition, we observed that vit1-1 displayed a short-root phenotype similar to the nramp3/nramp4 mutant when grown on Fe-deficient media in the presence of the Fe2+ chelator Ferrozine (Fig. 6 and Fig. S6). Over-expression of AtVTL1, AtVTL2 or AtVTL5 restored root growth to greater than the wild-type length (p>0.001), thus complementing the vit1-1 mutation.
Figure 6. Complementation of the vit1-1 mutant by over-expression of AtVTL1, AtVTL2 or AtVTL5.
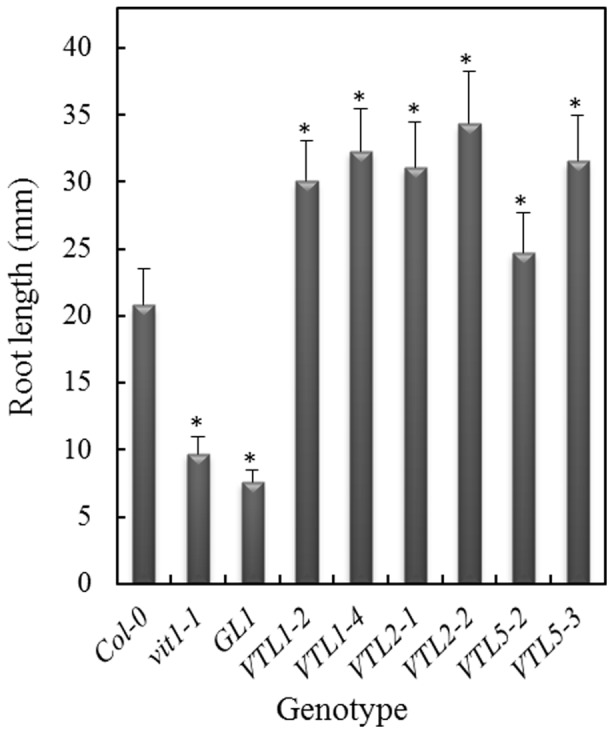
The three VTL genes were over-expressed under the control of the 35S promoter in the vit1-1 background. Root length was determined after 13 days of growth on agarose Petri plates as described in the Materials and Methods ion ES medium without added Fe and in the presence of the Fe2+ chelator, Ferrozine. Asterisks indicate significant differences from the wild-type (p<0.001). Shown are results from 2 independent transformants.
Regulation of AtVTL1, AtVTL2 and AtVTL5 Gene Expression
Finally, we investigated the response of AtVTL1, AtVTL2 and AtVTL5 to nutrient supply using quantitative qRT-PCR. Previously, we reported that the transcriptional activity of these genes positively correlated with the Fe supply [20]. Quantitative RT-PCR analyses confirmed these results (Fig. 7). Compared to Fe-sufficient controls (40 µM Fe), the transcript abundance of AtVTL1, AtVTL2 and AtVTL5 was decreased under Fe deficiency and increased in plants grown on media containing 120 µM Fe. Similarly, transcript abundance also decreased under Zn deficiency for AtVTL1, AtVTL2 and AtVTL5 (Fig. 7). However, when the Zn concentration was increased to 5 µM, the transcript abundance for AtVTL1, AtVTL2 and AtVTL5 was not significantly different from that of control plants grown on standard media (Fig. 7). The VTL genes were unable to restore growth in the Zn sensitive Δzrc1 mutant (Fig. S7). Thus, a function of AtVTL1, AtVTL2 or AtVTL5 in vacuolar Zn transport is unlikely. Since AtVTL1, AtVTL2 and AtVTL5 responded positively to Fe supply and since their over-expression complemented vacuolar Fe transport mutations in yeast and Arabidopsis mutants, we conclude that VTL genes encode protein involved in Fe homeostasis, presumably as Fe vacuolar transporters.
Figure 7. RT-qPCR of AtVTL1, AtVTL2 and AtVTL5 in response to Fe and Zn supply.
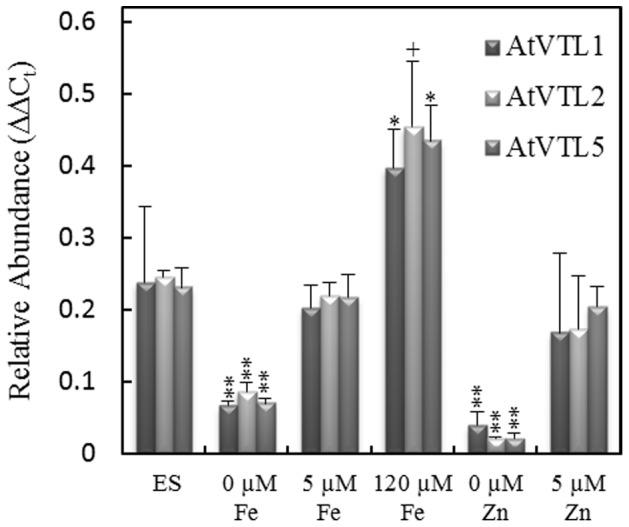
Seedlings were grown on agarose petri plates as described in the Materials and Methods. Growth conditions were: ES medium (ES); “0”, ES without Fe or Zn or at the concentrations indicated. RT-qPCR was standardized to the expression of the ACTIN2. Symbols indicate significant differences to expression in the ES medium in three biological replicates: **, p<0.001; *, p<0.01; and +, p<0.05.
Discussion
Heterologous expression of AtVIT1, OsVIT1, OsVIT2 and TgVIT1 in the Δccc1 mutant has been shown to complement the Δccc1 mutation by restoring Fe uptake into the vacuole, and thus, to protect the cell from the deleterious effects of high cytosolic Fe ([6], [18], [19]. Vacuolar Fe transporter activity has been reported for FPN2, NRAMP3 and NRAMP4. Whereas AtVIT1 and FPN2 transported Fe into vacuoles, the two NRAMPs were shown to be efflux carriers. Previously, we have identified five genes in Arabidopsis, which encoded proteins with significant sequence homology to AtVIT1 [20]. The transcript abundance of AtVTL1, AtVTL2 and AtVTL5 was rapidly decreased in roots under conditions of Fe deficiency [21], [28]. We have shown in this report, that heterologous expression of AtVTL1, AtVTL2 and AtVTL5 in yeast Δccc1 cells restored their ability to grow in the presence of 4 mM Fe. Since the heterologous expression of these genes in yeast correlated with increased Fe in the vacuolar fraction, we proposed that these three proteins increased Fe transport into the vacuole and thereby reduced toxic Fe in the cytoplasm of Δccc1 cells.
VTLs are Localized to the Arabidopsis Vacuolar Membrane
Transient expression of GFP::VTL1 and GFP::VTL2 chimeric proteins in onion epidermis cells resulted in a specific co-localization of GFP::VTL1 with the vacuolar marker vac-rk CD3-975. In the case of GFP::VTL2 transient expression, a prominent signal was co-localized with the vacuole marker, but GFP fluorescence was also found in the cytoplasm and associated with the plasma membrane, indicating possible localization of AtVTL2 on other membranes in addition to the vacuole. Attempts at localizing AtVTL5 have not been successful.
Evidence for a function of AtVTL1, 2 and 5 in Fe homeostasis was obtained by complementation of the nramp3/nramp4 and vit1-1 mutations. The NRAMP3 and NRAMP4 proteins act redundantly as vacuolar Fe efflux carriers. The abundance of both NRAMPs was increased during Fe deficiency, and in the double mutant, greening of cotyledons and early root growth of seedlings were delayed compared to controls when seeds were germinated under Fe deficiency [17]. This inhibition of root growth could be largely eliminated by supplying Fe to the growth medium. The root growth phenotype was complemented by over-expression of AtVTL1, 2 or 5 in the nramp3/nramp4 mutant. In addition the total Fe content of seeds from over-expressing AtVTL1, 2 or 5 plants was increased compared to the wild type and the mutant. It might have seemed paradoxical that a mutation in a vacuole efflux transport system could be complemented by a putative vacuolar uptake transporter; however, an increased vacuolar Fe concentration might have been sufficient to compensate for a decreased efflux caused by the mutations in NRAMP3 and NRAMP4.
The vit1-1 mutant also displayed a Fe deficiency-dependent inhibition of seedling root growth that is likely the result of a mis-localization of Fe in the seed radicle. Over-expression of AtVTL1, 2 or 5 might have restored the Fe supply to the growing root. In summary and based on the complementation activity in yeast, the increased vacuolar Fe concentration in yeast cells expressing AtVTL1, 2 or 5, the localization of AtVTL1 and 2 on the vacuolar membrane in onion epidermis cells and the restoration of seedling root growth in the nramp3/nramp4 and vit1-1 mutants, a critical role for the VTL proteins in the subcellular distribution of Fe is apparent.
AtVTLs and Metal Homeostasis
Low substrate specificity is commonly found in cation transports. For example, AtIRT1 is the predominant plasma membrane Fe2+ transporter and is essential for Fe uptake from the soil [3]. In the absence of Fe or in the presence of excess divalent cations, AtIRT1 can also catalyze the transport of Zn, Mn and Cd [3], [29]. Both NRAMP3 and NRAMP4 have been shown also to be vacuolar Mn exporters [30]; although, transcription was correlated with the Fe and not the Mn nutrient status. Lanquar et al. [30] envision a passive function of NRAMP3 and NRAMP4 in cycling of Mn from the vacuole to the plastid in mesophyll cells. The nramp3/nramp4 double mutant is hypersensitive to Zn and Cd, and as Sinclair and Krämer [31] suggested, the cause of the hypersensitivity appeared to be an indirect effect related to impaired Fe and Mn transport. Although the transcript data do support a role for the VTLs in Fe homeostasis, a role in homeostasis of other divalent cations analogous to the roles of NRAMP3 and NRAMP4 cannot be excluded.
Five Zn transport genes are among the Arabidopsis genes that response to Fe deficiency [32]. Four of these genes that transport Zn into the vacuole are strongly induced under Fe deficiency. The remaining gene, AtZIP3, was repressed and shown to catalyzed Zn uptake into cells. Zn has been shown to accumulate in roots grown under Fe-deficient conditions, likely the result of the Zn transport activity of IRT1 [3], [31]. It is noteworthy that the expression of AtVTL1, AtVTL2 and AtVTL5 was greatly decreased under Zn deficiency but unchanged when grown under Zn excess (Fig. 7). Expression of AtVTL1, AtVTL2 and AtVTL5 in the yeast Δzrc1, a vacuolar Zn transport mutant, did not complement this mutation (Fig. S7).
Although a direct role of the VTL proteins in Zn transport is lacking, an involvement of Zn in Fe homeostasis has been clearly demonstrated. Transcriptional regulation in strategy I plants to Fe deficiency is mediated in part by the bHLH transcription factors FIT (bHLH029) [33], [34], [35] and POPEYE (bHLH047, PYE) [36]. The central components of PYE regulation include PYE (At3g47640), IAA–LEUCINE-RESISTANT3 (ILR3, bHLH105, At5g54680) and BRUTUS (BTS, At3g47640). PYE is weakly and BTS is strongly induced under Fe deficiency [20], [36], and PYE and BTS both interact but with ILR3 in a yeast two-hybrid assay but not with each other [36]. Either a ternary complex of PYE-ILR3-BTS or the competition between PYE and BTS for ILR3 has been proposed as the regulatory switch in the PYE network [36]. PYE directly regulated genes that were associated with the response to Fe deficiency including: FRO3, NAS4 and ZIF1. The transcription of AtVTL1, 2 and 5 was increased 1.9, 16.9 and 2.9-fold, respectively, in the pye-1 knockout mutant [36], and roots of the pye-1 mutant had an elevated Fe content compared with controls. These observations are consistent with the positive correlation between the root Fe concentration and transcript abundance of AtVTL1, 2 and 5 (Fig. 7) [21].
In the ilr3-1 gain-of-function mutant, the transcript abundance of AtVTL1, 2 and 5 was decreased between 3- and 4-fold; an observation that can be explained by a gain-of-function in the PYE-ILR3-BTS repression [22]. In general, BTS and the rice homologs OsHRZs negatively regulate the Fe deficiency response in Arabidopsis and rice, and thus plants with decreased expression of these genes showed tolerance to Fe deficiency [36], [37]. Recombinant BTS has been shown to bind both Fe and Zn, primarily at the hemerythrin domain of the protein [36]. In a recent model, Kobayshi and Nishizawa [38] have proposed a mechanism for transcriptional regulation through the BTS/OsHRZ proteins based on binding of Zn and Fe to the hemerythrin domain of these proteins. In their model, Fe binding to BTS/OsHRZ in the absence of Zn would lead to repression PYE-regulated gene expression as seen in Fig. 7. The implications of their model are germane to the repression of VTL transcription under Zn deficiency observed in our experiments. Taken together, we have identified a group of putative Fe transporters that participate in the Fe homeostasis in Arabidopsis. As vacuolar Fe transporters these proteins may have importance in biofortification efforts in the future.
Supporting Information
Sucrose density gradient isolation of membrane in the yeast vacuolar fraction. The vacuolar fraction was isolated as described in the Materials and Methods, and the vacuoles ruptured by repeated pipetting. The membranes were layered onto a continuous, 10 to 60% sucrose gradient and centrifuged at 110,000xg in a swing-out rotor over-night. The gradient was fractionated into 1 ml fractions and marker enzymes for the vacuole (bafilomycin-sensitive ATPase), plasma membrane (vanadate-sensitive ATPase) and endoplasmic reticulum (cytochrome c reductase) were determined by the method of Luster and Buckhout (Plant Physiol. 1989; 91(3): 1014-9). The activity of the vanadate-sensitive ATPase and the cytochrome c reductase were below the limits of detection.
(TIF)
Complementation of the yeast Δccc1 (vacuolar Fe2+/Mn2+ transporter) mutant with his-tagged AtVTL1 and AtVTL2 genes. Cells were transformed with the empty vector (pUE) or the VTL gene containing a H6 tag under the control of the PGK promoter and grown on YPD medium containing 7.5 mM FeSO4.
(TIF)
A. Chlorophyll content in wild-type (Col-0) and wild-type plants over-expressing AtVTL1 grown in the Fe concentrations indicated. Shown are chlorophyll a and b and the chlorophyll a/b ratio. B. Analysis of the Fe3+-chelate reductase activity in Col-0 and Col-0 plants over-expressing AtVTL1 . Bars are standard error of the mean.
(TIF)
Semi-quantitative PCR of of AtVTL1 , AtVTL2 and AtVTL5 . Expression was determined in Col-0 (WT), the nramp3/nramp4 double mutant and in the double mutant over-expressing each of the VTL1, VTL2 or VTL5 genes. Expression was standardized to the level of ACTIN2 expression.
(TIF)
Root growth in the nramp3/nramp4 double mutant transformed with AtVTL1 , AtVTL2 or AtVTL5 . Seedlings were grown for 5 days on standard media lacking Fe (see Materials and Methods). Shown are results from two to three independent transformants taken from one repetition of the experiment reported in Fig. 4.
(TIF)
Root growth in the vit1-1 mutant transformed with AtVTL1 , AtVTL2 or AtVTL5 . Seedlings were grown for 13 days on standard media (see Materials and Methods) lacking Fe and in the presence of the Fe2+ chelator, Ferrozine. Shown are results from an experiment similar to that reported in Fig. 6.
(TIF)
Complementation of the Δzrc1 by heterologous expression of the VTL genes. Δzrc1 (vacuolar Zn2+ transporter) cells were transformed with each of the three VTL genes or the empty vector (pUE) under the control of the PGK promoter and grown on SD medium containing ZnSO4 at the concentrations indicated for 24 or 48 h at 30°C. Cells were plated at the densities indicated in the figure.
(TIF)
Acknowledgments
We thank Drs. Mary-Lou Guerinot, Jerry Kaplan and Sabastian Thomine for kindly providing the Saccharomyes and Arabidopsis mutants used in this study and Christin Schläwicke for determination of seed Fe and leaf chlorophyll. We gratefully acknowledge the excellent technical support of Marion Dewender and Susanne Olstowski-Jacoby.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft to TJB (BU 713: 4-1). The funders had no role in study design, data collection and analysis, decision to publish,or preparation of the manuscript.
References
- 1. Kosman DJ (2010) Redox cycling in iron uptake, efflux, and trafficking. J Biol Chem 285: 26729–26735 10.1074/jbc.R110.113217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eide D, Broderius M, Fett J, Guerinot ML (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acad Sci U S A 93: 5624–5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, et al. (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14: 1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barberon M, Zelazny E, Robert S, Conéjéro G, Curie C, et al. (2011) Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci USA 108: E450–458 10.1073/pnas.1100659108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397: 694–697 10.1038/17800 [DOI] [PubMed] [Google Scholar]
- 6. Eckhardt U, Buckhout TJ (1998) Iron assimilation in Chlamydomonas reinhardtii involves ferric reduction and is similar to Strategy I higher plants. Journal of Experimental Botany 49: 1219–1226. [Google Scholar]
- 7. Kosman DJ (2003) Molecular mechanisms of iron uptake in fungi. Mol Microbiol 47: 1185–1197. [DOI] [PubMed] [Google Scholar]
- 8. Rodriguez-Celma J, Lin W-D, Fu G-M, Abadia J, Lopez-Millan A-F, et al. (2013) Mutually exclusive alterations in secondary metabolism are critical for the uptake of insoluble iron compounds by Arabidopsis and Medicago truncatula. Plant Physiol 162: 1473–1485 10.1104/pp.113.220426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fourcroy P, Siso-Terraza P, Sudre D, Saviron M, Reyt G, et al. (2014) Involvement of the ABCG37 transporter in secretion of scopoletin and derivatives by Arabidopsis roots in response to iron deficiency. New Phytol 201: 155–167 10.1111/nph.12471 [DOI] [PubMed] [Google Scholar]
- 10. Schmid NB, Giehl RFH, Doll S, Mock H-P, Strehmel N, et al. (2014) Feruloyl-CoA 6′-Hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in arabidopsis. Plant Physiol 164: 160–172 10.1104/pp.113.228544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santi S, Schmidt W (2009) Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol 183: 1072–1084 10.1111/j.1469-8137.2009.02908.x [DOI] [PubMed] [Google Scholar]
- 12. Briat J-F, Duc C, Ravet K, Gaymard F (2010) Ferritins and iron storage in plants. Biochim Biophys Acta 1800: 806–814 10.1016/j.bbagen.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 13. Ravet K, Touraine B, Boucherez J, Briat J-F, Gaymard F, et al. (2009) Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J 57: 400–412 10.1111/j.1365-313X.2008.03698.x [DOI] [PubMed] [Google Scholar]
- 14. Morrissey J, Baxter IR, Lee J, Li L, Lahner B, et al. (2009) The ferroportin metal efflux proteins function in iron and cobalt homeostasis in Arabidopsis. Plant Cell 21: 3326–3338 10.1105/tpc.109.069401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schaaf G, Honsbein A, Meda AR, Kirchner S, Wipf D, et al. (2006) AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J Biol Chem 281: 25532–25540 10.1074/jbc.M601062200 [DOI] [PubMed] [Google Scholar]
- 16. Kim SA, Punshon T, Lanzirotti A, Li L, Alonso JM, et al. (2006) Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314: 1295–1298 10.1126/science.1132563 [DOI] [PubMed] [Google Scholar]
- 17. Lanquar V, Lelievre F, Bolte S, Hames C, Alcon C, et al. (2005) Mobilization of vacuolar iron by AtNRAMP3 and AtNRAMP4 is essential for seed germination on low iron. EMBO J 24: 4041–4051 10.1038/sj.emboj.7600864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang Y, Xu Y-H, Yi H-Y, Gong J-M (2012) Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J 72: 400–410 10.1111/j.1365-313X.2012.05088.x [DOI] [PubMed] [Google Scholar]
- 19. Momonoi K, Yoshida K, Mano S, Takahashi H, Nakamori C, et al. (2009) A vacuolar iron transporter in tulip, TgVit1, is responsible for blue coloration in petal cells through iron accumulation. Plant J 59: 437–447 10.1111/j.1365-313X.2009.03879.x [DOI] [PubMed] [Google Scholar]
- 20.Buckhout TJ, Yang TJW, Schmidt W (2009) Early iron-deficiency-induced transcriptional changes in Arabidopsis roots as revealed by microarray analyses. BMC Genomics 10. doi: 10.1186/1471-2164-10-147. [DOI] [PMC free article] [PubMed]
- 21. Gollhofer J, Schlawicke C, Jungnick N, Schmidt W, Buckhout TJ (2011) Members of a small family of nodulin-like genes are regulated under iron deficiency in roots of Arabidopsis thaliana. Plant Physiol Biochem 49: 557–564 10.1016/j.plaphy.2011.02.011 [DOI] [PubMed] [Google Scholar]
- 22. Rampey RA, Woodward AW, Hobbs BN, Tierney MP, Lahner B, et al. (2006) An Arabidopsis basic helix-loop-helix leucine zipper protein modulates metal homeostasis and auxin conjugate responsiveness. Genetics 174: 1841–1857 10.1534/genetics.106.061044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blair D, Diehl H (1961) Bathophenanthrolinedisulphonic acid and bathocuproinedisulphonic acid, water soluble reagents for iron and copper. Talanta 7: 163–174. [Google Scholar]
- 24.Rose M, Winston F, Hieter P (1990) Methods in yeast genetics: A laboratory course manual. Cold Spring Harbor, New York: Cold Spring Harbor Press.
- 25. Li L, Chen OS, McVey Ward D, Kaplan J (2001) CCC1 is a transporter that mediates vacuolar iron storage in yeast. J Biol Chem 276: 29515–29519 10.1074/jbc.M103944200 [DOI] [PubMed] [Google Scholar]
- 26. Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743. [DOI] [PubMed] [Google Scholar]
- 27. Nelson BK, Cai X, Nebenfuhr A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 10.1111/j.1365-313X.2007.03212.x [DOI] [PubMed] [Google Scholar]
- 28. Yang TJW, Lin W-D, Schmidt W (2010) Transcriptional profiling of the Arabidopsis iron deficiency response reveals conserved transition metal homeostasis networks. Plant Physiol 152: 2130–2141 10.1104/pp.109.152728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohen CK, Fox TC, Garvin DF, Kochian LV (1998) The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiol 116: 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lanquar V, Ramos MS, Lelievre F, Barbier-Brygoo H, Krieger-Liszkay A, et al. (2010) Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol 152: 1986–1999 10.1104/pp.109.150946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sinclair SA, Kramer U (2012) The zinc homeostasis network of land plants. Biochim Biophys Acta 1823: 1553–1567 10.1016/j.bbamcr.2012.05.016 [DOI] [PubMed] [Google Scholar]
- 32. Schmidt W, Buckhout TJ (2011) A hitchhiker's guide to the Arabidopsis ferrome. Plant Physiol Biochem 49: 462–470 10.1016/j.plaphy.2010.12.001 [DOI] [PubMed] [Google Scholar]
- 33. Colangelo EP, Guerinot ML (2004) The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response. Plant Cell 16: 3400–3412 10.1105/tpc.104.024315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jakoby M, Wang H-Y, Reidt W, Weisshaar B, Bauer P (2004) FRU (BHLH029) is required for induction of iron mobilization genes in Arabidopsis thaliana. FEBS Lett 577: 528–534 10.1016/j.febslet.2004.10.062 [DOI] [PubMed] [Google Scholar]
- 35. Yuan Y, Wu H, Wang N, Li J, Zhao W, et al. (2008) FIT interacts with AtbHLH38 and AtbHLH39 in regulating iron uptake gene expression for iron homeostasis in Arabidopsis. Cell Res 18: 385–397 10.1038/cr.2008.26 [DOI] [PubMed] [Google Scholar]
- 36. Long TA, Tsukagoshi H, Busch W, Lahner B, Salt DE, et al. (2010) The bHLH transcription factor POPEYE regulates response to iron deficiency in Arabidopsis roots. Plant Cell 22: 2219–2236 10.1105/tpc.110.074096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi T, Nagasaka S, Senoura T, Itai RN, Nakanishi H, et al.. (2013) Iron-binding Haemerythrin RING ubiquitin ligases regulate plant iron responses and accumulation. Nat Commun 4. doi: 10.1038/ncomms3792. [DOI] [PMC free article] [PubMed]
- 38.Kobayashi T, Nishizawa NK (2014) Iron sensors and signals in response to iron deficiency. Plant Sci 224C. doi: 10.1016/j.plantsci.2014.04.002. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sucrose density gradient isolation of membrane in the yeast vacuolar fraction. The vacuolar fraction was isolated as described in the Materials and Methods, and the vacuoles ruptured by repeated pipetting. The membranes were layered onto a continuous, 10 to 60% sucrose gradient and centrifuged at 110,000xg in a swing-out rotor over-night. The gradient was fractionated into 1 ml fractions and marker enzymes for the vacuole (bafilomycin-sensitive ATPase), plasma membrane (vanadate-sensitive ATPase) and endoplasmic reticulum (cytochrome c reductase) were determined by the method of Luster and Buckhout (Plant Physiol. 1989; 91(3): 1014-9). The activity of the vanadate-sensitive ATPase and the cytochrome c reductase were below the limits of detection.
(TIF)
Complementation of the yeast Δccc1 (vacuolar Fe2+/Mn2+ transporter) mutant with his-tagged AtVTL1 and AtVTL2 genes. Cells were transformed with the empty vector (pUE) or the VTL gene containing a H6 tag under the control of the PGK promoter and grown on YPD medium containing 7.5 mM FeSO4.
(TIF)
A. Chlorophyll content in wild-type (Col-0) and wild-type plants over-expressing AtVTL1 grown in the Fe concentrations indicated. Shown are chlorophyll a and b and the chlorophyll a/b ratio. B. Analysis of the Fe3+-chelate reductase activity in Col-0 and Col-0 plants over-expressing AtVTL1 . Bars are standard error of the mean.
(TIF)
Semi-quantitative PCR of of AtVTL1 , AtVTL2 and AtVTL5 . Expression was determined in Col-0 (WT), the nramp3/nramp4 double mutant and in the double mutant over-expressing each of the VTL1, VTL2 or VTL5 genes. Expression was standardized to the level of ACTIN2 expression.
(TIF)
Root growth in the nramp3/nramp4 double mutant transformed with AtVTL1 , AtVTL2 or AtVTL5 . Seedlings were grown for 5 days on standard media lacking Fe (see Materials and Methods). Shown are results from two to three independent transformants taken from one repetition of the experiment reported in Fig. 4.
(TIF)
Root growth in the vit1-1 mutant transformed with AtVTL1 , AtVTL2 or AtVTL5 . Seedlings were grown for 13 days on standard media (see Materials and Methods) lacking Fe and in the presence of the Fe2+ chelator, Ferrozine. Shown are results from an experiment similar to that reported in Fig. 6.
(TIF)
Complementation of the Δzrc1 by heterologous expression of the VTL genes. Δzrc1 (vacuolar Zn2+ transporter) cells were transformed with each of the three VTL genes or the empty vector (pUE) under the control of the PGK promoter and grown on SD medium containing ZnSO4 at the concentrations indicated for 24 or 48 h at 30°C. Cells were plated at the densities indicated in the figure.
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.