Abstract
Unlike most other bacteria, mycobacteria make fatty acids with the multidomain enzyme eukaryote-like fatty acid synthase I (FASI). Previous studies have demonstrated that the tuberculosis drug pyrazinamide and 5-chloro-pyrazinamide target FASI activity. Biochemical studies have revealed that in addition to C16:0, Mycobacterium tuberculosis FASI synthesizes C26:0 fatty acid, while the Mycobacterium smegmatis enzyme makes C24:0 fatty acid. In order to express M. tuberculosis FASI in a rapidly growing Mycobacterium and to characterize the M. tuberculosis FASI in vivo, we constructed an M. smegmatis Δfas1 strain which contained the M. tuberculosis fas1 homologue. The M. smegmatis Δfas1 (attB::M. tuberculosis fas1) strain grew more slowly than the parental M. smegmatis strain and was more susceptible to 5-chloro-pyrazinamide. Surprisingly, while the M. smegmatis Δfas1 (attB::M. tuberculosis fas1) strain produced C26:0, it predominantly produced C24:0. These results suggest that the fatty acid elongation that produces C24:0 or C26:0 in vivo is due to a complex interaction among FASI, FabH, and FASII and possibly other systems and is not solely due to FASI elongation, as previously suggested by in vitro studies.
Mycobacterium tuberculosis infects one-third of the world's population, and it is estimated that 1% of the world's population is newly infected with this organism each year (31). The mycobacterial cell wall is a complex structure that plays a role in both M. tuberculosis virulence and drug resistance (12, 16). These features of the mycobacterial cell wall are conferred by a wide variety of unique lipids that compose 60% of the cell wall. Mycolic acids (C74 to C90 α-alkyl β-hydroxyl fatty acids) are the major lipid components of the mycobacterial cell wall and the hallmark of mycobacteria and related species (16). Long-chain saturated fatty acids, which are precursors of cell membrane phospholipids, mycolic acids, and other complex lipids, are generated by the type I fatty acid synthase (FASI) in mycobacteria (4, 6, 16). Mycobacteria are unusual among prokaryotes in that they possess both FASI (typically found in parasites, fungi, and all higher eukaryotes) and the type II fatty acid synthase (FASII), which is found in most prokaryotes and plants. The multifunctional FASI enzyme is a monomer that contains seven separate domains with catalytic activities, including an active site for the prosthetic group 4′-phosphopantetheine of the acyl carrier protein (ACP) (4, 6). Mycobacterial FASI generates C16:0 from acetyl coenzyme A (acetyl-CoA) primers and elongates the molecules to produce C24:0/26:0 fatty acyl-CoA derivatives, which are the precursors of other fatty acid synthases and polyketide systems (8, 16). In contrast, FASII elongates the FASI products to produce meromycolate precursors, which are modified and condensed with C24:0/26:0 to form mycolic acids (8, 16). In vitro studies have shown that mycobacterial FASI produces a unique bimodal distribution of fatty acids (15, 23). In addition to C16:0, C24:0 is produced by the rapidly growing organism Mycobacterium smegmatis (23), and C26:0 is produced by the slow growers M. tuberculosis and Mycobacterium bovis (15).
Since fatty acid synthesis in bacteria is essential for cell survival, the enzymes involved in this pathway have emerged as promising targets for antimicrobial agents (14). The FASII enoyl-ACP reductase was identified as the target of isoniazid and ethionamide, which are first- and second-line tuberculosis drugs (1), as well as a universal bacterial target for triclosan, a consumer antimicrobial agent (13, 18-21, 28). The fungal metabolites cerulenin and thiolactomycin target the condensing enzymes of the bacterial FASII pathway (10, 17, 22, 24, 25). Two studies have shown that 5-chloro-pyrazinamide (5-Cl-PZA) (5, 32) and pyrazinamide (PZA) (32) inhibit M. tuberculosis FASI, indicating that FASI is also a drug target.
The use of PZA, a FASI inhibitor, in tuberculosis chemotherapy has greatly reduced the length of treatment necessary to cure a patient (3, 27). Therefore, new FASI inhibitors could be useful tools for treating tuberculosis. In order to develop a system that allows quick purification of the large quantities of M. tuberculosis FASI necessary for drug testing or structure-function studies, we constructed a recombinant M. smegmatis strain in which the native fas1 gene was deleted and replaced with the M. tuberculosis fas1 gene. In the course of analyzing this recombinant M. smegmatis ΔfasI (attB::M. tuberculosis fas1) strain, which was designated mc22700, we studied the in vivo elongation of C16:0 by mc22700. The data which are presented in this paper allowed us to challenge the concept that C16:0 elongation to produce C24:0 in M. smegmatis or to produce C26:0 in M. tuberculosis is FASI dependent, as described previously (4, 15, 23).
MATERIALS AND METHODS
Bacterial strains and media.
The M. smegmatis strains used in this study are described in Table 1. The strains were grown in Middlebrook 7H9 medium (Difco) supplemented with 10% (vol/vol) ADS enrichment (50 g of albumin, 20 g of dextrose, and 8.5 g of sodium chloride in 1 liter of water), 0.2% (vol/vol) glycerol, and 0.5% (vol/vol) Tween 80 or in Mueller-Hinton broth (Difco) supplemented with 0.5% (vol/vol) Tween 80. The solid media used were the media described above with 1.5% (wt/vol) agar added.
TABLE 1.
M. smegmatis strains used in this study
MIC determination.
The MIC was determined by a broth macrodilution test with an inoculum containing 105 CFU of each strain per ml in either Middlebrook 7H9 or Mueller-Hinton broth. The concentrations of 5-Cl-PZA used in these assays were 60, 40, 30, 25, 20, 10, and 5 μg/ml; a preparation containing no drug was used as a control. The assay was terminated for each strain independently when the optical density at 600 nm (OD600) in the no-drug tube reached 1 to 1.2. The MIC was defined as the first concentration of drug which resulted in no visible growth (OD600, <0.02).
Determination of generation time.
Each strain was grown to an OD600 of 0.5 in Middlebrook 7H9 broth as described above and was diluted 10-fold to obtain the starting inoculum. The OD600 of each culture was determined every 120 min for 16 h. In addition, aliquots of each culture were taken at every other time point and plated to determine the number of CFU. The generation time was obtained by plotting the growth curves resulting from both the OD600 and CFU values.
Transformation experiments.
M. smegmatis strains grown at 37°C to the mid-log phase (OD600, ∼0.7) were washed twice with 10% cold glycerol and resuspended in cold 10% glycerol (1 ml). A plasmid (1.5 μl) was added to each cold cell suspension (150 μl) to transform the strain, and the mixture was electroporated by using the following parameters: 2.5 V, 25 μF, and 1,000 Ω. Middlebrook 7H9 broth (1 ml) was added to the suspension, which was incubated at 37°C for 2 h before plating.
Deletion of M. smegmatis fasI by specialized transduction.
The allelic exchange substrate was formed by amplification of the 5′ and 3′ flanking regions of M. smegmatis fas1 from M. smegmatis genomic DNA. A 850-bp 5′ flanking region from nucleotide 386 of M. smegmatis fas1 to a noncoding sequence upstream from the fas1 start codon and a 739-bp 3′ flanking region from nucleotide 7384 of M. smegmatis to nucleotide 8123 of M. smegmatis fas1 were amplified (GenBank accession number AY205337). The amplified flanking regions were cloned with a TA cloning kit into the PCR2.1 vector (Invitrogen) for sequencing and subsequent cloning. The substrate used for allelic exchange was formed by cloning the 5′ and 3′ flanking regions of fas1 described above into pJSC285, a cloning vector containing a bacteriophage lambda cos site, a PacI site, and a kanamycin resistance gene flanked by resolvase sites. The allelic exchange substrate on plasmid pYUB978 was packaged into phMSG104 (11) to create phAE978, as previously described (2, 11). phAE978 was used to transduce mc2155 and mc22670 (32).
Transduction of M. smegmatis strains.
M. smegmatis bacilli were grown to an OD600 of 0.8 in Luria-Bertani medium supplemented with 0.2% glycerol and 0.1% Tween 80. The cultures (10 ml) were washed twice in mycobacteriophage buffer (50 mM Tris HCl [pH 7.6], 150 mM NaCl, 10 mM, MgCl2, 2 mM CaCl2) to remove any traces of the Tween 80 detergent. Adsorption of phAE978 mycobacteriophage to the washed bacilli was carried out at a multiplicity of infection of 10, and the mixture was incubated at 37°C, the nonpermissive temperature, for 30 min. The bacilli were pelleted, plated on Luria-Bertani medium plates containing 25 μg of kanamycin per ml, and incubated at 37°C. Kanamycin-resistant colonies were screened for allelic exchange by Southern blotting by using PCR products from both M. smegmatis and M. tuberculosis fas1 as probes.
Radiolabeling of fatty acids with [1-14C]acetate and analysis by HPLC.
Cultures of M. smegmatis strains (25 ml) were grown at 37°C to the mid-log phase (OD600, ∼0.8) in Middlebrook 7H9 broth and were labeled with [1-14C]acetate (0.3 μCi/ml) for 1 h. Cell pellets were saponified by using a 25% methanolic KOH solution for 3 h at reflux and then acidified. The fatty acids were extracted with chloroform and derivatized to UV-absorbing p-bromophenacyl fatty acids (30) by using an Alltech kit (catalog no. 18036). The p-bromophenacyl fatty acid esters were then analyzed by high-performance liquid chromatography (HPLC) by using a reverse-phase C18 column, a diode array detector, and an IN/US β-RAM flowthrough beta-gamma radiation detector. The elution system was CH3CN-H2O (83/17, vol/vol) for 20 min, followed by a linear increase to 100% CH3CN for 2 min and then 100% acetonitrile for another 16 min (flow rate, 2 ml/min). Use of this system resulted in separation of saturated and unsaturated fatty acids (from C12:0 to C26:0). The chromatogram's peaks were identified by comparison with the chromatograms of p-bromophenacyl fatty acid ester standards (30).
Nucleotide sequence accession number.
The nucleotide sequence of the M. smegmatis fatty acid synthase gene (fas1) and the ACP synthase gene (acpS) have been deposited in the GenBank database under accession number AY205337.
RESULTS
Construction of mc22700, an M. smegmatis Δfas1 mutant containing the M. tuberculosis fasI homologue.
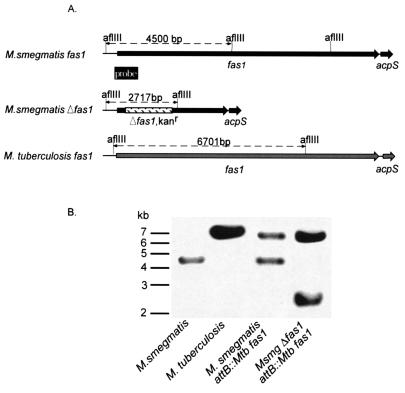
Since fatty acid synthases are essential for bacteria, we hypothesized that fas1 is an essential gene for mycobacteria. To delete the M. smegmatis fas1 gene, we first generated mc22670 (Table 1), an mc2155 M. smegmatis derivative containing the M. tuberculosis fas1 gene integrated into the attB site, by using pYUB970, a site-specific integrating cosmid (from a genomic library of M. tuberculosis H37Rv) bearing M. tuberculosis fas1 and its 5′ and 3′ flanking regions. The native M. smegmatis fas1 gene was then deleted by specialized transduction (2). The allelic exchange substrate was formed by amplification of the 5′ and 3′ flanking regions of M. smegmatis fas1 from M. smegmatis genomic DNA. Kanamycin-resistant (Kanr) transductants were observed for mc22670 but not for wild-type strain mc2155; these results were the results expected for an essential gene that was found to be a drug target (5, 32). Kanr colonies were screened for allelic exchange by Southern blotting by using PCR products from both M. smegmatis and M. tuberculosis fas1 as probes. The Kanr colonies were found to contain a shifted band that was consistent with allelic exchange with a Δfas1 Kanr construct, as shown in Fig. 1; this confirmed construction of mc22700, an M. smegmatis strain expressing M. tuberculosis fas1 (Table 1).
FIG. 1.
Allelic exchange in mc22670, a fas1 merodiploid strain of M. smegmatis, results in mc22700, an M. smegmatis strain bearing M. tuberculosis fas1. (A) Map of the fas1 genomic region of wild-type and mutant strains of M. smegmatis (following allelic exchange) and wild-type M. tuberculosis fas1, showing AflIII sites. (B) Southern blot of AflIII-digested genomic DNA from different strains probed with the fragments shown in panel A. Mtb, M. tuberculosis; Msmg, M. smegmatis.
The ability to exchange M. smegmatis FASI for the M. tuberculosis homologue provided an improved expression system with a rapidly growing, nonpathogenic mycobacterium strain. Moreover, it has been shown previously that M. smegmatis, unlike M. tuberculosis and M. bovis BCG, tolerates multiple copies of M. tuberculosis fas1 (32). Thus, overexpression of M. tuberculosis FASI under different promoters is feasible in M. smegmatis and could be followed by allelic exchange of the M. smegmatis fas1 gene by using the transduction system which we developed, as shown by the formation of strain mc22700.
Growth characteristics and 5-Cl-PZA susceptibility of mc22700.
Strain mc22700 differs from the parental M smegmatis strains in several interesting properties. First, mc22700 was found to have a longer generation time (250 min in broth, compared to 210 min for wild-type strain mc2155 or mc22670). The generation time was determined by measuring the OD600 and counting the actual CFU during exponential growth in Middlebrook 7H9 medium and then plotting the resulting growth curves. The slow-growth phenomenon was independent of the medium used. A lower growth rate was also observed in solid media, as single colonies of mc22700 were visualized 16 h later than single colonies of mc2155 or mc22670 were visualized.
We compared the susceptibilities of wild-type strain mc2155, strain mc22670, and strain mc22700 to 5-Cl-PZA. Previously, FASI was identified as a potential drug target for 5-Cl-PZA in M. tuberculosis (32). Another study confirmed that 5-Cl-PZA inhibits mycobacterial FASI (5). The MIC of 5-Cl-PZA were 10 μg/ml for the mc22700 strain and 25 μg/ml for mc2155 and mc22670, as previously described (32). The increased susceptibility of mc22700 to 5-Cl-PZA is consistent with the previous finding that M. tuberculosis is more susceptible to 5-Cl-PZA than M. smegmatis (9, 32). This finding complements the results of a previous study in which the effect of a multicopy fas1 gene on 5-Cl-PZA resistance was described (32). Multiple copies of M. tuberculosis fas1 conferred only modest resistance to 5-Cl-PZA in M. smegmatis (there was 2.5-fold increase in the MIC), while a single copy of M. tuberculosis fas1 (mc22670) did not confer 5-Cl-PZA resistance in M. smegmatis. In contrast, for a diploid strain containing one extra copy of M. smegmatis fas1, the MIC of this drug was fivefold greater (32). Taken together, these results show that M. tuberculosis is more susceptible to 5-Cl-PZA than M. smegmatis. Furthermore, the MICs of cerulenin, a FASI (and possibly FASII) inhibitor (22), and isoniazid, a FASII inhibitor (1, 29, 30), were the same for mc22700 and the parental M. smegmatis strains.
mc22700, unlike mc2155, produces C26:0 fatty acid, but C24:0 is still the predominant end product.
Previous biochemical studies showed that purified FASI of M. smegmatis produces C16:0/C24:0, whereas purified FASI from M. tuberculosis or M. bovis BCG produces C16:0/C26:0 (15, 23). To test the activity of M. tuberculosis FASI in synthesizing C16-C24:0/C26:0 fatty acids in vivo, we monitored [1-14C]acetate incorporation into lipids in wild-type strain mc2155, strain mc22670 (M. smegmatis [attB::M. tuberculosis fas1]), and mc22700 (M. smegmatis Δfas1 [attB::M. tuberculosis fas1]). This experiment was repeated three times, and the results of a typical experiment are shown in Table 2 and Fig. 2.
TABLE 2.
Percentages of saturated fatty acids (C16 to C26) in mc2155, mc22670, and mc22700a
| Strain | % of:
|
|||||
|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C20:0 | C22:0 | C24:0 | C26:0 | |
| mc2155 | 56.9 | 9.3 | 4.4 | 6.7 | 18.3 | 0.0 |
| mc22670 | 39.9 | 11.6 | 3.6 | 5.8 | 17.7 | 0.0 |
| mc22700 | 31.9 | 29.7 | 3.4 | 6.0 | 11.7 | 4.8 |
The cumulative percentages are not 100% because the values for other saturated and unsaturated fatty acids, such as C12:0, C14:0, and C18:1, are not included.
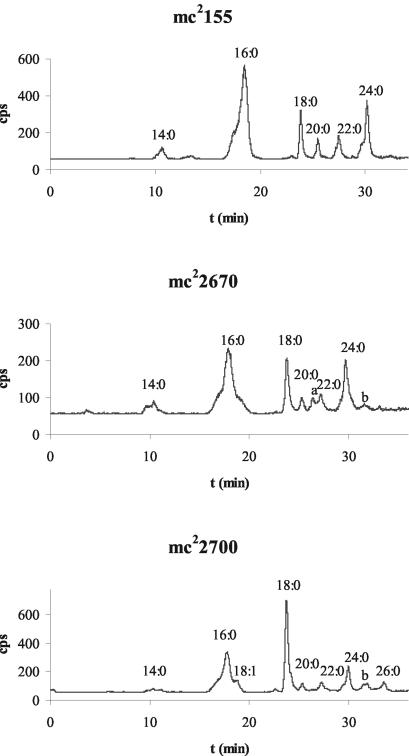
FIG. 2.
HPLC analysis of 1-14C-labeled fatty acids extracted from mc2155 and mc22700 after labeling with [1-14C]acetate for 30 min. The retention times (t) for the p-bromophenacyl fatty acid esters under the elution conditions described previously (30) are as follows: C14:0, 10.5 min; C16:0, 17.8 min; C18:1, 18.9 min; C18:0, 23.8 min; C20:0, 25.3 min; C22:1, 26.5 min (peak a); C22:0, 27.3 min; C24:0, 29.9 min; C26:1,31.6 min (peak b); and C26:0, 33.6 min.
We found that the characteristic C16:0-C24:0 fatty acid bimodal distribution in M. smegmatis was similar for wild-type strain mc2155 and the merodiploid fas1 strain mc22670 (Fig. 2). Surprisingly, the characteristic end product of M. tuberculosis FASI, C26:0, was not detected in mc22670, the strain containing both M. smegmatis fas1 and M. tuberculosis fas1. In contrast, mc22700 produced both C24:0 and C26:0. However, C24:0 was more abundant than C26:0 in this strain (Table 2 and Fig. 2) despite the deletion of M. smegmatis fas1 and replacement of this gene by M. tuberculosis fas1. The similarity of the fatty acid profiles of the merodiploid strain of M. smegmatis and the wild-type M. smegmatis strain is compatible with the dominance of M. smegmatis fas1 in the host strain over M. tuberculosis fas1. The production of larger amounts of C24:0 than of C26:0 in mc22700 was unexpected since in vitro studies of M. smegmatis and M. bovis BCG FASI demonstrated that M. smegmatis synthesizes C24:0 and M. bovis BCG synthesizes C26:0 (15, 23). Since the M. tuberculosis fas1 gene replaced M. smegmatis fas1 in mc22700, it was predicted that this strain would have a fatty acid profile similar to that of M. tuberculosis or M. bovis BCG strains. The presence of larger amounts of C24:0 than of C26:0 despite the deletion of M. smegmatis fas1 suggests that C16:0 elongation in vivo is not solely determined by FASI activity, as previously shown by in vitro studies in which purified FASI was used (15, 23).
Unlike the predominant synthesis of C24:0 over C26:0 in mc22700, the C18:0/C16:0 ratio was a direct result of the replacement of M. smegmatis fas1 by M. tuberculosis fas1 (Table 2). Introducing M. tuberculosis fas1 into a mycobacterial strain increased the amount of C18:0 synthesized by the strain. The C18:0/C16:0 ratios were found to be 0.16 for wild-type M. smegmatis, 0.29 for mc22670, and 0.93 for mc22700. Previous in vivo studies of M. smegmatis, M. bovis BCG, and M. tuberculosis showed that the C18:0/C16:0 ratios were 0.17 for M. smegmatis (30) and 0.6 to 1 for either M. bovis BCG (30) or M. tuberculosis (32). In vitro studies of FASI activity have shown that M. bovis BCG FASI produces more C18:0 than C16:0 (15), while M. smegmatis FASI produces more C16:0 than C18:0 (23). Thus, our in vivo results for recombinant strain mc22700 confirmed the previous finding that M. tuberculosis FASI generates more C18:0 than M. smegmatis FASI generates.
DISCUSSION
The profiles of C16:0-C24:0/26:0 synthesis are different for the wild-type strain of M. smegmatis and strain mc22700. In these strains, the only variable was the FASI system (M. smegmatis fas1 in mc2155 and M. tuberculosis fas1 in mc22700). The observation that mc22700 and mc2155 synthesize C26:0 and C24:0, respectively, and the observation that replacement of the M. smegmatis fas1 gene by the M. tuberculosis homologue in M. smegmatis does not result in the C16:0/C26:0 bimodal fatty acid profile observed in vitro (15) suggest that this fatty acid profile is determined not only by FASI but also by the interaction of FASI with FASII. Previous studies with M. tuberculosis FabH, a β-ketoacyl synthase that uses acyl-CoA as its substrate, suggested that this enzymatic system acts as an interface between the type I and type II fatty acid synthase systems by funneling acyl-CoA formed by the FASI system into the FASII elongating acyl-ACP primers (7). We hypothesize that the differences in the fatty acid profiles of mycobacterial species are due to FASI interactions with FabH and FASII. Further studies of fabH systems in M. smegmatis may delineate the differences in the fatty acid profiles of M. smegmatis and M. tuberculosis. Moreover, the origin of FASI (M. smegmatis or M. tuberculosis) seems to play an important role in the amount of C18:0 produced by a strain. The C18:0/C16:0 ratio found for mc22700 is similar to the ratio found for M. tuberculosis strains, while the C18:0/C16:0 ratio found for mc22670 is only reminiscent of M. tuberculosis FASI activity in the fas1 merodiploid strain.
M. tuberculosis FASI is an essential enzyme and is unique among bacterial species, and therefore it is an attractive drug target. The ability to express M. tuberculosis fas1 in M. smegmatis should provide a relatively rapid purification system for M. tuberculosis FASI, as previously described (4, 5, 15), without dependence on expression of the enzyme in Escherichia coli (which has been unsuccessful so far). Moreover, it has been shown previously that M. smegmatis, unlike M. tuberculosis and M. bovis BCG, tolerates multiple copies of M. tuberculosis fas1 (32). Repeated attempts to transform the slowly growing mycobacteria M. bovis BCG and M. tuberculosis with multiple copies of fas1 yielded no colonies, while M. smegmatis was readily transformed with multiple copies of fas1 (32). Thus, M. smegmatis is an optimal bacterial system for overexpression of M. tuberculosis FASI. The specialized transduction system for M. smegmatis fas1 described here should allow us to replace M. smegmatis FASI with overexpressed M. tuberculosis FASI under different promoters, which should facilitate large-scale purification. Although the FASI proteins from M. tuberculosis and M. smegmatis exhibit 90% homology, it is preferable to study the FASI protein from the pathogenic organism M. tuberculosis, especially if structure-function studies leading to rationale drug design for M. tuberculosis FASI inhibitors are planned. M. tuberculosis FASI studies should lead to development of new analogs of PZA and new classes of effective FASI inhibitors. In addition, the concern about emergence of drug resistance when universal bacterial sites are targeted should be eliminated as the FASI site is a unique site in bacteria. Improved agents that exhibit activity against mycobacterial FASI may provide new options for treating M. bovis, Mycobacterium avium, and drug-resistant M. tuberculosis infections.
Acknowledgments
We thank Jeff Cox for providing plasmid pJSC285.
This work was supported by grant AI43268 from the NIH.
REFERENCES
- 1.Banerjee, A., E. Dubnau, A. Quemard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. de Lisle, and W. R. Jacobs, Jr. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. [DOI] [PubMed] [Google Scholar]
- 2.Bardarov, S., S. Bardarov, Jr., M. S. Pavelka, Jr., V. Sambandamurthy, M. Larsen, J. Tufariello, J. Chan, G. Hatfull, and W. R. Jacobs, Jr. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148:3007-3017. [DOI] [PubMed] [Google Scholar]
- 3.Bass, J. B., Jr., L. S. Farer, P. C. Hopewell, R. O'Brien, R. F. Jacobs, F. Ruben, D. E. Snider, Jr., and G. Thornton. 1994. Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and The Centers for Disease Control and Prevention. Am. J. Respir. Crit. Care Med. 149:1359-1374. [DOI] [PubMed] [Google Scholar]
- 4.Bloch, K. 1977. Control mechanisms for fatty acid synthesis in Mycobacterium smegmatis. Adv. Enzymol. Relat. Areas Mol. Biol. 45:1-84. [DOI] [PubMed] [Google Scholar]
- 5.Boshoff, H. I., V. Mizrahi, and C. E. Barry III. 2002. Effects of pyrazinamide on fatty acid synthesis by whole mycobacterial cells and purified fatty acid synthase I. J. Bacteriol. 184:2167-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brindley, D. N., S. Matsumura, and K. Bloch. 1969. Mycobacterium phlei fatty acid synthase—a bacterial multienzyme complex. Nature 224:666-669. [Google Scholar]
- 7.Choi, K. H., L. Kremer, G. S. Besra, and C. O. Rock. 2000. Identification and substrate specificity of beta-ketoacyl (acyl carrier protein) synthase III (mtFabH) from Mycobacterium tuberculosis. J. Biol. Chem. 275:28201-28207. [DOI] [PubMed] [Google Scholar]
- 8.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 9.Cynamon, M. H., R. J. Speirs, and J. T. Welch. 1998. In vitro antimycobacterial activity of 5-chloropyrazinamide. Antimicrob. Agents Chemother. 42:462-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Agnolo, G., I. S. Rosenfeld, J. Awaya, S. Omura, and P. R. Vagelos. 1973. Inhibition of fatty acid synthesis by the antibiotic cerulenin. Specific inactivation of beta-ketoacyl-acyl carrier protein synthetase. Biochim. Biophys. Acta 326:155-156. [DOI] [PubMed] [Google Scholar]
- 11.Glickman, M. S., S. M. Cahill, and W. R. Jacobs, Jr. 2001. The Mycobacterium tuberculosis cmaA2 gene encodes a mycolic acid trans-cyclopropane synthetase. J. Biol. Chem. 276:2228-2233. [DOI] [PubMed] [Google Scholar]
- 12.Glickman, M. S., J. S. Cox, and W. R. Jacobs, Jr. 2000. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 5:717-727. [DOI] [PubMed] [Google Scholar]
- 13.Heath, R. J., J. Li, G. E. Roland, and C. O. Rock. 2000. Inhibition of the Staphylococcus aureus NADPH-dependent enoyl-acyl carrier protein reductase by triclosan and hexachlorophene. J. Biol. Chem. 275:4654-4659. [DOI] [PubMed] [Google Scholar]
- 14.Heath, R. J., S. W. White, and C. O. Rock. 2001. Lipid biosynthesis as a target for antibacterial agents. Prog. Lipid Res. 40:467-497. [DOI] [PubMed] [Google Scholar]
- 15.Kikuchi, S., D. L. Rainwater, and P. E. Kolattukudy. 1992. Purification and characterization of an unusually large fatty acid synthase from Mycobacterium tuberculosis var. bovis BCG. Arch. Biochem. Biophys. 295:318-326. [DOI] [PubMed] [Google Scholar]
- 16.Kolattukudy, P. E., N. D. Fernandes, A. K. Azad, A. M. Fitzmaurice, and T. D. Sirakova. 1997. Biochemistry and molecular genetics of cell-wall lipid biosynthesis in mycobacteria. Mol. Microbiol. 24:263-270. [DOI] [PubMed] [Google Scholar]
- 17.Kremer, L., J. D. Douglas, A. R. Baulard, C. Morehouse, M. R. Guy, D. Alland, L. G. Dover, J. H. Lakey, W. R. Jacobs, Jr., P. J. Brennan, D. E. Minnikin, and G. S. Besra. 2000. Thiolactomycin and related analogues as novel anti-mycobacterial agents targeting KasA and KasB condensing enzymes in Mycobacterium tuberculosis. J. Biol. Chem. 275:16857-16864. [DOI] [PubMed] [Google Scholar]
- 18.Kuo, M. R., H. R. Morbidoni, D. Alland, S. F. Sneddon, B. B. Gourlie, M. M. Staveski, M. Leonard, J. S. Gregory, A. D. Janjigian, C. Yee, J. M. Musser, B. Kreiswirth, H. Iwamoto, R. Perozzo, W. R. Jacobs, Jr., J. C. Sacchettini, and D. A. Fidock. 2003. Targeting tuberculosis and malaria through inhibition of enoyl reductase: compound activity and structural data. J. Biol. Chem. 278:20851-20859. [DOI] [PubMed] [Google Scholar]
- 19.Marcinkeviciene, J., W. Jiang, L. M. Kopcho, G. Locke, Y. Luo, and R. A. Copeland. 2001. Enoyl-ACP reductase (FabI) of Haemophilus influenzae: steady-state kinetic mechanism and inhibition by triclosan and hexachlorophene. Arch. Biochem. Biophys. 390:101-108. [DOI] [PubMed] [Google Scholar]
- 20.McMurry, L. M., P. F. McDermott, and S. B. Levy. 1999. Genetic evidence that InhA of Mycobacterium smegmatis is a target for triclosan. Antimicrob. Agents Chemother. 43:711-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parikh, S. L., G. Xiao, and P. J. Tonge. 2000. Inhibition of InhA, the enoyl reductase from Mycobacterium tuberculosis, by triclosan and isoniazid. Biochemistry 39:7645-7650. [DOI] [PubMed] [Google Scholar]
- 22.Parrish, N. M., F. P. Kuhajda, H. S. Heine, W. R. Bishai, and J. D. Dick. 1999. Antimycobacterial activity of cerulenin and its effects on lipid biosynthesis. J. Antimicrob. Chemother. 43:219-226. [DOI] [PubMed] [Google Scholar]
- 23.Peterson, D. O., and K. Bloch. 1977. Mycobacterium smegmatis fatty acid synthetase. Long chain transacylase chain length specificity. J. Biol. Chem. 252:5735-5739. [PubMed] [Google Scholar]
- 24.Slayden, R. A., and C. E. Barry. 2002. The role of KasA and KasB in the biosynthesis of meromycolic acids and isoniazid resistance in Mycobacterium tuberculosis. Tuberculosis (Edinburgh) 82:149-160. [DOI] [PubMed] [Google Scholar]
- 25.Slayden, R. A., R. E. Lee, J. W. Armour, A. M. Cooper, I. M. Orme, P. J. Brennan, and G. S. Besra. 1996. Antimycobacterial action of thiolactomycin: an inhibitor of fatty acid and mycolic acid synthesis. Antimicrob. Agents Chemother. 40:2813-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snapper, S. B., R. E. Melton, S. Mustafa, T. Kieser, and W. R. Jacobs, Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911-1919. [DOI] [PubMed] [Google Scholar]
- 27.Steele, M. A., and R. M. Des Prez. 1988. The role of pyrazinamide in tuberculosis chemotherapy. Chest 94:845-850. [DOI] [PubMed] [Google Scholar]
- 28.Surolia, N., and A. Surolia. 2001. Triclosan offers protection against blood stages of malaria by inhibiting enoyl-ACP reductase of Plasmodium falciparum. Nat. Med. 7:167-173. [DOI] [PubMed] [Google Scholar]
- 29.Takayama, K., L. Wang, and H. L. David. 1972. Effect of isoniazid on the in vivo mycolic acid synthesis, cell growth, and viability of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2:29-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vilcheze, C., H. R. Morbidoni, T. R. Weisbrod, H. Iwamoto, M. Kuo, J. C. Sacchettini, and W. R. Jacobs, Jr. 2000. Inactivation of the inhA-encoded fatty acid synthase II (FASII) enoyl-acyl carrier protein reductase induces accumulation of the FASI end products and cell lysis of Mycobacterium smegmatis. J. Bacteriol. 182:4059-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. March 2004, revision date. Tuberculosis. Fact sheet no. 104. [Online.] World Health Organization, Geneva, Switzerland. http://www.who.int/mediacentre/factsheet/who104/en/index.html.
- 32.Zimhony, O., J. S. Cox, J. T. Welch, C. Vilcheze, and W. R. Jacobs, Jr. 2000. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat. Med. 6:1043-1047. [DOI] [PubMed] [Google Scholar]