Abstract
It is reported that 6% of children and 3% of adults have food allergies, with studies suggesting increased prevalence worldwide over the last few decades. Despite this, our diagnostic capabilities and techniques for managing patients with food allergies remain limited. We have conducted a systematic review of literature published within the last 5 years on the diagnosis and management of food allergies. While the gold standard for diagnosis remains the double-blind, placebo-controlled food challenge, this assessment is resource intensive and impractical in most clinical situations. In an effort to reduce the need for the double-blind, placebo-controlled food challenge, several risk-stratifying tests are employed, namely skin prick testing, measurement of serum-specific immunoglobulin E levels, component testing, and open food challenges. Management of food allergies typically involves allergen avoidance and carrying an epinephrine autoinjector. Clinical research trials of oral immunotherapy for some foods, including peanut, milk, egg, and peach, are under way. While oral immunotherapy is promising, its readiness for clinical application is controversial. In this review, we assess the latest studies published on the above diagnostic and management modalities, as well as novel strategies in the diagnosis and management of food allergy.
Keywords: skin prick testing, oral challenge, specific IgE, component testing, oral immunotherapy, epinephrine autoinjector
Introduction
European studies estimate the lifetime prevalence of food allergy is 17.3% and the point prevalence 6%.1 Recent studies suggest an increased prevalence worldwide over the last few decades of food allergy and food-induced anaphylaxis.2,3 Despite the increasing prevalence of food allergy, our diagnostic and management strategies have remained relatively unchanged over time. The double-blind, placebo-controlled food challenge (DBPCFC) is considered the gold standard for diagnosis of food allergies, but is rarely employed by physicians outside of an academic context. It is estimated that DBPCFCs have a false negative rate ranging from 2%–5% and a false positive rate near 5.4%–12.9%.4 The open food challenge (OFC) is a more viable option for most clinicians, though it is not without its own pitfalls.
Most clinicians rely on skin prick testing (SPT) and serum-specific immunoglobulin E (sIgE) testing to establish the diagnosis of food allergy. SPT is readily performed in the clinical setting, and a near infinite number of foods can be evaluated, although extracts are not yet standardized. It is typically the first test used in the evaluation of food allergy. Measurement of sIgE for a wide variety of foods is available in most centers. These tests both evaluate the presence of IgE, which determines sensitization but does not necessarily correlate with clinical reactivity. In an effort to improve diagnostic accuracy, component testing allows the allergist to examine IgE levels against a particular protein of the culprit food. Examining sensitization profiles to specific food allergen components aims to distinguish between sensitized and truly reactive patients.5 Some proposed novel diagnostic tests are in development, but are not yet ready for clinical application.
Management of food allergy relies primarily on allergen avoidance, with prompt emergency care for accidental exposure. Food allergen avoidance is not always effective, as allergens such as milk and egg may be hidden in foods. An estimated 10%–20% of individuals with a diagnosis of anaphylaxis experience recurrent reactions.3,6,7 Despite clear guidelines advising prompt use of an epinephrine autoinjector (EAI) in anaphylaxis, many patients and families do not use an EAI, possibly due to inadequate knowledge and anxiety.8,9 Moreover, the diagnosis of food allergy and the need to carry an EAI are associated with negative effects on quality of life for patients and families alike.10 A recent review found that there were no robust studies examining the effectiveness of injectable epinephrine, antihistamines, systemic glucocorticosteroids, or methylxanthines in the management of anaphylaxis.11 As prompt use of an EAI is the most important step in the acute management of anaphylaxis, we have focused on this treatment modality. Depending on the allergen, many individuals will have lifelong food allergies. Hence, it would be advantageous to have a treatment strategy that allows for food reintroduction and obviates the need to carry an EAI. As such, recent developments in immunotherapy for foods are a very exciting prospect. Incorporating baked milk and egg in the diet may be viewed as a form of immunotherapy for these allergens, while there are other protocols under investigation to desensitize and potentially induce tolerance through gradual introduction of the raw allergen. Other allergens under investigation as candidates for immunotherapy include peanut and peach. In this systematic review, we aim to assess existing and new diagnostic modalities and management options for food allergy. In particular, we will focus on literature published in the last 5 years.
Methods
We searched the PubMed database for scientific literature published between January 13, 2009 and January 13, 2014 using the following search criteria: “food allergy” AND “management” OR “diagnosis”. We used the following filters in our search: clinical trial, abstract available, and studies done on humans in any language. Of the available articles, we selected those that were relevant to this review based on the abstract. A team of six readers then further reviewed these articles. The articles have been summarized in regard to food allergy diagnosis and management in Tables 1 and 2, respectively. Upon initial search of PubMed using the above terms, 12,139 titles appeared. After applying the filters as described, 217 titles remained. Once abstracts had been reviewed, 100 articles were warranted for inclusion in the study (Figure 1).
Table 1.
Diagnosis of food allergy
| Modality | Allergen | Type of study | Authors, publication year | Reference | Findings | Comments |
|---|---|---|---|---|---|---|
| SPT | Milk | Retrospective cohort | Bellini et al, 2011 | 12 | Used end-point prick test to help predict results of OFC in milk-allergic children. Wheal diameter >4.5 mm in SPT with a 1/10,000 dilution of fresh milk was best able to discriminate milk-allergic patients from tolerant ones. | End-point titration is an accessible and safe test and should be considered in the diagnosis of milk allergy. This study used a cohort of patients with known milk allergy, so results may not be generalizable. |
| SPT and specific IgE | Peanut | Prospective cohort | Johannsen et al, 2011 | 14 | Study of 49 preschoolers with peanut sensitization but unknown reactivity. Half of the children reacted to challenge. SPT wheal diameter of >7 mm predicted positive challenge with 83% sensitivity and 84% NPV. Specific IgE >2 kUA/L showed sensitivity of 79% and 80% NPV. Using a combination of SPT and specific IgE, results increased sensitivity to 96% and NPV to 95%. | Half of peanut-sensitized preschoolers will pass OFC. If SPT wheal diameter is <7 mm and specific IgE <2 kUA/L, there is a 5% chance of reaction to the OFC. |
| SPT | Egg | Cross-sectional | Tripodi et al, 2009 | 13 | 1:256 dilution of egg extract used for SPT with wheal size >3 mm was 95% specific and 100% sensitive in distinguishing a positive from a negative OFC. | As extracts are not standardized, these study results may not be reproducible. |
| SPT and specific IgE | Milk and egg | Retrospective cohort | Mehl et al, 2012 | 17 | 23% of children with milk allergy had discordant SPT and specific IgE results, as did 10% with egg allergy. For milk-allergic children with a positive OFC, 84% had a positive SPT response (wheal >3 mm) and 87% had specific IgE >0.35 kUA/L. For egg-allergic children, 93% with a positive SPT response had a positive OFC, and 96% with positive challenge had specific IgE >0.35 kUA/L. | SPT and specific IgE should not be used interchangeably. Low thresholds were used to qualify a positive SPT or specific IgE in this study. |
| Specific IgE, component testing | Peanut | Cross-sectional | Chiang et al, 2010 | 22 | 89.5% of Asian children with peanut allergy had detectable Ara h 1 or Ara h 2. | Milk and egg SPT and sIgE levels associated with a positive OFC were similar in Asian children with peanut allergy and Western counterparts. This study did not confirm peanut allergy with oral challenge. |
| Specific IgE | Peanut | Cross-sectional | van Nieuwaal et al, 2010 | 18 | 90% failed peanut challenge at specific IgE 24.8 kUA/L, and95% failed at 43.8 kUA/L. | This study reported higher specific IgE cutoff levels than others, perhaps due to examining a different population. |
| Specific IgE, component testing | Peanut | Experimental technique | Lin et al, 2012 | 23 | Specific peptides Ara h 2_10, Ara h 2_18, Ara h 1_16, and Ara h 3_140 had 90% sensitivity and 95% specificity. | This study used a novel approach to the diagnosis of peanut allergy but is not yet ready for clinical application. |
| Specific IgE, component testing | Peanut | Cross-sectional | Asarnoj et al, 2012 | 21 | 89.5% of children who were Ara h 8 reactive could safely consume peanut. | Most children who were Ara h 8-positive and reacted had mild reactions, though some had systemic reactions. |
| Specific IgE, component testing | Peanut | Cross-sectional | Eller and Bindslev-Jensen, 2013 | 20 | Best correlation between IgE and clinical thresholds was with using Ara h 2 levels >1.63 kUA/L which had a specificity of 100% and sensitivity of 70%. | Ara h 2 may be helpful in distinguishing between peanut allergy and sensitization. |
| Specific IgE, component testing | Peanut | Cross-sectional | Lieberman et al, 2013 | 19 | IgE to peanut (>0.35 kUA/L) was the most sensitive test (93%) for predicting results of OFC. Ara h 2 was most specific (92%) and had the best PPV (94%). | This study used patients from three sites, some of whom were on oral immunotherapy for peanut. |
| Salivary specific IgA | Peanut | Prospective cohort | Kulis et al, 2012 | 24 | Salivary peanut-specific IgA increased in patients receiving SLIT compared to controls, but did not change significantly over a 12-month period of treatment. | Increased levels of salivary peanut-specific IgA are induced by the SLIT intervention although it is not clear if it might serve to predict effectiveness of SLIT. |
| Specific IgE, component testing | Egg | Retrospective cohort | Montesinos et al, 2010 | 25 | Egg white-specific IgE of 1.52, 1.35, and 2.59 kUA/L predicted reactivity in patients aged 2–3, 3–4, and 4–5 years, respectively. | Retrospective study finding that children who outgrow egg allergy had significantly lower specific IgE to egg white, OVA, and OVM. |
| Specific IgE, component testing | Egg | Cross-sectional | Alessandri et al, 2012 | 27 | 94% of Gal d 1-negative patients tolerated boiled egg and95% of Gal d 1-positive patients reacted to raw egg. | Gal d 1 IgE reactivity is a good predictor of clinical egg allergy. This study does not discuss baked egg versus boiled egg. |
| Specific IgE, component testing | Egg | Cross-sectional | Caubet et al, 2012 | 26 | In children who were allergic to both raw and baked egg there were higher ratios of specific IgE/IgG for OVA and OVM antigens compared to children who could tolerate baked egg. IgG4 levels themselves did not differ significantly between the two groups. | IgE/IgG4 ratio may be useful in diagnosis of baked egg-tolerant children. |
| Specific IgE, SPT | Sesame | Cross-sectional | Permaul et al, 2009 | 28 | Specific IgE >7 kUA/L was >90% specific. SPT wheal diameter of >6 mm was >90% specific. | This study offers SPT and specific IgE values to help predict the likelihood of positive reaction in OFC to sesame. |
| Specific IgE | Wheat and soy | Cross-sectional | Komata et al, 2009 | 29 | Median specific IgE in wheat-allergic children was 4.31 kUA/L, and in soybean-allergic children 3.89 kUA/L. | Increasing levels of specific IgE are associated with increased risk of failed OFC in wheat- and soybean-allergic patients. In wheat allergy, age influences this relationship, with younger children being more likely to react when specific IgE levels were low. |
| OFC | Milk | Cross-sectional | Correa et al, 2010 | 111 | OFC in Brazilian children on a milk-free diet to assess for immediate and delayed reactions (up to 30 days). 23.1% of patients had a positive challenge. | This study did not assess IgE-mediated illness. Those with a history of anaphylaxis were excluded from the study. |
| OFC | Milk | Prospective cohort | Mendonça et al, 2012 | 37 | 46 children with a clinical history of reaction to milk and a positive SPT response underwent challenge. Challenge was positive in 41.3%. Cutaneous symptoms were the most common (73.7%), followed by respiratory (57.9%) and gastrointestinal (36.8%). Reactions were classified as mild (57.9%), moderate (36.8%), or severe (5.3%). Epinephrine was not used in any of the patients. | OFC is safe and effective in establishing the diagnosis of milk allergy. |
| SPT, specific IgE, OFC | Milk(baked) | Retrospective chart review | Bartnikas et al, 2012 | 39 | Among 35 baked milk challenges, 83% passed. Of the six who failed, 50% passed the initial challenge but developed symptoms at home with ongoing exposure. Children with an SPT wheal diameter of <12 mm were 90% likely to pass the baked milk challenge. No child with wheal diameter of <7 mm failed the baked milk challenge. | Most milk-allergic children tolerate baked milk. Some children who initially pass the baked milk challenge may develop symptoms with ongoing exposure at home. |
| OFC | Milk | Prospective cohort | Dambacher et al, 2013 | 38 | 116 participants underwent OFC for milk. In 66%, diagnosis of milk allergy was rejected. Of the remaining patients, 32% had an acute reaction, 39% had a late reaction, and 29% had both reactions. Infants had a higher minimum eliciting dose than older children. | OFC is important in establishing the diagnosis of milk allergy and helps to avoid unnecessary elimination diets. |
| OFC | Milk, egg, peanut | Retrospective chart review | Mudd et al, 2009 | 112 | 41% of patients who failed their initial OFC to milk, peanut, or egg passed a subsequent challenge to the same food. Severe reaction with the initial challenge was a predictor of failure of subsequent challenge with milk. | As a retrospective chart review, there may be several uncontrolled factors. |
| OFC | Milk, egg, cod, soy, wheat | Cross-Sectional | Winberg et al, 2013 | 44 | Validation study of recipes used in DBPCFC for milk, egg, soy, cod, and wheat. 275 children aged 8–10 or 14–15 years could not detect any sensorial differences between the active culprit food and placebo for any of the challenge foods. | Provides a range of validated recipes for DBPCFC for several foods that are easy to prepare and use the same liquid test vehicle. |
| OFC | Egg | Prospective cohort | Escudero et al, 2013 | 43 | Challenged egg-allergic patients to both dried egg white and raw egg white. 25% of patients reacted to both dried egg white and raw egg white. 75% did not react to either. | Dried egg white is suitable for use in OFC and has some advantages over raw egg. |
| OFC | Peanut | Prospective cohort | Glaumann et al, 2013 | 36 | To evaluate the reproducibility of OFC, 27 peanut-allergic patients underwent a DBPCFC followed by a single-blind OFC. A novel test, basophil allergen threshold sensitivity (CD-sens), evaluating basophil allergen threshold sensitivity, was also used. 48% did not react to either challenge; 52% reacted at both. CD-sens testing was not reproducible. | OFCs are 100% reproducible for both negative and positive tests. Peanut allergen sensitivity threshold was not reproducible using the CD-sens test. |
| OFC | Retrospective chart review | Lieberman et al, 2011 | 34 | 701 OFCs performed in 521 patients. Reactions were elicited in 132 (18.8%) cases. 1.7% of the reactions required treatment with epinephrine. | OFC performed in an outpatient setting under appropriate supervision is safe. It is an important step in establishing the diagnosis of food allergy and avoiding unnecessary elimination diets. | |
| OFC | Retrospective chart review | Fleischer et al, 2011 | 32 | Following OFCs in children with elimination diets based on immunoassays, 84%–93% were able to reintroduce foods they had been avoiding. | This study examined a population of children referred to a specialized care centre. OFC is an essential element in the diagnosis of food allergy. Relying solely on immunoassays can result in unnecessary elimination diets. | |
| OFC | Retrospective chart review | Calvani et al, 2012 | 35 | Of 544 OFCs analyzed, 48.3% were positive, of which 65.7% were mild. 31.9% of those who reacted had multiorgan involvement, and 2.4% had anaphylaxis. Antihistamines were the most commonly used treatment during challenge. | OFCs are safe when performed by an allergist in the appropriate setting. | |
| OFC | Review | Järvinen and Sicherer, 2012 | 33 | Food-specific IgE tests are helpful biomarkers of allergy, but lack sensitivity and specificity. OFC remains the standard for diagnosis of reactivity. IgE testing does not assess for non-IgE-mediated reactions. | Review article providing an overview on technique and interpretation of OFCs. | |
| OFC | Review | Asero et al, 2009 | 4 | While DBPCFC is the gold standard for diagnosis of food allergy, it is clinically cumbersome and difficult to perform. Open challenges have fewer disadvantages. | Discretion is needed when deciding whether a patient needs a food challenge, and whether it should be DBPCFC or open. | |
| OFC | Prospective cohort | van der Velde et al, 2012 | 41 | Health-related QOL scores improved after DBPCFC, with greater improvement after negative challenge than positive challenge. There was longitudinal validity of the Food Allergy Quality of Life Questionnaire. | When indicated, OFC should be performed, as health-related QOL improves following this assessment. | |
| OFC | Peanut, tree nuts | Prospective cohort | Knibb et al, 2012 | 42 | 40 patients and their mothers completed questionnaires before and 3–6 months after OFC. Both parent and child experienced improved food-related QOL after challenge. Patient anxiety levels were decreased after challenge, while parental anxiety remained the same. Improvement was independent of challenge outcome, despite coexisting food allergies in 50% of children. | OFC is associated with increased parental anxiety on the day of the challenge but leads to improved QOL and decreased patient anxiety following challenge. |
| OFC | Cross-sectional | Indinnimeo et al, 2013 | 40 | Reported QOL was worse among those with a history of anaphylaxis and aged >3 years. Duration of exclusion diet had a significant impact on QOL for milk-allergic children but not children with other food allergies. | Elimination diets negatively impact QOL. They must only be employed when necessary and for the shortest duration possible. | |
| Gut tryptase | Cross-sectional | Hagel et al, 2013 | 113 | Patients with gastrointestinal symptoms of food allergy had elevated levels of tryptase in gut mucosa compared to controls. | Novel approach in investigation of food allergy, but clinical relevance and application may not warrant its use. | |
| Specific IgG | Cross-sectional | Zeng et al, 2013 | 30 | Food-specific IgG is variable in symptomatic and healthy Chinese adults. | Specific IgG is not a reliable means of diagnosing food allergy. |
Abbreviations: DBPCFC, double-blind, placebo-controlled food challenge; IgA, immunoglobulin A; IgE, immunoglobulin E; IgG4, immunoglobulin G, type 4; NPV, negative predictive value; OFC, open food challenge; OVA, ovalbumin; OVM, ovomucoid; PPV, positive predictive value; QOL, quality of life; SLIT, sublingual immunotherapy; SPT, skin prick test.
Table 2.
Management of food allergy
| Modality | Allergen | Type of study | Authors, publication year | Reference | Findings | Comments |
|---|---|---|---|---|---|---|
| Prevention in pregnancy | Peanut | Retrospective | Sicherer et al, 2010 | 46 | In a cohort of infants with likely milk or egg allergy, frequent peanut consumption (OR 2.9), IgE levels to milk and egg, male sex, and non-white race were associated with peanut-sIgE >5 kUA/L. | Maternal peanut consumption during pregnancy may be associated with sensitization of infants, although allergy was not evaluated. |
| Prevention in pregnancy | Peanut | Retrospective | DesRoches et al, 2010 | 48 | Mothers of peanut-allergic children reported higher consumption of peanut during pregnancy and breastfeeding (OR 4.22, 1.57–11.30 [95% CI]; 0.28, 1.31–3.97 [95% CI]). | Recall bias may have influenced the results of this study. |
| Prevention in pregnancy | RCT | West et al, 2012 | 51 | Higher maternal vitamin C intake showed a trend toward a reduced association with wheeze, although it was not statistically significant (P=0.06). Increased maternal copper intake was associated with reduced risk of wheeze and eczema, but not food allergy. | Maternal antioxidant intake may influence the development of allergic disease in children. Particularly, increased dietary copper may reduce wheeze and eczema at 1 year. There was no significant effect on food allergy. | |
| Prevention in pregnancy | RCT | Palmer et al, 2012 | 52 | No significant difference at age 1 year between children of mothers who were supplemented with polyunsaturated fatty acids during pregnancy and controls. | Polyunsaturated fatty acid supplementation during pregnancy does not reduce the risk of allergic disease in childhood. | |
| Prevention in pregnancy | Peanut, tree nuts, sesame | Cross-sectional | Hsu et al, 2013 | 47 | Maternal consumption of tree nut and sesame seed during the first two trimesters was associated with a 60% higher risk of having a child sensitized to tree nut, peanut, or sesame seed. Children with asthma and environmental allergies had increased risk of sensitization. | Maternal consumption of tree nut and sesame seed leads to greater risk of having a child who is sensitized to tree nut, peanut, or sesame seed. This study did not evaluate allergy to these foods. |
| Prevention in infancy | Egg | Cross-sectional | Koplin et al, 2010 | 59 | Compared with introduction at 4–6 months of age, later introduction was associated with higher risk of egg allergy (OR 1.6 for 10–12 months, 3.4 for >12 months). At age 4–6 months, first exposure to cooked egg (boiled, scrambled, fried, or poached) reduced the risk of egg allergy compared to first exposure to egg in baked goods (egg-containing cakes or biscuits or similar products) (OR 0.2). Duration of breastfeeding and age of introduction of solids were not associated with egg allergy. | Introduction of cooked egg between 4 and 6 months of age may be protective. Optimal timing of introduction of foods to induce tolerance remains under investigation. |
| Prevention in infancy | Milk | Prospective cohort | Katz et al, 2010 | 58 | Incidence of IgE-mediated milk allergy was 0.5%. The mean age of introduction of cow’s milk was different between healthy infants (61.6 days) and milk-allergic infants (116.1 days). | Early exposure to cow’s milk may promote tolerance. |
| Prevention in infancy | RCT | Jensen et al, 2012 | 53 | High-risk children randomized to receive probiotics or placebo for first 6 months of life. Long-term follow-up showed no significant difference in allergic disease between the groups at age 5 years. | Early probiotic supplementation does not reduce the risk of allergic disease in childhood. | |
| Prevention in infancy | RCT | D’Vaz et al, 2012 | 56 | There was no association between fish oil supplementation and allergic disease. | Fish oil supplementation does not prevent childhood allergic disease. | |
| Prevention in infancy | Case-control | Grimshaw et al, 2013 | 57 | Infants diagnosed with food allergy at 2 years were introduced to solids earlier (<16 weeks) and were less likely to be receiving breast milk when cow’s milk was introduced. | Optimal timing for introduction of foods to induce tolerance is unknown. | |
| Prevention in infancy | RCT | Loo et al, 2014 | 55 | Infants from birth to 6 months of age received cow’s milk formula with or without probiotic supplements. At the age of 5 years, there were no significant differences between the groups in the proportion of children who developed asthma, allergic rhinitis, eczema, or food allergy. | Probiotic supplementation during infancy does not protect against the development of allergic disease in childhood. | |
| Prevention in infancy | RCT | West et al, 2013 | 54 | 25% of patients in the probiotic treatment group versus 35% of controls had some type of allergic disease. | Probiotic supplementation during infancy does not protect against allergic disease at age 8–9 years. | |
| Prevention in childhood | Cross-sectional | DeMuth et al, 2013 | 60 | Children presenting to allergy clinic with a history of parent-reported antacid use had a higher prevalence of food allergy (57%) compared to controls (32%). Patients who had taken antacids also had higher mean peanut-sIgE levels than controls (11.0 ± 5.0 kUA/L versus 2.0 ± 5.5 kUA/L; P= 0.01) | In a cohort of children presenting to allergy clinic, parent-reported antacid use was associated with an increased risk of food allergy. | |
| Prevention – allergen avoidance | Milk | Cross-sectional | Boyano-Martínez et al, 2009 | 67 | 40% of children with milk allergy had accidental exposure resulting in allergic reactions: 53% mild, 32% moderate, 15% severe. | Accidental exposures to milk in milk-allergic children are common. |
| Prevention – allergen avoidance | Milk | Prospective cohort | Tuokkola et al, 2010 | 66 | 85% of families adhered to the milk-elimination diet. Older children and those who were mono-sensitized were more likely to have some milk in their diet. | It is unclear whether the children in the study were avoiding milk for IgE-mediated allergy or intolerance. The participants in the study were receiving special infant formula reimbursement, so this may partially account for the high rate of adherence. |
| Prevention – allergen avoidance | Milk (cow versus camel) | Prospective cohort | Ehlayel et al, 2011 | 68 | 80% of cow’s milk-allergic children had a negative SPT response to camel milk and could safely ingest it. Children with a positive SPT response to camel milk were not challenged. | Patients in this study were not challenged to cow’s milk to confirm allergy; it is possible they acquired tolerance. Camel milk could be a safe alternative to cow’s milk, but this may not be practical depending on cultural influences. |
| Prevention – allergen avoidance | Cross-sectional | Sakellariou et al, 2010 | 71 | The percentage of people able to correctly identify more than 50% of the terms associated with a given allergen were: 4.8% of the general population, 2.9% of parents of food-allergic children, and 39.5% of health care professionals without a history of food allergy. Females, those with higher education, and those with a history of food allergy scored more highly. | Consumer confusion regarding labeling for food allergies is prevalent. Labeling must state exact terms for each allergen, and “may contain” statements should be limited to avoid misleading labels. | |
| Prevention – allergen avoidance | Review | Turner et al, 2011 | 73 | A review of food-labeling practices detailing both the consumers’ and manufacturers’ perspectives. Overly stringent labeling measures reduce options for allergic individuals, leading to anxiety and affecting QOL. | The current system of food labeling is not beneficial for manufacturers, consumers, or health professionals. A standardized risk assessment tool such as VITAL could help improve food-labeling practices. | |
| Prevention – allergen avoidance | Seafood | Cross-sectional | Ng et al, 2011 | 65 | 25% of seafood-allergic patients seen in allergy clinic were unable to recall dietary advice provided. Nonetheless 89% of all parents implemented a safe diet, but over half of the 89% followed a more stringent elimination diet than needed. 1/5 had allergic reactions to seafood after diagnosis. Prescription of an EAI was associated with improved adherence. | Food allergy has a detrimental effect on QOL. Many parents have difficulty recalling dietary advice given and implement more stringent diets than necessary. |
| Prevention – allergen avoidance | Prospective cohort | Pulcini et al, 2011 | 74 | A survey of Mississippi, USA public school nurses found that 97% of schools had at least one food-allergic student. 30% of schools had food allergy action plans. Action plans were more likely to exist when the nurse had received information from a physician. | Most schools have food-allergic students, but relatively few have action plans for these students. | |
| Prevention – allergen avoidance | Cross-sectional | Ben-Shoshan et al, 2012 | 72 | Precautionary statement “not suitable” was most effective in deterring purchase of a product. Individuals directly affected by food allergy were more likely to avoid products with “may contain”, “may be processed on the same equipment as products containing”, and “not suitable for” on the label than indirectly affected subjects. Households reporting a moderate/severe allergy were more likely to avoid. | The effect of labeling on allergic patients is complex and multifactorial. A standardized labeling process with fewer variations may be helpful for consumers in identifying which products to avoid. | |
| Prevention – allergen avoidance | Cross-sectional | Kim et al, 2012 | 75 | 71% of schools in Korea relied on parental reports of food allergy. 47% of participating schools had experienced student visits to a school health room due to food allergy within the previous year. >80% relied on self-care without school-wide measures for food allergies. | In Korea, most schools do not have a plan for managing food allergies among students. The authors suggest that such plans be implemented. | |
| Prevention – allergen avoidance | Cross-sectional | Ercan et al, 2012 | 76 | 52% of teachers knew which of their students had allergic disease. Pollen was thought to be the most common agent to cause anaphylaxis (54%), followed by food (47%). Among foods, egg (30.4%) and strawberries (25.3%) were thought to be the two leading causes. Only 10% were aware of an EAI, and 4% knew where to apply it. 25% of teachers knew all of the symptoms of anaphylaxis and 6% reported there was a management plan for anaphylaxis in the school. | Primary school teachers are not well informed about food allergy and anaphylaxis. Training programs on the subject should be implemented. | |
| Prevention – allergen avoidance | Milk | Cross-sectional | Berni Canani et al, 2013 | 114 | Examined new amino acid formula in 60 patients with IgE or non-IgE-mediated reaction to milk. No patients had immediate or delayed reactions. Fecal concentration of calprotectin and eosinophil cationic protein remained stable after exposure to the new amino acid formula. | Provides a safe amino acid formula alternative for children with IgE- and non-IgE-mediated reactions to cow’s milk. |
| Prevention – allergen avoidance | Prospective cohort | Zurzolo et al, 2013 | 70 | 65% of products had warning labels for an allergen that was not listed in the ingredients. Most common were tree nuts (36.2%), followed by peanuts (34.1%), sesame (27.5%), and egg (22.6%). “May contain traces of” was the most common type of statement used (29%). | The use of precautionary labeling for food allergy is high, leading to restricted diets for consumers with allergies. | |
| EAI | Telephone survey | Soller et al, 2011 | 115 | Telephone survey that identified 3.2% of participants as having a probable food allergy. Of these, 45% had an EAI. Factors that increased the probability of having an EAI were: marriage/partner, children, female, multiple food allergies, history of being treated with epinephrine, and confirmatory testing. | Health care providers and patients need more education on the recognition and management of anaphylaxis. | |
| EAI | Cross-sectional | DeMuth and Fitzpatrick, 2011 | 77 | 59% had an EAI in clinic on follow-up visit, of which 79% reported having received training in its use. Having EAI training was associated with having an EAI available. Fewer children aged >5 years reported having an EAI at school during lunch (25%) than those <5 years (42%). | Many children do not have an EAI available at all times. EAI training improves the likelihood of EAI carriage. | |
| EAI | Cross-sectional | Segal et al, 2012 | 79 | Evaluated 141 patients: 9.9% had used epinephrine previously. 37% had a valid device with them at follow-up visit. 62%–87% incorrectly performed steps in use of an EAI. 41 participants were reevaluated 1 year later, after which time the mean scores improved from 4.71 to 6.7. | Many patients do not carry valid EAIs and are not skilled in their use. Repeated instruction may improve this skill. | |
| EAI | Prospective | Spina et al, 2012 | 78 | Assessed influence of periodic checks by school nurse on EAI carrying among adolescents. Periodic checks did not influence the rate of carrying, but those who did carry EAIs were more likely to have unexpired medication with periodic reminders. | Carriages rates of EAIs are low among adolescents. We must work to develop effective interventions to improve this. | |
| EAI | Cross-sectional | Simons et al, 2012 | 116 | Most allergists expect patients aged 12–14 years to describe anaphylaxis symptoms (95.4%), demonstrate use of an EAI (93.1%), carry an EAI (88.1%), learn to self-inject an EAI (84.5%) and be able to self-inject (78.6%). | Most pediatric allergists expect their patients to be able to self-administer an EAI between the ages of 12 and 14 years. There are no patient data on optimal timing of transfer of responsibility. | |
| EAI | Cross-sectional | Pinczower et al, 2013 | 80 | Used FAQOL to assess impact of prescription of an EAI. Being aged 7–12 years, having four or more food allergies, and history of anaphylaxis had strongest impact on QOL. Patients issued an EAI reported lower QOL. | We must weigh the risks and benefits of prescribing EAIs as they are associated with reduced QOL. | |
| EAI | Peanut | Prospective cohort | Chad et al, 2013 | 8 | 56% of parents of children with peanut allergy expressed fear about using EAI. Fear was attributed to concern about hurting the child, using the EAI incorrectly, or a bad outcome. Parents of children with a history of severe reaction, long duration of disease, or who were satisfied with EAI training were less likely to be afraid. | Many parents of peanut-allergic children are afraid to use an EAI. Parent training can help decrease fear associated with EAIs. |
| FAHF-2 | RCT | Wang et al, 2010 | 63 | Among 18 active intervention and placebo subjects, there were no significant differences in vital signs, physical examination, laboratory data, pulmonary function test results, and electrocardiogram data obtained before and after treatment visits. | FAHF-2 was safe and well tolerated in patients with food allergy. | |
| FAHF-2 | Open-label | Patil et al, 2011 | 64 | 14/18 patients completed the study, taking FAHF-2 for 6 months. There were no significant differences in laboratory parameters, PFT results, or electrocardiogram findings before or after treatment. There was a significant reduction in basophil CD63 expression at 6 months. There was a trend toward reduction of eosinophil and basophil numbers after treatment. | This study demonstrates further safety and tolerability of FAHF-2. There may be some immunologic modulatory effect. | |
| FAHF-2 | Review | Wang and Li, 2012 | 62 | Review of FAHF-2 from development to Phase I clinical trials. | ||
| Complementary and alternative medicine | Survey | Nakano et al, 2012 | 61 | 8.4% of food-allergic children in Japan used complementary and alternative medicine. Herbal teas were most common (22%), followed by Chinese herbal medicine (18.5%) and lactic acid bacteria (16%). 13.6% felt that complementary and alternative medicine was very effective, and 11.1% thought it caused some type of side effect. | Among food-allergic Japanese children, 8.4% are using complementary and alternative medicine to treat their food allergy. | |
| OIT | Peanut | RCT | Anagnostou et al, 2014 | 81 | 62% of patients receiving OIT and none of the control group achieved desensitization. The control patients went on to receive OIT in a second phase, during which 54% achieved desensitization. GI side effects were the most common. | This study demonstrates successful desensitization using peanut flour in a majority of participants; however, it does not evaluate long-term tolerance. |
| OIT | Peanut | RCT | Varshney et al, 2011 | 82 | 16 OIT patients ingested a maximum dose of 5 g of peanut (20 peanuts). Placebo subjects tolerated a median dose of 280 mg. OIT patients had reduced SPT wheal size and IL-13 and increased peanut-specific IgG4 | Demonstrates desensitization and immunomodulation with peanut OIT, but does not examine long-term tolerance. |
| OIT | Peanut | RCT | Sampson et al, 2011 | 117 | Of nine patients in the omalizumab treatment group, 44% tolerated >1 g peanut versus 20% in the placebo group (n=5). Mild and moderate adverse events were reported in both groups (88.9% placebo, 76.5% omalizumab). | The study was stopped early, and most did not meet predefined study endpoints. Some evidence to suggest that omalizumab helps to increase the threshold tolerated in peanut OIT. |
| SLIT | Peanut | RCT | Fleischer et al, 2013 | 83 | After 44 weeks of treatment, 70% of subjects receiving SLIT tolerated peanut OFC compared with 15% of controls. | SLIT safely induced a modest level of desensitization in the majority of 40 subjects aged 12–37 years. A significant increase in dose was tolerated, but no correlation was found between clinical improvement and sIgE levels. |
| SLIT, OIT | Peanut | RCT | Chin et al, 2013 | 84 | Comparison of two previously published trials (Varshney et al82 and Skripak et al99) regarding peanut OIT versus SLIT. | Retrospective comparison of two RCTs for OIT and SLIT in peanut-allergic children. OIT provided higher dose thresholds than SLIT. |
| OIT | Peanut | Prospective cohort | Vickery et al, 2014 | 88 | Of 39 enrolled subjects, 24 completed a protocol involving 5 years of peanut OIT. Among them, 12 (50%) had sustained unresponsiveness to peanut 1 month after discontinuing OIT. Compared to those who failed challenge, those who passed had smaller SPT wheal diameters, and lower levels of sIgE, Ara h 1, and Ara h 2. | This study demonstrates that 50% of patients treated with peanut OIT for 5 years have sustained unresponsiveness 1 month after discontinuing treatment. While these results are promising, longer-term follow-up data would be helpful in determining acquisition of tolerance. |
| OIT | Peanut | Prospective cohort | Jones et al, 2009 | 86 | 27/29 patients undergoing OIT were able to reach target dose of peanut. Throughout treatment, SPT wheal size and sIg E decreased, while IgG4 increased. | Demonstrated clinical desensitization and immune regulation with OIT to peanut. |
| OIT | Peanut | Prospective cohort | Hofmann et al, 2009 | 91 | 20/28 patients completed the study. On escalation day, 79% had URT, 68% GI symptoms, and 18% mild wheezing. After build-up phase, risk of reaction was 46% (29% URT, 24% cutaneous). Risk of reaction during home dosing was 3.5% (URT 1.2%, skin 1.1%). Treatment was given in 0.7% of the home doses. | This study examined the safety of peanut OIT. Most reactions occurred during escalation. Reactions with home dosing were rare. |
| OIT | Peanut | Prospective cohort | Blumchen et al, 2010 | 90 | 23 children received OIT with a rush protocol, after which a median dose of 0.15 g Peanut was tolerated. 22/23 patients received long-term protocol. After 7 months of treatment, 14 reached protective dose (0.5 g peanut). | Rush protocol was not effective and was associated with adverse reactions. Long-term build-up is safe and effective. |
| OIT | Peanut | Prospective cohort | Yu et al, 2012 | 92 | 24 patients received 6,662 doses. 84% of symptoms were mild, 13% moderate, and 3% severe (GI reactions requiring epinephrine). | In this study examining the safety of peanut OIT, most reactions were mild, though some patients had severe reactions requiring treatment with epinephrine. |
| OIT | Peanut | Retrospective cohort | Wasserman et al, 2014 | 89 | 352 patients received 240,351 doses of peanut OIT. There were 95 reactions requiring treatment with epinephrine; three required two doses. 298 patients achieved the target maintenance dose (85%). | This retrospective chart review examines data from five clinics conducting peanut OIT using different agents (peanut, peanut butter, or peanut flour). It demonstrates that a significant number of patients have reactions requiring epinephrine, and that most are able to reach the target maintenance dose. |
| OIT | Peanut | Editorial | Mansfield, 2013 | 87 | Pro/con editorial of peanut OIT | The author argues that peanut OIT is ready for clinical practice. |
| OIT | Peanut | Systematic review | Sheikh et al, 2012 | 118 | Systematic review of six case series studies with a total of 85 participants. | Many participants increase their threshold dose while on treatment. Adverse reactions were common and, though usually mild, some were life-threatening. |
| Novel immunotherapy | Peanut | Experimental technique | Pascal et al, 2013 | 119 | Identified four regions of Ara h 2 that induced T-cell proliferation in peanut-allergic children. These could potentially be used for a peptide-based vaccine for food allergy. | Used a novel approach to treatment of food allergy, but requires further study before it is ready for clinical application. |
| OIT | Milk | RCT | Pajno et al, 2013 | 94 | Children desensitized with OIT were randomized to one of two feeding regimens: daily or twice weekly. There was no difference in adverse reactions among the two groups. | Once desensitization is achieved, a twice-weekly maintenance regimen is as effective as daily maintenance. |
| OIT | Milk | Prospective cohort | Nadeau et al, 2011 | 95 | Pilot study in eleven milk-allergic children, using omalizumab to perform rapid oral desensitization. 9/10 achieved target dose of 1 g during rush desensitization. Over 7–11 weeks, 9/10 reached 2 g target. | Rush milk desensitization with omalizumab was generally successful and well tolerated. All children had adverse events, but most were mild and did not require treatment. There was one event requiring epinephrine. |
| OIT | Milk | Prospective cohort | Vázquez-Ortiz et al, 2013 | 97 | 81 children enrolled. 71.6% had complete desensitization and 20.9% partial desensitization. 95% of children had reactions, of which 91% had a single affected organ system. 20 children (25%) accounted for 78% of reactions that were frequent, persistent, and unpredictable. sIgE >50, SPT wheal diameter of >9 mm, or Sampson severity grade 2, 3, or 4 at initial DBPCFC are risk factors for reaction persistence. | While many children can achieve desensitization with milk OIT, there is a high rate of adverse events. Development of tolerance was not discussed. |
| OIT | Milk | Prospective cohort | García-Ara et al, 2013 | 96 | 36 patients were classified according to milk-sIgE and treated with two dosing protocols. 100% of patients with sIgE <3.5 kUA/L and 88% of patients with sIgE >3.5 kUA/L were desensitized. 75% of patients had adverse events during induction, and 60% during maintenance. | Tolerance was achieved earlier with lower sIgE. Adverse events were more frequent with higher sIgE. |
| OIT | Milk | Prospective cohort | Keet et al, 2013 | 98 | 5-year follow up of 32 patients from two studies (Skripak et al99 and Keet et al100). 31% were tolerating full servings of milk with minimal to no symptoms. | Achievement of long-term tolerance through milk OIT is low. |
| OIT – formula | Milk | Prospective cohort | Berni Canani et al, 2013 | 69 | 260 milk-allergic children evaluated. Rate of acquisition of tolerance was higher in groups that received extensively hydrolyzed casein formula (43.6%) or EHCF + Lactobacillus rhamnosus GG (78.9%) compared to rice, soy, and amino acid formulas. | ECHF accelerates acquisition of tolerance in milk-allergic children. |
| OIT – formula | Milk | RCT | Reche et al, 2010 | 101 | 92 infants with IgE-mediated milk allergy were randomized to receive hydrolyzed rice formula or EHCF. One infant in the EHCF group had an immediate reaction. No statistically significant difference in acquisition of milk tolerance between the two groups. | Hydrolyzed rice formula is a safe alternative for children with milk allergy. |
| OIT | Milk | Review | Yeung et al, 2012 | 93 | Cochrane Review examining five studies with a total of 196 participants (106 treatment, 90 control). Primary outcome of studies was desensitization; tolerance was not assessed. 62% of the OIT group were able to tolerate a full serving of milk versus 8% of controls. 25% of the OIT group could ingest a partial serving of milk versus none in the control group. | Side effects in milk OIT are common, but the majority are mild. Long-term tolerance has not yet been assessed. Maintaining desensitization requires regular consumption of the highest tolerated dose of milk. |
| OIT | Egg | RCT | Burks et al, 2012 | 103 | After 10 months of egg OIT, 55% of the treatment group and none of the placebo group passed OFC. After 22 months, 75% of the OIT group were desensitized. After 2 months of avoiding egg consumption, only 28% passed OFC. | Egg OIT can successfully desensitize most children, but sustained unresponsiveness is achieved only in a small subset. |
| OIT | Egg | RCT | Meglio et al, 2013 | 104 | Of a group of ten egg-allergic children treated with raw egg OIT, 80% achieved the target dose over a 6-month period. One failed and another achieved partial desensitization. 20% of the control group achieved tolerance after 6 months. | This study demonstrates effective desensitization but does not examine long-term tolerance. While this study is encouraging, it had small numbers and a short duration. |
| OIT | Egg | Prospective cohort | Itoh et al, 2010 | 106 | Rush OIT in six egg-allergic children, whereby after 12 days all participants reached the target dose of one whole egg, after which they remained on maintenance twice a week. At 12 months, there was decreased egg-sIgE and increased IgG4. There were no serious reactions and no participants required epinephrine. | Rush OIT to egg was well tolerated in the group of six children. |
| OIT | Egg | Prospective cohort | García Rodríguez et al, 2011 | 107 | In rush OIT in 23 egg-allergic children over 5–10 days, 78.3% had reactions, but none were serious. 20/23 were successfully desensitized. At 6 months, there were decreases in sIgE and SPT size and increases in IgG4 and CD4+ FoxP3+ cells. | Describes a protocol for egg desensitization that is tolerated by most egg-allergic patients as well as immunologic changes possibly associated with desensitization. |
| OIT | Egg | Prospective cohort | Leonard et al, 2012 | 102 | 89% of patients challenged to baked egg were tolerant. 53% of those became regular egg tolerant as well. Among those who reacted to baked egg, 61% subsequently tolerated it and 26% tolerated regular egg. Those who tolerated baked egg had lower egg-sIgE. In subjects ingesting baked egg, egg SPT wheal size and ovalbumin- and ovomucoid-sIgE all decreased, while ovalbumin and ovomucoid-specific IgG4 increased. | Baked egg challenges should be performed among egg-allergic children, as most egg-allergic children tolerate baked egg and inclusion of baked egg in the diet may hasten the resolution of egg allergy. |
| OIT | Egg | Prospective cohort | Ojeda et al, 2012 | 108 | 80.6% of the subjects in the intention-to-treat group achieved desensitization to egg. Four cases required treatment with epinephrine at home. Baked egg-tolerant patients were more likely to achieve raw egg tolerance. | This study demonstrates effective desensitization but does not examine long-term tolerance. The authors’ definition of anaphylaxis as involving three or more organ systems may explain why there were few cases of anaphylaxis. |
| OIT | Egg | Prospective cohort | Tortajada-Girbés et al, 2012 | 105 | 19 egg-allergic children were given increasing amounts of egg at weekly intervals. 89.5% of subjects achieved tolerance to the target dose. There was no anaphylaxis during the OIT phase, though two participants withdrew from the study as they had anaphylaxis with the initial dose. | This study demonstrates effective desensitization to egg but does not examine long-term tolerance. |
| SLIT | Peach | RCT | García et al, 2010 | 110 | In describing sensitization profiles of peach-allergic individuals, rPru p 3 was the most recognized (83.3%), followed by nArt v 3 (25.9%), rMal d 4 (24.1%), and rMal d 1 (18.5%). sIgE to rPru p 3 rose in both groups but remained elevated only in the treatment group. Peach SPT wheal size decreased in the treatment group. | rPru p 3 was the most common antigen recognized by peach-allergic individuals. This study demonstrates a decrease in SPT wheal size in the treatment group, but they did not perform OFC. |
| SLIT | Peach | RCT | Fernández-Rivas et al, 2009 | 109 | After 6 months of SLIT with Pru p 3 extract, the treatment group tolerated higher doses of peach, had smaller SPT, and increased IgG4. No systemic reactions were observed, but local reactions were common. | Demonstrates effective desensitization and safety of SLIT to peach using Pru p 3 extract. |
| Effect of cooking method on peanut allergens | Peanut | Experimental technique | Kim et al, 2013 | 120 | Examined effect of cooking method on peanut proteins. Ara h 2 and 3 were the most important epitopes. Ara h 2 was enhanced by boiling, roasting, and frying peanuts and decreased by pickling (vinegar). Also, there was less IgE binding in vinegar. Ara h 3 was enhanced by all treatments. | Cooking methods will alter epitopes in peanut, although the clinical significance of this is not established. |
| Milk | Review | Restani et al, 2009 | 121 | Choices in cow’s milk (filtration not effective); alternatives to cow’s milk (donkey, mare, camel, etc); comorbid beef allergy. | Milk allergy is an increasing problem in infancy. Further research should be targeted to evaluating management of milk allergy. | |
| Review | Sanz et al, 2011 | 5 | Use of microarray component-based diagnosis in food allergy. | Microarray technology to evaluate sIgE to foods is a helpful advance in food allergy diagnosis. |
Abbreviations: DBPCFC, double-blind, placebo-controlled food challenge; EAI, epinephrine autoinjector; EHCF, extensively hydrolyzed cow’s milk formula; FAHF-2, food allergy herbal formula 2; FAQOL, food allergy health–related quality of life; GI, gastrointestinal; IgE, immunoglobulin E; IgG4, immunoglobulin G, type 4; OFC, open food challenge; OIT, oral immunotherapy; OR, odds ratio; PFT, pulmonary function test; QOL, quality of life; RCT, randomized controlled trial; sIgE, specific IgE; SLIT, sublingual immunotherapy; SPT, skin prick test; URT, upper respiratory tract; VITAL, Voluntary Incidental Trace Allergen Labelling.
Figure 1.
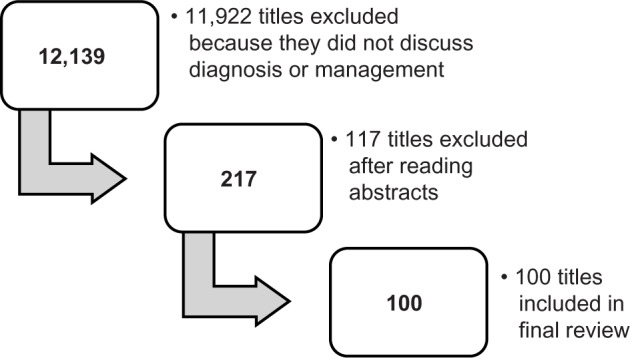
Results of the database search for literature about food allergy diagnosis and management.
Diagnosis
SPT
SPT is the primary diagnostic modality employed by most allergists. It is relatively inexpensive, can be done in the office, results are available immediately, and almost any food can be tested in this manner. Typically, an extract or fresh food is placed on the volar aspect of the forearm and the skin is pricked with an instrument. Fresh food testing can also be accomplished using the “prick-to-prick” method, where the testing device first pricks the food to be tested and is then used to prick the patient. A positive test will result in wheal formation and erythema, indicating sensitization to the allergen tested. Two studies have examined the use of end-point prick testing, or using dilutions of extract or fresh food in SPT, in predicting the outcome of the OFC. In a cohort of patients known to be milk allergic, Bellini et al12 reported that SPT with a wheal diameter greater than 4.5 mm with a 1/10,000 dilution of fresh milk was the best test for discriminating between milk-tolerant and milk-reactive subjects. They proposed using diluted milk after SPT with milk extract to help decide who should proceed to the OFC, noting that those with a positive SPT to the 1/10,000 dilution should avoid the challenge. However, their results reveal that 50% of children with a negative SPT response to diluted milk will have a positive challenge. Tripodi et al13 conducted a similar study using egg extract; however, their results may not be reproducible as extracts were not standardized and may have contained different levels of allergen. Johannsen et al14 evaluated SPT and sIgE to peanut as predictors of OFC outcomes in sensitized preschoolers, demonstrating that 50% of sensitized children could safely ingest peanut. Further, with a combined SPT wheal diameter of <7 mm and sIgE <2 kUA/L for peanut, there is a 5% chance they will react to an OFC. It is reported that a SPT wheal diameter of 8 mm as the threshold for sesame.15 Other researchers suggest a SPT wheal diameters of 8 mm and 7 mm for milk and egg thresholds, respectively.16
sIgE and component testing
Measurement of IgE levels to a specific antigen is another commonly employed method in the diagnosis of food allergies. Like SPT, it assesses sensitization rather than clinical food allergy.17 Component testing strives to delineate sensitized patients from those who will react to a given food. Peanut is one of the more extensively investigated foods in this regard. In evaluating peanut-sIgE, van Nieuwaal et al18 found higher cutoffs for predicting OFC failure compared to previous studies. Ninety percent of participants failed the peanut challenge at an sIgE of 24.8 kUA/L, and 95% at 43.8 kUA/L. The authors attributed these findings to their study population, many of whom were diagnosed with peanut allergy without undergoing DBPCFC. In distinguishing peanut sensitization from reactivity, elevated levels of Ara h 2 tend to be associated with a reactive phenotype,19,20 whereas 89.5% of children with elevated Ara h 8 can safely ingest peanut.21 In comparing peanut-sIgE, Ara h 2, and OFC results, sIgE was the most sensitive test 0.93 (93%), while Ara h 2 was the most specific and had the best positive predictive value.19 Researchers have shown similar results in peanut-allergic Asian children.22 In an effort to develop a more accurate test, Lin et al examined specific sequences of the peanut components and found that using a combination of four peptides Ara h 1, 2, and 3 had 90% sensitivity and 95% specificity.23 The biomarkers generally used to monitor the effect of immunotherapy are sIgE and SPT wheal size. Kulis et al24 examined the effect of peanut sublingual immunotherapy (SLIT) on salivary immunoglobulin A levels and found a transient elevation in the treatment group, but, by 1 year, there was no significant difference between the two groups.
Most children with egg allergy will outgrow it. Montesinos et al examined the relationships between sIgE directed towards egg white, ovalbumin, and ovomucoid, and demonstrated that these biomarkers were lower in subjects who outgrew their egg allergy.25 Another recent finding in egg allergy is that many egg-allergic children are able to tolerate baked egg. sIgE to egg white, ovalbumin, and ovomucoid tends to be lower in children who are tolerant to baked egg.26,27
Further studies have examined the role of diagnostic testing in sesame, wheat, and soy allergy. For sesame, sIgE >7 kUA/L or an SPT wheal size >6 mm were both >90% specific in predicting the results of an OFC.28 In a Japanese cohort, the median sIgE level in allergic children was 4.31 kUA/L for wheat and 3.89 kUA/L for soybean.29
It has been previously hypothesized that immunoglobulin G (IgG)-mediated reactions may be involved with food hypersensitivity, and, as such, some health care practitioners measure food-specific IgG levels. However, in a cohort of 5,394 Chinese adults, there was no relationship between food-specific IgG levels and allergic symptoms.30 Given that increased IgG levels to food allergens may indicate tolerance rather than allergy, this test is not used by allergists in their evaluation.31
Food challenges
Food challenges involve feeding the patient incremental doses of a suspected food and observing them for clinical reaction. Ideally, this is done in a double-blind, placebo-controlled manner. However, it is more practical to administer an OFC that is neither blinded nor placebo controlled. Food challenges may be performed to confirm the diagnosis of allergy or to monitor for resolution, and are often necessary due to the poor sensitivity and specificity of SPT and sIgE testing. Fleischer et al found that most children diagnosed with food allergy on the basis of immunoassays were able to reintroduce the suspected food into their diet following challenge.32 Food challenges are also useful in establishing the diagnosis of non-IgE-mediated processes that cannot be detected by SPT or sIgE testing.33 When performed in an appropriate setting, OFCs are an extremely safe procedure. In an evaluation of 701 OFCs performed in 521 patients, 18.8% elicited a reaction. Only 1.7% of those who reacted required treatment with epinephrine.34 Calvani et al reported similar results: among 544 OFCs, 48.3% of patients reacted, although 65.7% had mild reactions; only 2.7% required treatment with epinephrine.35 OFCs have been demonstrated to be a highly reproducible and valid strategy for establishing the diagnosis of food allergy. A recent study exemplified this, with 100% correlation between a positive DBPCFC and a positive single-blind OFC among patients with peanut allergy.36
OFCs have been established as a safe and effective way to diagnose milk allergy.37 An OFC for milk is important for several reasons. Many children will become milk tolerant with time. In fact, between 58.7% and 66% of children with suspected milk allergy will be tolerant to an OFC.37,38 Similar to those with an egg allergy, most milk-allergic children can tolerate baked milk. Bartnikas et al reported that 83% of the milk-allergic children they challenged were able to tolerate baked milk in their diet. In particular, they found that no child with an SPT wheal diameter <7 mm failed the baked milk challenge, and that 90% of those with a wheal diameter under 12 mm passed the baked milk challenge.39 Elimination diets are detrimental to health as they can be associated with nutritional deficiencies and increased anxiety among patients and families. Liberalizing the diet to include safe foods that are tolerated by the patient is essential in improving quality of life.40 OFCs are associated with a transient increase in parental anxiety, but, in the long-term, parents and patients report improved quality of life.41,42
There are several methods by which OFC may be conducted, with many clinicians using individualized protocols. These protocols can vary in terms of timing, dosing, the agent used, and the definition of a positive or negative challenge. In an egg OFC, Escudero et al challenged patients to both dried egg white and raw egg white. They found that 25% of the patients reacted to both, and 75% of the patients reacted to neither, indicating that dried egg white can be used to evaluate raw egg.43 Dried egg white offers several advantages over raw egg white, including storage capacity and palatability. Similarly, Winberg et al sought to validate recipes for use in DBPCFCs to egg, milk, cod, soy, and wheat. Using the same liquid test vehicle for each allergen, they found that children were unable to differentiate test from control doses.44 This provides clinicians with validated recipes that are easy to prepare and effective in concealing antigen.
OFCs and blind food challenges to evaluate immediate IgE-mediated food allergy are typically started with 0.1% to 1% of the total challenge food. If known, the initial OFC dose should be lower than the expected threshold dose. According to one approach, the total amount that should be administered during a gradually escalating OFC equals 8–10 g of a dry food, 16–20 g of meat or fish, and 100 mL of wet food (eg, apple sauce). The recommended dosing interval is 15 minutes.45
Management
Primary prevention
There are many theories regarding the origins of allergy. While we do not fully understand the etiology of allergic disease, much research has focused on how it may be prevented. In a cohort of infants with likely milk or egg allergy, Sicherer et al found that maternal peanut consumption during pregnancy, as well as sIgE to milk and peanut, were linked to an increased risk of having peanut-sIgE >5 kUA/L.46 Similar findings have been reported for tree nut and sesame seeds.47 It is important to note that these studies evaluated sensitization rather than reactivity. Mothers of peanut-allergic children reported higher consumption of peanuts during pregnancy and breastfeeding compared to mothers of non-allergic subjects.48 However, the retrospective design of the study makes recall bias a likely explanation for this finding. In two recent cohort studies assessing clinical peanut allergy, it was reported that higher maternal peanut intake during pregnancy was associated with a reduced risk of peanut-allergic reaction in offspring.49,50
Other studies have assessed the influence of maternal antioxidants consumption and polyunsaturated fatty acid supplementation during pregnancy on allergic disease in children. West et al reported that increased dietary levels of copper during pregnancy were associated with lower rates of wheeze and eczema, but had no effect on food allergy in offspring.51 There was no association between polyunsaturated fatty acid supplementation during pregnancy and prevention of allergic disease in offspring.52
Beyond pregnancy, several studies have assessed interventions in infancy that may prevent the development of food allergy. Three studies have assessed the role of probiotic supplementation during infancy on allergic disease in childhood but failed to establish an association.53–55 Similarly, no association was found between fish oil supplementation in infancy and allergic disease.56 Other studies have examined how the timing of food introduction influences acquisition of tolerance. In a case-control study, Grimshaw et al reported that infants with food allergy at 2 years of age tended to have been introduced to solids at less than 16 weeks and were less likely to have received breast milk when cow’s milk was introduced.57 Other studies have examined the timing of introduction of specific foods, namely milk and egg. In a prospective cohort, Katz et al found an overall incidence of IgE-mediated milk allergy of 0.5%. The median age of introduction among milk-tolerant infants was 61.6 days, compared to 116.1 days for milk-allergic infants.58 Koplin et al reported that, compared to introduction of egg between 4 and 6 months of age, delayed introduction was associated with a higher risk of egg allergy, with an odds ratio (OR) of 1.6 for those introduced to egg at 10–12 months and 3.4 for those introduced when older than 12 months.59 While the optimal timing for the introduction of foods to induce tolerance remains under investigation, the above studies suggest that delaying food introduction beyond 4–6 months of age may increase the risk for food allergy rather than prevent its development.
Fewer studies have examined factors in childhood that are associated with the development of allergic disease. In a cross-sectional study examining children in an allergy clinic, DeMuth et al found that history of taking antacid medication in children according to parental report was associated with an increased prevalence of food allergy.60 This may be explained by the effects of antacids on gastric pH and subsequent influence on the digestion of proteins.
Complementary and alternative medicines
An estimated 8.4% of Japanese children use complementary and alternative medicines in the management of their food allergies. Among these, herbal teas are the most common (22%), followed by Chinese herbal medicine (18.5%) and lactic acid bacteria (16%).61 Of these therapies, most research has focused on a type of Chinese herbal medicine, food allergy herbal formula 2 (FAHF-2). FAHF-2 is a nine-herb formula manufactured in China using a standardized process and monitored for contaminants using high-performance liquid chromatography.62 Two studies have demonstrated the safety and tolerability of FAHF-2 and offered some evidence of an immunologic modulatory effect. Neither of these studies examined the clinical effect of this formulation.63,64
Allergen avoidance
Several steps are required for the allergic patient to successfully avoid a particular allergen. They must be appropriately diagnosed, clearly understand what foods to avoid, and be able to identify the allergen on a label. For children, there is an added layer of complexity as their caregivers must be proficient in these skills as well.
There is anxiety associated with the diagnosis of food allergy. A study of seafood-allergic children found that 25% of their parents could not recall dietary advice provided by a physician. Nonetheless, 89% of them implemented a safe diet, although it was often more restrictive than needed. Approximately one in five patients had recurrent reactions following diagnosis. Prescription of an EAI was associated with improved adherence to the diet.65 In studies of milk-allergic children, 85% adhered to a milk-free diet, although this cohort received special infant formula reimbursement, which may bias the sample. Moreover, it is unclear whether these children were avoiding milk because of IgE-mediated reactions.66 Milk is prevalent in many foods and may be a “hidden” allergen. Accordingly, accidental exposures to milk are common and observed in up to 40% of children with milk allergy, although these reactions are usually mild (53%).67 There are several options available for milk-allergic patients. One study examined camel milk as an alternative and found it was well tolerated.68 Berni Canani et al examined a new amino acid formula and found that this was a safe alternative for children with milk allergy.69
Labeling practices to alert consumers to food allergens are varied among manufacturers, with many products bearing a “may contain” precautionary label. In an Australian study, 65% of products had precautionary labels for an allergen that was not listed in the ingredients.70 There is a high degree of consumer confusion when it comes to food labeling, with less than 5% of the general population able to correctly identify more than 50% of the terms associated with a given allergen.71 Among food labels, “not suitable” was found to be the most effective in deterring purchase of a product among food-allergic individuals and members of their households.72 Current food-labeling practices benefit neither the consumer nor the manufacturer. The use of a standardized process would be beneficial to both these groups.73
Young children with food allergies require assistance from adults to efficiently avoid allergens. Food is consumed at school, and the teacher plays an important role in the safety of food-allergic children. A survey of Mississippi, USA schools found that 97% had at least one food-allergic child, but only 30% had action plans for these students. Schools were more likely to have action plans when the school nurse had received appropriate information from a physician.74 Similar findings were reported in a Korean study, where 71% of schools relied on parental report of food allergy and 47% had experienced student visits to a school health room due to food allergy. More than 80% relied on self-care without school-wide measures for food allergies.75 Further, there are many misconceptions among teachers regarding food allergy and anaphylaxis. In a survey of primary school teachers, pollen was thought to be the most common agent to cause anaphylaxis. Among foods, egg and strawberry were the leading suspects. Only 10% of teachers surveyed were aware of EAI and only 4% knew how to administer it.76 Training programs on the recognition and management of anaphylaxis should be implemented for teachers and other caregivers. Anaphylaxis action plans can be written by physicians and provided for distribution in daycares and schools.
EAIs
Most individuals with IgE-mediated food allergy are advised to carry an EAI in case of accidental exposure. There are many barriers to the successful use of an EAI, including the ability to recognize the symptoms of anaphylaxis, the availability of an EAI, and understanding of how to use the EAI. There are additional psychological factors at play, as many patients and parents with an EAI do not use it during anaphylaxis, mostly for reasons relating to fear.8 In almost 50% of cases, an EAI is not carried by individuals with food allergy.77–79 Barriers to EAI availability include having the device on one’s person and having a device that has not expired. Similarly, many patients are not skilled in the use of their EAIs, with 62%–87% demonstrating errors in use. Repeated instruction can improve both self-carry practices and the individual’s ability to use an EAI.77,79 While prescription of an EAI is often necessary, it does impact quality of life. Pinczower et al found that prescription of an EAI negatively impacted health-related quality of life, along with being allergic to multiple foods, a history of severe reaction, and patient age 7–12 years.80
Immunotherapy
Immunotherapy is an attractive option for the treatment of food allergies, as its goal is to induce tolerance in the subject. Patients are considered to be tolerant when they can safely consume the food without following a daily oral food regimen to maintain clinical non-reactivity. In most oral immunotherapy (OIT) protocols, small amounts of allergen are administered orally to patients in gradually increasing amounts, with the immediate goal to induce desensitization. With desensitization, the treated patient manifests a decreased response to the ingested food allergens but must continue to take daily food doses.
Peanut is one of the most common food allergens, and is of great concern given that accidental ingestion of even a very small amount can cause life-threatening reactions and that peanut allergy is typically life-long.
Allergen-specific OIT for peanut allergy aims to induce desensitization and, potentially, tolerance to peanut. However, at present, there is still considerable uncertainty about the effectiveness and safety of this approach.
Three randomized controlled studies have been published on this subject. Anagnostou et al conducted a randomized controlled trial (RCT) of peanut OIT using peanut flour in two phases. In the first phase, 62% of 39 participants achieved desensitization after 6 months of OIT, defined as a negative peanut challenge to 1,400 mg of peanut protein. In the control group, who avoided peanuts, there were no participants who achieved desensitization. In the second phase, the control group underwent OIT, with 54% achieving desensitization. Side effects were generally mild, with gastrointestinal complaints being the most common.81 Varshney et al randomized 28 subjects to receive either OIT with peanut flour or placebo. Initial escalation, build-up, and maintenance phases were followed by an OFC at 1 year with titrated skin prick tests and laboratory studies performed at regular intervals. Three subjects in the treatment group withdrew early because of side effects, but all remaining peanut subjects (n=16) ingested the maximum cumulative dose of 5,000 mg (approximately 20 peanuts) after 1 year versus 280 mg in the placebo group (P<0.001). Several immunological changes accompanied successful completion of the OIT protocol: decreased SPT wheal size and Th2 cytokine production and increased IgG4 and Treg cells. There was no significant change in peanut-sIgE levels. Adverse effects were frequent, but the majority were mild.82
Fleischer et al also published an RCT in 2013 to investigate safety, efficacy, and immunologic effects of peanut SLIT in 40 peanut-allergic children. After 44 weeks of SLIT, 14 of 20 (70%) subjects receiving peanut SLIT were responders, compared with three of 20 (15%) subjects receiving placebo (P<0.001). In peanut SLIT responders, the median successful consumed dose increased from 3.5 to 496 mg. After 68 weeks of SLIT, the median successful consumed dose significantly increased to 996 mg. With regard to side effects, of 10,855 peanut doses administered through to week 44, 63.1% of patients were symptom free; excluding oropharyngeal symptoms, 95.2% were symptom free.83
In 2013, Chin et al published a letter to an editor to retrospectively compare two RCTs of OIT versus SLIT in peanut-allergic children. They found that, after 2 years, OIT was associated with greater immunological changes in sIgE and IgG4 levels basophil activation, and IgE/IgG4 ratio. Clinically, they found that dose thresholds were lower and more variable during DBPCFC at 12 months in SLIT versus OIT.84 Additionally, other small, uncontrolled trials show suggestive evidence that OIT can increase the threshold dose for peanut exposure.85–87
There are few studies published on long-term outcomes of peanut OIT. Vickery et al prospectively followed a group of patients who underwent 5 years of treatment with peanut OIT. They found that 12 of 24 patients (50%) had sustained unresponsiveness to peanut 1 month after discontinuing therapy.88 Adverse effects are common, but OIT appears to be relatively safe if administered in a carefully monitored setting. Nevertheless, there remains concern about safety, with several studies having evaluated this. In a retrospective chart review examining data from five clinics performing peanut OIT, Wasserman et al reported that, among 352 patients receiving 240,351 doses of OIT, there were 95 reactions requiring treatment with epinephrine.89 Two studies have evaluated home dosing, and reported reactions in 2.6%–3.7% of total daily home doses.90,91 In one of these studies, two reactions required epinephrine.91 In the RCT published by Varshney et al,82 47% of the patients had mild-to-moderate side effects requiring antihistamines during the initial rush phase, with two patients requiring epinephrine. During the build-up phase and home doses, none of the peanut OIT patients required epinephrine.
Hofmann et al found that patients were more likely to develop significant allergic symptoms during the initial escalation day than during other phases. Upper respiratory tract (79%) and abdominal (68%) symptoms were the most frequent symptoms at that phase. The risk of having any symptom after the build-up phase was 46% and the risk of reaction with home dosing was 3.5%.91
Yu et al presented data of an ongoing Phase I single-center trial of peanut OIT. Symptoms were mostly mild (84%) and self-resolved or were resolved with antihistamines; 13% were moderate and 3% were severe. Of the severe symptoms, three gastrointestinal reactions required epinephrine. Abdominal pain was the most common reaction, followed by oropharyngeal and lip pruritus. Respiratory symptoms were rare.92
OIT has been explored for other common food allergens including milk and egg. In 2012, Yeung et al published a Cochrane Review on milk OIT (MOIT). At that time, the authors identified five RCTs in order to compare OIT to placebo or avoidance. A total of 196 (106 treatment and 90 control) children were enrolled in these studies. The primary outcome of these studies was successful desensitization, but long-term tolerance was not assessed. According to this Cochrane Review, MOIT was proved to be an effective method of inducing (partially) desensitization. Yeung et al reported that 62% of the children in the MOIT group could tolerate 200 mL of milk versus 8% in the control group. Twenty-five percent of the children tolerated 10–184 mL of milk versus 0% in the control group. In general, the authors described low-quality studies with small numbers and different treatment protocols.93
Since that publication, there has been only one RCT performed, which was by Pajno et al in 2013 and which compared two different maintenance regimens (daily milk versus milk twice a week) over 1 year following successful cow’s milk desensitization. No difference was found in clinical efficacy or adverse effects between the two groups. The levels of sIgG4, sIgE and SPT wheal size were comparable between the intervention and control groups.94
In 2011, Nadeau et al published a pilot study in which they performed rush desensitization to milk using omalizumab. This protocol was successful in 9/10 of the patients. Although all of the patients experienced adverse reactions, most of these were mild and only one case required epinephrine.95
A major pitfall for OIT in general is the frequency of adverse events. Although most are mild and self-limited, there is a risk of severe reactions necessitating treatment with epinephrine. Two studies examined predictors of achieving tolerance and adverse effects. García-Ara et al evaluated the efficacy and safety of an OIT protocol according to the level of sIgE. They found that the lower the sIgE level at baseline, the earlier tolerance was achieved, and that adverse effects were more frequent when sIgE levels were higher.96 Vázquez-Ortiz et al identified three variables associated with reaction persistence throughout the OIT: cow milk-sIgE levels of at least 50 kUA/L, SPT wheal size of ≥9 mm and Sampson severity grades 2, 3 and 4 at baseline food challenge.97
Currently, there is no evidence that MOIT can induce long-term tolerance. In a letter to the editor, Keet et al98 published a follow-up of two studies of MOIT (cow’s milk) after 5 years to evaluate cow’s milk consumption, symptoms, and potential predictors of long-term outcomes. The first study, undertaken by Skripak et al, was a double-blind, placebo-controlled trial with 20 children.99 The second, performed by Keet et al, was an open-label randomized trial of OIT versus SLIT with 30 children.100 Sixteen subjects were eligible from each study and they were followed up after a median of 4.5 years.
Thirty one percent of patients could tolerate full servings of cow’s milk with minimal or no symptoms. There are several limitations of this report as there was no follow-up serology or SPT, no control group, and no data for quality of life before and after treatment.98
Two studies have evaluated dietary management strategies other than OIT in milk-allergic children. The first study, published in 2010 by Reche et al, compared the clinical tolerance of a hydrolyzed rice protein formula with an extensively hydrolyzed cow’s milk formula (EHCF) in infants with IgE-mediated cow’s milk allergy. The authors found no significant differences regarding tolerance achievement, adverse reactions, sIgE level, growth, or clinical tolerance.101 In a 2013 trial comparing five feeding regimens in milk-allergic children (EHCF, EHCF + Lactobacillus rhamnosus GG, hydrolyzed rice formula, soy formula, and amino acid formula), milk-allergic children who received EHCF alone or in combination with L. rhamnosus GG achieved tolerance at 12 months. Significantly more children achieved tolerance after 12 months than their peers who received hydrolyzed rice formula, soy formula, or amino acid-based formula.69 Both studies were relatively small and larger sample sizes may be needed.
As many egg-allergic patients tolerate baked egg, Leonard et al examined the role of baked egg in acquisition of tolerance. They reported that 89% of egg-allergic patients tolerated baked egg and, over time, 53% became regular egg tolerant, compared to 26% of the control group. Those who tolerated baked egg had lower egg white-sIgE levels. Additionally, inclusion of baked egg in the diet appears to hasten the acquisition of tolerance to regular egg.102
To date, two RCTs of egg OIT have been published. In 2012, Burks et al reported successful desensitization in 55% of their treatment group (n=40) and none of the placebo group (n=15). After 22 months, this increased to 75% of the OIT group. After discontinuation of OIT and avoidance of all egg products for 6–8 weeks, only 28% of the OIT group tolerated egg in an OFC. This study demonstrates successful desensitization, but long-term tolerance was achieved in less than one-third of participants.103 Meglio et al published similar data for a group of ten egg-allergic children, demonstrating that 80% were desensitized over a 6-month period, but they did not examine acquisition of tolerance.104 Tortajada-Girbés et al published a prospective cohort trial of OIT involving 19 egg-allergic children. Most participants (89.5%) reached the target dose of egg and were able to tolerate egg in meals on a weekly basis. There were no severe reactions during this trial, although two participants withdrew as they experienced anaphylaxis with the initial dose of OIT.105 Two small studies have examined a rush protocol for the administration of egg OIT, demonstrating successful desensitization over 5–12 days. Many participants had reactions during OIT, but these were generally mild or moderate in nature.106,107 Ojeda et al examined a home-based egg OIT protocol wherein participants were pretreated with antihistamines. They found that 80.6% of subjects in the intention-to-treat group achieved complete desensitization, tolerating the target dose of one egg, while 3.2% reached incomplete tolerance and 16.2% achieved no tolerance. Children who were tolerant to baked egg at baseline were more likely to achieve tolerance to raw egg throughout the study.108
Two studies have examined SLIT for peach. After 6 months of SLIT with a Pru p 3 extract, the treatment group tolerated higher doses of peach and had a smaller SPT wheal size and increased specific IgG4.109 In a separate study, García et al demonstrated that, among peach-allergic individuals, the most commonly recognized antigen is Pru p 3 (83.3%). sIgE to Pru p 3 rose in both the SLIT treatment group and the control group, but remained elevated only in the treatment group. SPT wheal size decreased in the treatment group.110
Conclusion
While the mainstays of diagnosis and management in food allergy remain relatively unchanged, there are several emerging modalities that offer exciting prospects for the future. Though we rely heavily on SPT, component testing will likely contribute to improved diagnostic accuracy. OFCs are safe when performed appropriately and improve patients’ quality of life. While management strategies are currently limited to allergen avoidance and emergency treatment of accidental exposures, immunotherapy trials offer great promise for developing desensitization. Future studies exploring strategies to induce tolerance are required. Improved diagnostic capabilities and management techniques will revolutionize food allergy diagnosis and management for physicians and patients alike in years to come.
Acknowledgments
Dr Ben-Shoshan is the recipient of the Fonds de recherche du Québec Junior 1 award and the AllerGen NCE emerging clinician scientist award.
Footnotes
Disclosure
Dr Ben-Shoshan is a consultant for Sanofi and Novartis. The authors report no other conflicts of interest in this work.
References
- 1.Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A, EAACI Food Allergy and Anaphylaxis Guidelines Group Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69(8):992–1007. doi: 10.1111/all.12423. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Shoshan M, Turnbull E, Clarke A. Food allergy: temporal trends and determinants. Curr Allergy Asthma Rep. 2012;12(4):346–372. doi: 10.1007/s11882-012-0274-3. [DOI] [PubMed] [Google Scholar]
- 3.Decker WW, Campbell RL, Manivannan V, et al. The etiology and incidence of anaphylaxis in Rochester, Minnesota: a report from the Rochester Epidemiology Project. J Allergy Clin Immunol. 2008;122(6):1161–1165. doi: 10.1016/j.jaci.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asero R, Fernandez-Rivas M, Knulst AC, Bruijnzeel-Koomen CA. Double-blind, placebo-controlled food challenge in adults in everyday clinical practice: a reappraisal of their limitations and real indications. Curr Opin Allergy Clin Immunol. 2009;9(4):379–385. doi: 10.1097/ACI.0b013e32832d9049. [DOI] [PubMed] [Google Scholar]
- 5.Sanz ML, Blázquez AB, Garcia BE. Microarray of allergenic component-based diagnosis in food allergy. Curr Opin Allergy Clin Immunol. 2011;11(3):204–209. doi: 10.1097/ACI.0b013e3283466fe4. [DOI] [PubMed] [Google Scholar]
- 6.González-Pérez A, Aponte Z, Vidaurre CF, Rodríguez LA. Anaphylaxis epidemiology in patients with and patients without asthma: a United Kingdom database review. J Allergy Clin Immunol. 2010;125(5):1098–1104. e1. doi: 10.1016/j.jaci.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Mullins RJ. Anaphylaxis: risk factors for recurrence. Clin Exp Allergy. 2003;33(8):1033–1040. doi: 10.1046/j.1365-2222.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 8.Chad L, Ben-Shoshan M, Asai Y, et al. A majority of parents of children with peanut allergy fear using the epinephrine auto-injector. Allergy. 2013;68(12):1605–1609. doi: 10.1111/all.12262. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Shoshan M, La Vieille S, Eisman H, et al. Anaphylaxis treated in a Canadian pediatric hospital: incidence, clinical characteristics, triggers, and management. J Allergy Clin Immunol. 2013;132(3):739–741. e3. doi: 10.1016/j.jaci.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Primeau MN, Kagan R, Joseph L, et al. The psychological burden of peanut allergy as perceived by adults with peanut allergy and the parents of peanut-allergic children. Clin Exp Allergy. 2000;30(8):1135–1143. doi: 10.1046/j.1365-2222.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 11.Dhami S, Panesar SS, Roberts G, et al. EAACI Food Allergy and Anaphylaxis Guidelines Group Management of anaphylaxis: a systematic review. Allergy. 2014;69(2):168–175. [Google Scholar]
- 12.Bellini F, Ricci G, Dondi A, Piccinno V, Angelini F, Pession A. End point prick test: could this new test be used to predict the outcome of oral food challenge in children with cow’s milk allergy? Ital J Pediatr. 2011;37:52. doi: 10.1186/1824-7288-37-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tripodi S, Businco AD, Alessandri C, Panetta V, Restani P, Matricardi PM. Predicting the outcome of oral food challenges with hen’s egg through skin test end-point titration. Clin Exp Allergy. 2009;39(8):1225–1233. doi: 10.1111/j.1365-2222.2009.03250.x. [DOI] [PubMed] [Google Scholar]
- 14.Johannsen H, Nolan R, Pascoe EM, et al. Skin prick testing and peanut-specific IgE can predict peanut challenge outcomes in preschoolchildren with peanut sensitization. Clin Exp Allergy. 2011;41(7):994–1000. doi: 10.1111/j.1365-2222.2011.03717.x. [DOI] [PubMed] [Google Scholar]
- 15.Peters RL, Allen KJ, Dharmage SC, et al. HealthNuts Study Skin prick test responses and allergen-specific IgE levels as predictors of peanut, egg, and sesame allergy in infants. J Allergy Clin Immunol. 2013;132(4):874–880. doi: 10.1016/j.jaci.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 16.Hill DJ, Heine RG, Hosking CS. The diagnostic value of skin prick testing in children with food allergy. Pediatr Allergy Immunol. 2004;15(5):435–441. doi: 10.1111/j.1399-3038.2004.00188.x. [DOI] [PubMed] [Google Scholar]
- 17.Mehl A, Niggemann B, Keil T, Wahn U, Beyer K. Skin prick test and specific serum IgE in the diagnostic evaluation of suspected cow’s milk and hen’s egg allergy in children: does one replace the other? Clin Exp Allergy. 2012;42(8):1266–1272. doi: 10.1111/j.1365-2222.2012.04046.x. [DOI] [PubMed] [Google Scholar]
- 18.van Nieuwaal NH, Lasfar W, Meijer Y, et al. Utility of peanut-specific IgE levels in predicting the outcome of double-blind, placebo-controlled food challenges. J Allergy Clin Immunol. 2010;125(6):1391–1392. doi: 10.1016/j.jaci.2010.01.057. [DOI] [PubMed] [Google Scholar]
- 19.Lieberman JA, Glaumann S, Batelson S, Borres MP, Sampson HA, Nilsson C. The utility of peanut components in the diagnosis of IgE-mediated peanut allergy among distinct populations. J Allergy Clin Immunol Pract. 2013;1(1):75–82. doi: 10.1016/j.jaip.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Eller E, Bindslev-Jensen C. Clinical value of component-resolved diagnostics in peanut-allergic patients. Allergy. 2013;68(2):190–194. doi: 10.1111/all.12075. [DOI] [PubMed] [Google Scholar]
- 21.Asarnoj A, Nilsson C, Lidholm J, et al. Peanut component Ara h 8 sensitization and tolerance to peanut. J Allergy Clin Immunol. 2012;130(2):468–472. doi: 10.1016/j.jaci.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Chiang WC, Pons L, Kidon MI, Liew WK, Goh A, Wesley Burks A. Serological and clinical characteristics of children with peanut sensitization in an Asian community. Pediatr Allergy Immunol. 2010;21(2 Pt 2):e429–e438. doi: 10.1111/j.1399-3038.2009.00930.x. [DOI] [PubMed] [Google Scholar]
- 23.Lin J, Bruni FM, Fu Z, et al. A bioinformatics approach to identify patients with symptomatic peanut allergy using peptide microarray immunoassay. J Allergy Clin Immunol. 2012;129(5):1321–1328. e5. doi: 10.1016/j.jaci.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulis M, Saba K, Kim EH, et al. Increased peanut-specific IgA levels in saliva correlate with food challenge outcomes after peanut sublingual immunotherapy. J Allergy Clin Immunol. 2012;129(4):1159–1162. doi: 10.1016/j.jaci.2011.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montesinos E, Martorell A, Félix R, Cerdá JC. Egg white specific IgE levels in serum as clinical reactivity predictors in the course of egg allergy follow-up. Pediatr Allergy Immunol. 2010;21(4 Pt 1):634–639. doi: 10.1111/j.1399-3038.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- 26.Caubet JC, Bencharitiwong R, Moshier E, Godbold JH, Sampson HA, Nowak-Węgrzyn A. Significance of ovomucoid- and ovalbumin-specific IgE/IgG(4) ratios in egg allergy. J Allergy Clin Immunol. 2012;129(3):739–747. doi: 10.1016/j.jaci.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 27.Alessandri C, Zennaro D, Scala E, et al. Ovomucoid (Gal d 1) specific IgE detected by microarray system predict tolerability to boiled hen’s egg and an increased risk to progress to multiple environmental allergen sensitisation. Clin Exp Allergy. 2012;42(3):441–450. doi: 10.1111/j.1365-2222.2011.03915.x. [DOI] [PubMed] [Google Scholar]
- 28.Permaul P, Stutius LM, Sheehan WJ, et al. Sesame allergy: role of specific IgE and skin-prick testing in predicting food challenge results. Allergy Asthma Proc. 2009;30(6):643–648. doi: 10.2500/aap.2009.30.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komata T, Söderström L, Borres MP, Tachimoto H, Ebisawa M. Usefulness of wheat and soybean specific IgE antibody titers for the diagnosis of food allergy. Allergol Int. 2009;58(4):599–603. doi: 10.2332/allergolint.09-OA-0096. [DOI] [PubMed] [Google Scholar]
- 30.Zeng Q, Dong SY, Wu LX, et al. Variable food-specific IgG antibody levels in healthy and symptomatic Chinese adults. PLoS One. 2013;8(1):e53612. doi: 10.1371/journal.pone.0053612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavine E. Blood testing for sensitivity, allergy or intolerance to food. CMAJ. 2012;184(6):666–668. doi: 10.1503/cmaj.110026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fleischer DM, Bock SA, Spears GC, et al. Oral food challenges in children with a diagnosis of food allergy. J Pediatr. 2011;158(4):578–583. e1. doi: 10.1016/j.jpeds.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 33.Järvinen KM, Sicherer SH. Diagnostic oral food challenges: procedures and biomarkers. J Immunol Methods. 2012;383(1–2):30–38. doi: 10.1016/j.jim.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Lieberman JA, Cox AL, Vitale M, Sampson HA. Outcomes of office-based, open food challenges in the management of food allergy. J Allergy Clin Immunol. 2011;128(5):1120–1122. doi: 10.1016/j.jaci.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calvani M, Berti I, Fiocchi A, et al. Oral food challenge: safety, adherence to guidelines and predictive value of skin prick testing. Pediatr Allergy Immunol. 2012;23(8):755–761. doi: 10.1111/pai.12016. [DOI] [PubMed] [Google Scholar]
- 36.Glaumann S, Nopp A, Johansson SG, Borres MP, Nilsson C. Oral peanut challenge identifies an allergy but the peanut allergen threshold sensitivity is not reproducible. PLoS One. 2013;8(1):e53465. doi: 10.1371/journal.pone.0053465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendonça RB, Franco JM, Cocco RR, et al. Open oral food challenge in the confirmation of cow’s milk allergy mediated by immunoglobulin E. Allergol Immunopathol (Madr) 2012;40(1):25–30. doi: 10.1016/j.aller.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Dambacher WM, de Kort EH, Blom WM, Houben GF, de Vries E. Double-blind placebo-controlled food challenges in children with alleged cow’s milk allergy: prevention of unnecessary elimination diets and determination of eliciting doses. Nutr J. 2013;12:22. doi: 10.1186/1475-2891-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartnikas LM, Sheehan WJ, Hoffman EB, et al. Predicting food challenge outcomes for baked milk: role of specific IgE and skin prick testing. Ann Allergy Asthma Immunol. 2012;109(5):309–313. e1. doi: 10.1016/j.anai.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Indinnimeo L, Baldini L, De Vittori V, et al. Duration of a cow-milk exclusion diet worsens parents’ perception of quality of life in children with food allergies. BMC Pediatr. 2013;13:203. doi: 10.1186/1471-2431-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Velde JL, Flokstra-de Blok BM, de Groot H, et al. Food allergy-related quality of life after double-blind, placebo-controlled food challenges in adults, adolescents, and children. J Allergy Clin Immunol. 2012;130(5):1136–1143. e2. doi: 10.1016/j.jaci.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 42.Knibb RC, Ibrahim NF, Stiefel G, et al. The psychological impact of diagnostic food challenges to confirm the resolution of peanut or tree nut allergy. Clin Exp Allergy. 2012;42(3):451–459. doi: 10.1111/j.1365-2222.2011.03905.x. [DOI] [PubMed] [Google Scholar]
- 43.Escudero C, Sánchez-García S, Rodríguez del Río P, et al. Dehydrated egg white: an allergen source for improving efficacy and safety in the diagnosis and treatment for egg allergy. Pediatr Allergy Immunol. 2013;24(3):263–269. doi: 10.1111/pai.12052. [DOI] [PubMed] [Google Scholar]
- 44.Winberg A, Nordström L, Strinnholm Å, et al. New validated recipes for double-blind placebo-controlled low-dose food challenges. Pediatr Allergy Immunol. 2013;24(3):282–287. doi: 10.1111/pai.12061. [DOI] [PubMed] [Google Scholar]
- 45.Nowak-Wegrzyn A, Assa’ad AH, Bahna SL, Bock SA, Sicherer SH, Teuber SS, Adverse Reactions to Food Committee of American Academy of Allergy, Asthma and Immunology Work Group report: oral food challenge testing. J Allergy Clin Immunol. 2009;123(Suppl 6):S365–S383. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 46.Sicherer SH, Wood RA, Stablein D, et al. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. J Allergy Clin Immunol. 2010;126(6):1191–1197. doi: 10.1016/j.jaci.2010.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu JT, Missmer SA, Young MC, et al. Prenatal food allergen exposures and odds of childhood peanut, tree nut, or sesame seed sensitization. Ann Allergy Asthma Immunol. 2013;111(5):391–396. doi: 10.1016/j.anai.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 48.DesRoches A, Infante-Rivard C, Paradis L, Paradis J, Haddad E. Peanut allergy: is maternal transmission of antigens during pregnancy and breastfeeding a risk factor? J Investig Allergol Clin Immunol. 2010;20(4):289–294. [PubMed] [Google Scholar]
- 49.Bunyavanich S, Rifas-Shiman SL, Platts-Mills TA, et al. Peanut, milk, and wheat intake during pregnancy is associated with reduced allergy and asthma in children. J Allergy Clin Immunol. 2014;133(5):1373–1382. doi: 10.1016/j.jaci.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frazier AL, Camargo CA, Jr, Malspeis S, Willett WC, Young MC. Prospective study of peripregnancy consumption of peanuts or tree nuts by mothers and the risk of peanut or tree nut allergy in their offspring. JAMA Pediatr. 2014;168(2):156–162. doi: 10.1001/jamapediatrics.2013.4139. [DOI] [PubMed] [Google Scholar]
- 51.West CE, Dunstan J, McCarthy S, et al. Associations between maternal antioxidant intakes in pregnancy and infant allergic outcomes. Nutrients. 2012;4(11):1747–1758. doi: 10.3390/nu4111747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer DJ, Sullivan T, Gold MS, et al. Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants’ allergies in first year of life: randomised controlled trial. BMJ. 2012;344:e184. doi: 10.1136/bmj.e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jensen MP, Meldrum S, Taylor AL, Dunstan JA, Prescott SL. Early probiotic supplementation for allergy prevention: long-term outcomes. J Allergy Clin Immunol. 2012;130(5):1209–1211. e5. doi: 10.1016/j.jaci.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 54.West CE, Hammarström ML, Hernell O. Probiotics in primary prevention of allergic disease – follow-up at 8–9 years of age. Allergy. 2013;68(8):1015–1020. doi: 10.1111/all.12191. [DOI] [PubMed] [Google Scholar]
- 55.Loo EX, Llanora GV, Lu Q, Aw MM, Lee BW, Shek LP. Supplementation with probiotics in the first 6 months of life did not protect against eczema and allergy in at-risk Asian infants: a 5-year follow-up. Int Arch Allergy Immunol. 2014;163(1):25–28. doi: 10.1159/000356338. [DOI] [PubMed] [Google Scholar]
- 56.D’Vaz N, Meldrum SJ, Dunstan JA, et al. Postnatal fish oil supplementation in high-risk infants to prevent allergy: randomized controlled trial. Pediatrics. 2012;130(4):674–682. doi: 10.1542/peds.2011-3104. [DOI] [PubMed] [Google Scholar]
- 57.Grimshaw KE, Maskell J, Oliver EM, et al. Introduction of complementary foods and the relationship to food allergy. Pediatrics. 2013;132(6):e1529–e1538. doi: 10.1542/peds.2012-3692. [DOI] [PubMed] [Google Scholar]
- 58.Katz Y, Rajuan N, Goldberg MR, et al. Early exposure to cow’s milk protein is protective against IgE-mediated cow’s milk protein allergy. J Allergy Clin Immunol. 2010;126(1):77–82. e1. doi: 10.1016/j.jaci.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 59.Koplin JJ, Osborne NJ, Wake M, et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J Allergy Clin Immunol. 2010;126(4):807–813. doi: 10.1016/j.jaci.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 60.DeMuth K, Stecenko A, Sullivan K, Fitzpatrick A. Relationship between treatment with antacid medication and the prevalence of food allergy in children. Allergy Asthma Proc. 2013;34(3):227–232. doi: 10.2500/aap.2013.34.3657. [DOI] [PubMed] [Google Scholar]
- 61.Nakano T, Shimojo N, Okamoto Y, et al. The use of complementary and alternative medicine by pediatric food-allergic patients in Japan. Int Arch Allergy Immunol. 2012;159(4):410–415. doi: 10.1159/000338936. [DOI] [PubMed] [Google Scholar]
- 62.Wang J, Li XM. Chinese herbal therapy for the treatment of food allergy. Curr Allergy Asthma Rep. 2012;12(4):332–338. doi: 10.1007/s11882-012-0265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang J, Patil SP, Yang N, et al. Safety, tolerability, and immunologic effects of a food allergy herbal formula in food allergic individuals: a randomized, double-blinded, placebo-controlled, dose escalation, phase 1 study. Ann Allergy Asthma Immunol. 2010;105(1):75–84. doi: 10.1016/j.anai.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patil SP, Wang J, Song Y, et al. Clinical safety of Food Allergy Herbal Formula-2 (FAHF-2) and inhibitory effect on basophils from patients with food allergy: extended phase I study. J Allergy Clin Immunol. 2011;128(6):1259–1265. e2. doi: 10.1016/j.jaci.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ng IE, Turner PJ, Kemp AS, Campbell DE. Parental perceptions and dietary adherence in children with seafood allergy. Pediatr Allergy Immunol. 2011;22(7):720–728. doi: 10.1111/j.1399-3038.2011.01189.x. [DOI] [PubMed] [Google Scholar]
- 66.Tuokkola J, Kaila M, Kronberg-Kippilä C, et al. Cow’s milk allergy in children: adherence to a therapeutic elimination diet and reintroduction of milk into the diet. Eur J Clin Nutr. 2010;64(10):1080–1085. doi: 10.1038/ejcn.2010.132. [DOI] [PubMed] [Google Scholar]
- 67.Boyano-Martínez T, García-Ara C, Pedrosa M, Díaz-Pena JM, Quirce S. Accidental allergic reactions in children allergic to cow’s milk proteins. J Allergy Clin Immunol. 2009;123(4):883–888. doi: 10.1016/j.jaci.2008.12.1125. [DOI] [PubMed] [Google Scholar]
- 68.Ehlayel MS, Hazeima KA, Al-Mesaifri F, Bener A. Camel milk: an alternative for cow’s milk allergy in children. Allergy Asthma Proc. 2011;32(3):255–258. doi: 10.2500/aap.2011.32.3429. [DOI] [PubMed] [Google Scholar]
- 69.Berni Canani R, Nocerino R, Terrin G, et al. Formula selection for management of children with cow’s milk allergy influences the rate of acquisition of tolerance: a prospective multicenter study. J Pediatr. 2013;163(3):771–777. e1. doi: 10.1016/j.jpeds.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Zurzolo GA, Mathai ML, Koplin JJ, Allen KJ. Precautionary allergen labelling following new labelling practice in Australia. J Paediatr Child Health. 2013;49(4):E306–E310. doi: 10.1111/jpc.12138. [DOI] [PubMed] [Google Scholar]
- 71.Sakellariou A, Sinaniotis A, Damianidou L, Papadopoulos NG, Vassilopoulou E. Food allergen labelling and consumer confusion. Allergy. 2010;65(4):534–535. doi: 10.1111/j.1398-9995.2009.02209.x. [DOI] [PubMed] [Google Scholar]
- 72.Ben-Shoshan M, Sheth S, Harrington D, et al. Effect of precautionary statements on the purchasing practices of Canadians directly and indirectly affected by food allergies. J Allergy Clin Immunol. 2012;129(5):1401–1404. doi: 10.1016/j.jaci.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 73.Turner PJ, Kemp AS, Campbell DE. Advisory food labels: consumers with allergies need more than “traces” of information. BMJ. 2011;343:d6180. doi: 10.1136/bmj.d6180. [DOI] [PubMed] [Google Scholar]
- 74.Pulcini JM, Marshall GD, Jr, Naveed A. Presence of food allergy emergency action plans in Mississippi. Ann Allergy Asthma Immunol. 2011;107(2):127–132. doi: 10.1016/j.anai.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 75.Kim S, Yoon J, Kwon S, Kim J, Han Y. Current status of managing food allergies in schools in Seoul, Korea. J Child Health Care. 2012;16(4):406–416. doi: 10.1177/1367493512448128. [DOI] [PubMed] [Google Scholar]
- 76.Ercan H, Ozen A, Karatepe H, Berber M, Cengizlier R. Primary school teachers’ knowledge about and attitudes toward anaphylaxis. Pediatr Allergy Immunol. 2012;23(5):428–432. doi: 10.1111/j.1399-3038.2012.01307.x. [DOI] [PubMed] [Google Scholar]
- 77.DeMuth KA, Fitzpatrick AM. Epinephrine autoinjector availability among children with food allergy. Allergy Asthma Proc. 2011;32(4):295–300. doi: 10.2500/ajra.2011.32.3458. [DOI] [PubMed] [Google Scholar]
- 78.Spina JL, McIntyre CL, Pulcini JA. An intervention to increase high school students’ compliance with carrying auto-injectable epinephrine: a MASNRN study. J Sch Nurs. 2012;28(3):230–237. doi: 10.1177/1059840511431459. [DOI] [PubMed] [Google Scholar]
- 79.Segal N, Garty BZ, Hoffer V, Levy Y. Effect of instruction on the ability to use a self-administered epinephrine injector. Isr Med Assoc J. 2012;14(1):14–17. [PubMed] [Google Scholar]
- 80.Pinczower GD, Bertalli NA, Bussmann N, et al. The effect of provision of an adrenaline autoinjector on quality of life in children with food allergy. J Allergy Clin Immunol. 2013;131(1):238–240. e1. doi: 10.1016/j.jaci.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 81.Anagnostou K, Islam S, King Y, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383(9925):1297–1304. doi: 10.1016/S0140-6736(13)62301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Varshney P, Jones SM, Scurlock AM, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127(3):654–660. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fleischer DM, Burks AW, Vickery BP, et al. Consortium of Food Allergy Research (CoFAR) Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol. 2013;131(1):119.e1–127.e7. doi: 10.1016/j.jaci.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chin SJ, Vickery BP, Kulis MD, et al. Sublingual versus oral immunotherapy for peanut-allergic children: a retrospective comparison. J Allergy Clin Immunol. 2013;132(2):476–478. e2. doi: 10.1016/j.jaci.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clark AT, Islam S, King Y, Deighton J, Anagnostou K, Ewan PW. Successful oral tolerance induction in severe peanut allergy. Allergy. 2009;64(8):1218–1220. doi: 10.1111/j.1398-9995.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 86.Jones SM, Pons L, Roberts JL, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300. 300. e1–e97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mansfield LE. Oral immunotherapy for peanut allergy in clinical practice is ready. Allergy Asthma Proc. 2013;34(3):205–209. doi: 10.2500/aap.2013.34.3666. [DOI] [PubMed] [Google Scholar]
- 88.Vickery BP, Scurlock AM, Kulis M, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133(2):468–475. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wasserman RL, Factor JM, Baker JW, et al. Oral immunotherapy for peanut allergy: multipractice experience with epinephrine-treated reactions. J Allergy Clin Immunol Pract. 2014;2(1):91–96. doi: 10.1016/j.jaip.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 90.Blumchen K, Ulbricht H, Staden U, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126(1):83–91. e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 91.Hofmann AM, Scurlock AM, Jones SM, et al. Safety of a peanut oral immunotherapy protocol in children with peanut allergy. J Allergy Clin Immunol. 2009;124(2):286–291. 291. e1–e6. doi: 10.1016/j.jaci.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu GP, Weldon B, Neale-May S, Nadeau KC. The safety of peanut oral immunotherapy in peanut-allergic subjects in a single-center trial. Int Arch Allergy Immunol. 2012;159(2):179–182. doi: 10.1159/000336391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yeung JP, Kloda LA, McDevitt J, Ben-Shoshan M, Alizadehfar R. Oral immunotherapy for milk allergy. Cochrane Database Syst Rev. 2012;11:CD009542. doi: 10.1002/14651858.CD009542.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pajno GB, Caminiti L, Salzano G, et al. Comparison between two maintenance feeding regimens after successful cow’s milk oral desensitization. Pediatr Allergy Immunol. 2013;24(4):376–381. doi: 10.1111/pai.12077. [DOI] [PubMed] [Google Scholar]
- 95.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow’s milk allergy. J Allergy Clin Immunol. 2011;127(6):1622–1624. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.García-Ara C, Pedrosa M, Belver MT, Martín-Muñoz MF, Quirce S, Boyano-Martínez T. Efficacy and safety of oral desensitization in children with cow’s milk allergy according to their serum specific IgE level. Ann Allergy Asthma Immunol. 2013;110(4):290–294. doi: 10.1016/j.anai.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 97.Vázquez-Ortiz M, Alvaro-Lozano M, Alsina L, et al. Safety and predictors of adverse events during oral immunotherapy for milk allergy: severity of reaction at oral challenge, specific IgE and prick test. Clin Exp Allergy. 2013;43(1):92–102. doi: 10.1111/cea.12012. [DOI] [PubMed] [Google Scholar]
- 98.Keet CA, Seopaul S, Knorr S, Narisety S, Skripak J, Wood RA. Long-term follow-up of oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2013;132(3):737–739. e6. doi: 10.1016/j.jaci.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Skripak JM, Nash SD, Rowley H. A randomized, double-blind, placebo-controlled study of milk oral immunotherapy for cow’s milk allergy. J Allergy Clin Immunol. 2008;122(6):1154–1160. doi: 10.1016/j.jaci.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129(2):448–455. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reche M, Pascual C, Fiandor A, et al. The effect of a partially hydrolysed formula based on rice protein in the treatment of infants with cow’s milk protein allergy. Pediatr Allergy Immunol. 2010;21(4 Pt 1):577–585. doi: 10.1111/j.1399-3038.2010.00991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Leonard SA, Sampson HA, Sicherer SH, et al. Dietary baked egg accelerates resolution of egg allergy in children. J Allergy Clin Immunol. 2012;130(2):473–480. e1. doi: 10.1016/j.jaci.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burks AW, Jones SM, Wood RA, et al. Consortium of Food Allergy Research (CoFAR) Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367(3):233–243. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meglio P, Giampietro PG, Carello R, Gabriele I, Avitabile S, Galli E. Oral food desensitization in children with IgE-mediated hen’s egg allergy: a new protocol with raw hen’s egg. Pediatr Allergy Immunol. 2013;24(1):75–83. doi: 10.1111/j.1399-3038.2012.01341.x. [DOI] [PubMed] [Google Scholar]
- 105.Tortajada-Girbés M, Porcar-Almela M, Martorell-Giménez L, Tallón-Guerola M, Gracia-Antequera M, Codoñer-Franch P. Specific oral tolerance induction (SOTI) to egg: our experience with 19 children. J Investig Allergol Clin Immunol. 2012;22(1):75–77. [PubMed] [Google Scholar]
- 106.Itoh N, Itagaki Y, Kurihara K. Rush specific oral tolerance induction in school-age children with severe egg allergy: one year follow up. Allergol Int. 2010;59(1):43–51. doi: 10.2332/allergolint.09-OA-0107. [DOI] [PubMed] [Google Scholar]
- 107.García Rodríguez R, Urra JM, Feo-Brito F, et al. Oral rush desensitization to egg: efficacy and safety. Clin Exp Allergy. 2011;41(9):1289–1296. doi: 10.1111/j.1365-2222.2011.03722.x. [DOI] [PubMed] [Google Scholar]
- 108.Ojeda P, Ojeda I, Rubio G, Pineda F. Home-based oral immunotherapy protocol with pasteurized egg for children allergic to hen’s egg. Isr Med Assoc J. 2012;14(1):34–39. [PubMed] [Google Scholar]
- 109.Fernández-Rivas M, Garrido Fernández S, Nadal JA, et al. Randomized double-blind, placebo-controlled trial of sublingual immunotherapy with a Pru p 3 quantified peach extract. Allergy. 2009;64(6):876–883. doi: 10.1111/j.1398-9995.2008.01921.x. [DOI] [PubMed] [Google Scholar]
- 110.García BE, González-Mancebo E, Barber D, et al. Sublingual immunotherapy in peach allergy: monitoring molecular sensitizations and reactivity to apple fruit and Platanus pollen. J Investig Allergol Clin Immunol. 2010;20(6):514–520. [PubMed] [Google Scholar]
- 111.Correa FF, Vieira MC, Yamamoto DR, Speridião Pda G, de Morais MB. Open challenge for the diagnosis of cow’s milk protein allergy. J Pediatr (Rio J) 2010;86(2):163–166. doi: 10.2223/JPED.1967. [DOI] [PubMed] [Google Scholar]
- 112.Mudd K, Paterakis M, Curtin-Brosnan J, Matsui E, Wood R. Predicting outcome of repeat milk, egg, or peanut oral food challenges. J Allergy Clin Immunol. 2009;124(5):1115–1116. doi: 10.1016/j.jaci.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 113.Hagel AF, deRossi T, Zopf Y, et al. Mast cell tryptase levels in gut mucosa in patients with gastrointestinal symptoms caused by food allergy. Int Arch Allergy Immunol. 2013;160(4):350–355. doi: 10.1159/000341634. [DOI] [PubMed] [Google Scholar]
- 114.Berni Canani R, Nocerino R, Leone L, et al. Tolerance to a new free amino acid-based formula in children with IgE or non-IgE-mediated cow’s milk allergy: a randomized controlled clinical trial. BMC Pediatr. 2013;13:24. doi: 10.1186/1471-2431-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Soller L, Fragapane J, Ben-Shoshan M, et al. Possession of epinephrine auto-injectors by Canadians with food allergies. J Allergy Clin Immunol. 2011;128(2):426–428. doi: 10.1016/j.jaci.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 116.Simons E, Sicherer SH, Simons FE. Timing the transfer of responsibilities for anaphylaxis recognition and use of an epinephrine auto-injector from adults to children and teenagers: pediatric allergists’ perspective. Ann Allergy Asthma Immunol. 2012;108(5):321–325. doi: 10.1016/j.anai.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 117.Sampson HA, Leung DY, Burks AW, et al. A phase II, randomized, double-blind, parallel-group, placebo-controlled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011;127(5):1309–1310. e1. doi: 10.1016/j.jaci.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 118.Sheikh A, Nurmatov U, Venderbosch I, Bischoff E. Oral immunotherapy for the treatment of peanut allergy: systematic review of six case series studies. Prim Care Respir J. 2012;21(1):41–49. doi: 10.4104/pcrj.2011.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pascal M, Konstantinou GN, Masilamani M, Lieberman J, Sampson HA. In silico prediction of Ara h 2 T cell epitopes in peanut-allergic children. Clin Exp Allergy. 2013;43(1):116–127. doi: 10.1111/cea.12014. [DOI] [PubMed] [Google Scholar]
- 120.Kim J, Lee JY, Han Y, Ahn K. Significance of Ara h 2 in clinical reactivity and effect of cooking methods on allergenicity. Ann Allergy Asthma Immunol. 2013;110(1):34–38. doi: 10.1016/j.anai.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 121.Restani P, Ballabio C, Di Lorenzo C, Tripodi S, Fiocchi A. Molecular aspects of milk allergens and their role in clinical events. Anal Bioanal Chem. 2009;395(1):47–56. doi: 10.1007/s00216-009-2909-3. [DOI] [PubMed] [Google Scholar]


