Abstract
Background
Autoantibodies to oxidized low-density lipoprotein (oxLDL) are a heterogeneous group of antibodies that are controversially discussed to be either pathogenic or protective. Biochemical and anthropometric measurements correlated with increased levels of these antibodies are also controversial, especially in conditions of impaired glucose tolerance and type 2 diabetes mellitus. The present study was conducted to evaluate levels of oxLDL antibodies and their correlation with obesity in different glycemic situations.
Methods
Two hundred and seventy-four adult males were classified into three subgroups: group 1 (n=125), comprising a control group of nondiabetic subjects; group 2 (n=77), comprising subjects with impaired glucose tolerance; and group 3 (n=72), comprising patients with type 2 diabetes mellitus. Body mass index was calculated, and measurement of oxLDL and oxLDL antibodies was performed.
Results
Higher mean concentrations of oxLDL were found in the type 2 diabetes mellitus and impaired glucose tolerance groups (143.5±21.9 U/L and 108.7±23.7 U/L, respectively). The mean value for the control group was 73.5±27.5 U/L (P<0.001). Higher mean concentrations of anti-oxLDL antibodies were observed in the type 2 diabetes mellitus and impaired glucose tolerance groups (55.7±17.8 U/L and 40.4±17.6 U/L, respectively). The mean value for the control group was 20.4±10 U/L (P<0.001). Levels of anti-oxLDL antibodies were found to be positively and significantly correlated with body mass index in the control group (r=0.46), impaired glucose tolerance (r=0.51), type 2 diabetes mellitus group (r=0.46), and in the whole study population (r=0.44; P<0.001).
Conclusion
Anti-oxLDL antibody levels were increased in subjects with type 2 diabetes mellitus and impaired glucose tolerance and were positively correlated with obesity and body mass index.
Keywords: anti-oxidized low-density lipoprotein antibodies, obesity, body mass index, diabetes, impaired glucose tolerance
Introduction
Native low-density lipoprotein (LDL) particles become pathogenic,1 immunogenic,2,3 and atherogenic4,5 when oxidized. Current clinical research points to the oxidation of LDL as a causative and initiating event in many pathological conditions.6 The serum titer of autoantibodies to oxidized LDL (oxLDL) has been shown to be associated with and may predict progression of atherosclerosis,7 myocardial infarction, and coronary artery disease.8 Anti-oxLDL antibodies are also shown to be independent predictors for development of type 2 diabetes mellitus (DM) in women.9
Oxidative modification of LDL is an irreversible process that leads to alterations in lipoprotein structure and function, and takes place in two stages. In the first stage (mild oxidation), LDL lipids are oxidized without any transformation of the molecular structure of apolipoprotein (Apo) B-100. In the second stage (advanced oxidation), LDL lipids are further oxidized, and oxidative changes in amino acids, proteolysis, and cross-linking of Apo B-100 occur.10 Therefore, oxLDL exists in multiple forms, characterized by different degrees of oxidation, including minimally modified LDL, which is still recognized by the LDL receptor, and fully or extensively oxLDL, which is recognized by scavenger receptors. Thus, oxLDL might represent the elephant that is described by blind men.11 Whereas native LDL has no effect on the immune system, modified lipoproteins are immunogenic.12 OxLDL and malondialdehyde-modified LDL (MDA-LDL) are more addressed in biomedical research. Human autoantibodies to oxLDL have been purified and characterized. The predominant isotype of oxLDL antibodies are immunoglobulin (Ig)G1 and IgG3.13 The immune complexes formed by oxLDL and their antibodies have been shown to have proinflammatory properties.14 Investigation of circulating oxLDL antibodies for their protective or pathogenic role is controversial. Considerable debate has arisen, with some groups suggesting a positive correlation between oxLDL antibody levels and atherosclerosis or vascular disease, and others disagreeing with this correlation and even showing an inverse correlation between these antibodies and cardiovascular disease.
Hunt et al,15 Crisby et al,16 Lopes-Virella et al,17 and others have demonstrated the pathogenic role of oxLDL antibodies and their immune complexes. OxLDL antibodies are able to activate the complement system via the classical pathway and to induce FcR-mediated phagocytosis.17 On the other hand, several groups have proposed a protective role for the humoral immune response to modified LDL. Santos et al suggest that circulating anti-oxLDL antibodies could have a protective role in atherosclerosis.18 Garrido-Sanchez et al found that patients with coronary disease and disorders of carbohydrate metabolism have much lower levels of IgG anti-oxLDL antibodies than normoglycemic patients,19 supporting the protective role of these antibodies. Human anti-oxLDL antibodies play an important role in the regulation of oxLDL levels. Supporting the proposed protective effect, these antibodies have been found in children and healthy adults.20
Recent studies on the prevalence of DM indicate that there were 171 million people with the disease worldwide in the year 2000, and this figure is projected to increase to 366 million by the year 2030.21 DM is a strong risk factor for microvascular and macrovascular disease.22 Thus, it is associated with reduced life expectancy and significant morbidity.23
The role of oxLDL and their antibodies as a risk factor has attracted considerable attention.24 Previous epidemiological research evaluating the possible associations between serum oxLDL antibody levels and nutritional factors showed that oxLDL antibody levels are related to the percentage of kilocalories derived from lipids.25 Obesity is a well-known risk factor for diabetes and coronary artery disease. The present study investigated the relationship between anti-oxLDL antibodies and obesity in different glycemic situations.
Subjects and methods
The study sample was selected from the outpatient clinics of the main hospitals and government offices in Makkah Al-Mukarama, Kingdom of Saudi Arabia. The study protocol was approved by the biomedical ethics committee at the Faculty of Medicine, Umm Al-Qura University, Makkah, Kingdom of Saudi Arabi.
Approximately 274 adult male subjects aged 18–55 years agreed to participate and were enrolled, subjected to the study investigations, informed about the nature of the study, and signed their ethical consent. They completed a structured questionnaire, after which measurements of weight, height, and blood pressure were performed, with body mass index (BMI) calculated as weight (kg) divided by height (m2).
The study population was classified into three groups according to the recommendations of the American Diabetes Association for diagnosis of diabetes and classification of glucose tolerance.26 Group 1 comprised normal nondiabetic subjects, group 2 comprised subjects with impaired glucose tolerance (IGT), and group 3 comprised subjects with type 2 DM. In each group, participants were subcategorized as obese (BMI ≥30) or nonobese (BMI <30). Potential participants were excluded if they had a known history of coronary heart disease or cardiovascular complications of type 2 DM, had known insulin-dependent DM, were younger than 18 years or older than 55 years, or had conditions with known familial hypercholesterolemia.
Samples were collected from all study participants using standard procedures for collection and storage. Briefly, blood samples were drawn from the antecubital vein into ethylenediamine tetraacetic acid-containing vacuum tubes, for serum, blood was collected in plan tubes and left for 30 minutes, centrifuged for 15 minutes at 3,000 rpm, and the serum sample was obtained. All samples were correctly labeled and sent directly to the biochemistry laboratory.
Measurement protocols
Oxidized low-density lipoproteins
OxLDL levels were measured using a competitive enzyme-linked immunosorbent assay (ELISA) kit (Mercodia AB, Uppsala, Sweden). This protocol uses Holvoet et al monoclonal antibody, 4E6, which is specific for oxidatively modified LDL.27 The 4E6 antibody is directed against a conformational epitope in the Apo B-100 of LDL that is generated as a consequence of aldehyde substitution of the lysine residues of Apo B-100. The principle of the procedure is based on the fact that the oxLDL in the sample competes with a fixed amount of oxLDL bound to the microtiter well for binding to the biotin-labeled specific antibodies 4E6. After a washing step, the biotin-labeled antibody bound to the well is detected by horseradish peroxidase-conjugated streptavidin. After a second incubation and an additional washing step, the bound conjugate is detected by reaction with 3,3′,5,5′-tetramethylbenzidine. The reaction is then stopped by adding acid to give a colorimetric end point that is read spectrophotometrically.
Oxidized low-density lipoprotein antibodies
OxLDL antibodies were measured using a commercially available ELISA kit (IMMCO Diagnostics, Amerhurst, NY, USA). This test kit is designed for research use only. As described by Craig et al,28 the test is performed as a solid-phase immunoassay. Microwells are coated with oxLDL antigen followed by a blocking step to reduce nonspecific protein binding during the assay run. Controls, calibrators, and patient serum are incubated in the antigen-coated wells to allow specific antibodies in the samples to bind to the antigen. Unbound antibodies and other serum proteins are removed by washing the microwells. Bound antibodies are detected by adding an enzyme-labeled antihuman IgG conjugate to the microwells. Unbound conjugate is removed by washing. A specific enzyme substrate (3,3′,5,5′-tetramethylbenzidine) is then added to the wells, and the presence of antibodies is detected by a color change. The reaction is stopped, and the intensity of the color change, which is proportional to the concentration of antibody, is read at 450 nm. The results are expressed in ELISA units per milliliter. The interassay and intraassay coefficients of variation range from 5% to 13%.
Routine biochemistry
Glucose and glycosylated hemoglobin were measured using standard procedures and available commercial kits in a fully automated system (COBAS® Integra 400 Plus, Roche Diagnostics, Indianapolis, IN, USA). All assays were done following the procedures recommended for instrument operation, calibration, quality control, and assay guidelines. The instrument was calibrated using the calibrator for automated systems (Roche Diagnostics). Results were expressed in mg/dL for all parameters, except for glycosylated hemoglobin, which was expressed as a percentage.29
Statistical analysis
Descriptive statistics, t-tests, and one-way analysis of variance were used to compare the concentrations of oxLDL antibodies and other metabolic parameters between the control, IGT, and type 2 DM groups and between the obese and nonobese subgroups. The Pearson correlation procedure was used to test the correlation between anti-oxLDL antibody levels and BMI. P<0.05 was considered to be statistically significant. All statistical methods were performed using Statistical Package for the Social Sciences for Windows version 20 software (IBM Corporation, Armonk, NY, USA).
Results
The study included 274 men aged 18–55 years who lived in Makka Al-Mukaram. There were 125 normal controls, 77 subjects with IGT, and 72 patients with type 2 DM. As shown in Table 1, our data indicate an increased mean BMI in the IGT group when compared with the control group and the type 2 DM group (31.1±5.1 kg/m2 versus 28.3±4.9 kg/m2 and 30.2±5.1 kg/m2, respectively; P<0.001). In each group, the mean age of obese and nonobese subjects was similar (Table 1). Although fasting blood sugar, 2-hour postprandial glucose, and glycosylated hemoglobin showed a significant increase in the IGT and type 2 DM groups compared with controls, there was no significant difference in these parameters between the obese and nonobese subgroups (Table 1).
Table 1.
Results for age, BMI, glucose tolerance, lipid profile, oxLDL, and oxLDL antibodies in the study groups
| Control (n=125) | IGT (n=77) | Type 2 DM (n=72) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Nonobese (n=81) |
Obese (n=44) |
Nonobese (n=37) |
Obese (n=40) |
Nonobese (n=36) |
Obese (n=36) |
|
| Age, years | 34±9 | 34±8 | 40±10 | 39±9 | 42±8 | 43±10 |
| 34±9 | 39±10cc | 42±9c | ||||
| BMI, kg/m2 | 25.5±2.7 | 33.6±3.4 | 27.0±2.3 | 34.9±3.7 | 26.3±2.5 | 34.1±4.0 |
| 28.3±4.9 | 31.1±5.1 | 30.2±5.1 | ||||
| FBS, mg/dL | 91±10 | 91±11 | 116±23 | 116±19 | 196±67 | 189±63 |
| 91±10 | 116±21cc | 193±65cc | ||||
| 2-hour PP, mg/dL | 108±18 | 113±18 | 171±15 | 163±21 | 289±67 | 305±88 |
| 109±18 | 167±19cc | 298±79cc | ||||
| HbA1c, % | 5.0±0.6 | 5.1±0.7 | 6.9±1.2 | 6.5±1.3 | 8.7±1.8 | 8.3±2.4 |
| 5.0±0.6 | 6.7±1.2cc | 8.5±2.1cc | ||||
| Cholesterol, mg/dL | 199±57 | 201±45 | 236±63 | 244±55 | 271±88 | 253±68 |
| 200±53 | 240±59cc | 262±79cc | ||||
| TG, mg/dL | 165±81 | 151±73 | 227±147 | 201±83 | 266±184 | 231±126 |
| 160±78 | 213±118c | 248 ±158cc | ||||
| HDL, mg/dL | 48.8±16 | 48.7±14.8 | 49.5±15 | 56.6±18 | 48.2±17.2 | 44.7±14.2 |
| 48.7±16 | 53.2±17 | 46.5±16 | ||||
| LDL, mg/dL | 116±32 | 121±29 | 140±43 | 142±32 | 156±50 | 149±48 |
| 118±31 | 141±38cc | 153±49cc | ||||
| LDL/HDL | 2.6±0.9 | 2.6±0.8 | 3.1±1.5 | 2.8±1.1 | 3.5±1.3 | 3.5±1.5 |
| 2.6±0.9 | 2.9±1.3 | 3.5±1.4cc | ||||
| OxLDL (U/L) | 71±27.2 | 77±28.1 | 109±20.5 | 108±26.5 | 143±25.3 | 144±18.3 |
| 73.5±27.5 | 108.7±23.7cc | 143.5±21.9cc | ||||
| OxLDL antibodies (U/L) | 17.7±6.4 | 25.4±13.2a | 34.6±9.8 | 45.7±21b | 51.8±15.2 | 59.6±19.4 |
| 20.4±10.0 | 40.4 ±17.6cc | 55.7±17.8cc | ||||
Notes: Results are expressed as the mean ± standard deviation.
P<0.001 when obese compared with nonobese in the control group;
P<0.01 when obese compared with nonobese in the IGT group;
P<0.01 when compared with the control group;
P<0.001 when compared with the control group.
Abbreviations: BMI, body mass index; FBS, fasting blood sugar; PP, postprandial blood sugar; TG, triglycerides; oxLDL, oxidized low-density lipoprotein; DM, diabetes mellitus; HDL, high-density lipoprotein; IGT, impaired glucose tolerance; LDL, low density lipoprotein; HbA1c, Hemoglobin A1c.
OxLDL was found to be significantly increased in the IGT group when compared with controls (P<0.001), with more elevation observed in diabetic subjects. However, when we compared this parameter between obese and nonobese subjects in the same group, there was no significant difference between the means of each subgroup.
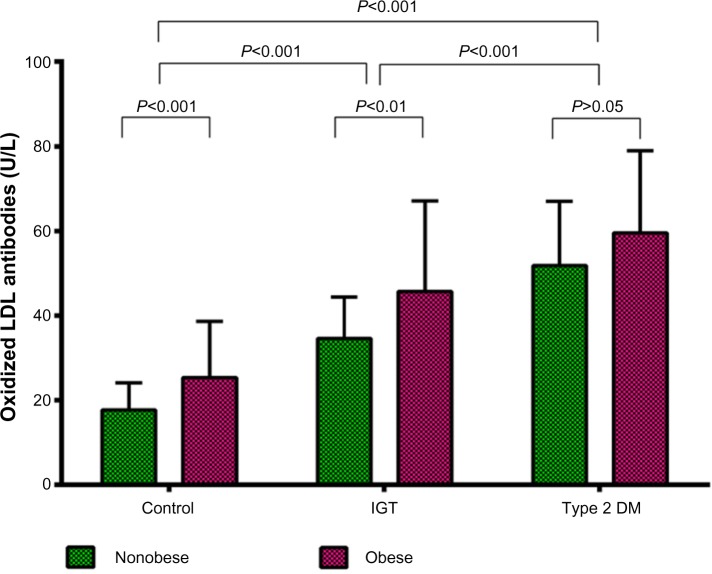
Mean anti-oxLDL antibody levels were increased significantly in the IGT group (40.4±17.6 U/L, P<0.001) when compared with controls, and an even greater mean increase was found in the type 2 DM group (55.7±17.8 U/L, P<0.001). Elevated mean levels of these antibodies were found in obese controls (25.4±13.2 U/L) when compared with nonobese controls (17.7±6.4, P<0.001), as shown in Table 1. In the IGT group, mean anti-oxLDL antibody levels were also significantly (P<0.01) increased in obese (45.7±21 U/L) compared with nonobese (34.6±9.8 U/L) subjects. The difference in mean anti-oxLDL antibody levels between the obese and nonobese subgroups was not significant in the type 2 DM group (Figure 1).
Figure 1.
Anti-oxidized LDL antibodies in the study groups.
Abbreviations: LDL, low-density lipoprotein; DM, diabetes mellitus; IGT, impaired glucose tolerance.
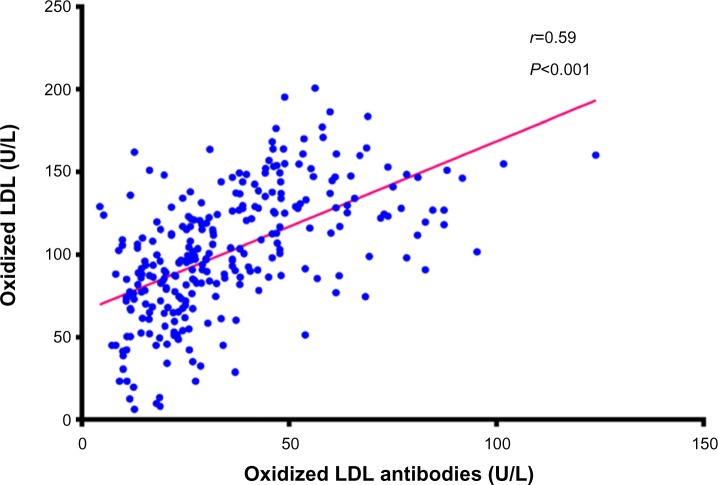
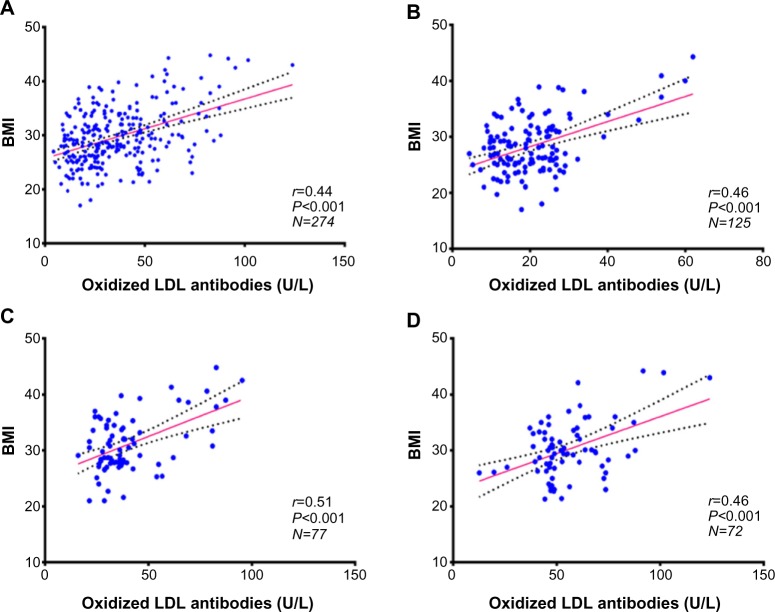
Figure 2 shows a positive correlation between oxLDL levels and oxLDL antibody levels in the study population (r=0.59, P<0.001). A positive correlation also existed between oxLDL antibodies and BMI in the control group (r=0.46, P<0.001), the IGT group (r=0.51, P<0.001), the type 2 DM group (r=0.46, P<0.001), and in the whole study population (r=0.44, P<0.001) as shown in Figure 3.
Figure 2.
Correlation between oxidized LDL and anti-oxidized LDL antibodies.
Abbreviation: LDL, low-density lipoprotein.
Figure 3.
Correlation between anti-oxidized LDL antibodies and body mass index.
Notes: (A) In all the study population; (B) in the control group; (C) in the IGT group; (D) in the type 2 DM group.
Abbreviations: LDL, low-density lipoprotein; DM, diabetes mellitus; IGT, impaired glucose tolerance; BMI, body mass index.
Discussion
Modified lipoproteins are immunogenic in humans and in animal models.12 Of all the modified forms of LDL, oxLDL and MDA-LDL are the most extensively investigated in biomedical research. OxLDL, because they are no longer recognized by LDL receptors, form immunogenic epitopes leading to formation of antibodies; the predominant isotype of oxLDL antibodies is IgG, of subclasses 1 and 3.13 These antibodies are attracting considerable attention in biomedical research in terms of their pathogenic or protective clinical role and the biochemical and anthropometric measures associated with elevated levels of these antibodies. The present study investigated the association between increased levels of anti-oxLDL antibodies and obesity in different glycemic situations.
Our data indicated higher levels of anti-oxLDL antibodies in the type 2 DM group (P<0.001). This finding cannot answer the question about the pathogenic or protective role of these antibodies, but it may give some clues. Diabetic individuals are believed to have a higher risk of cardiovascular disease than those without diabetes, and higher concentrations of anti-oxLDL antibodies seem to be pathogenic via formation of immune complexes. There is a significant body of evidence supporting a pathogenic role for immune complexes formed by oxLDL (oxLDL-IC) and their corresponding antibodies, in both atherosclerosis and macrovascular disease. OxLDL antibodies are able to activate the complement system via the classical pathway and induce FcR-mediated phagocytosis.17 Hunt et al indicate that high levels of oxLDL-IC and advanced glycation end products (AGE)-LDL-IC are important predictors of carotid intima-medial thickening in patients with type 1 diabetes.15 Crisby et al showed that MDA-LDL IgM levels were associated with an increased risk of cardiovascular events in patients with a previous myocardial infarction.16 Further, increased levels of oxLDL-IC have been found to be associated with development of coronary artery calcification.17
In contrast, Garrido-Sanchez et al found that patients with coronary disease and disorders of carbohydrate metabolism have much lower levels of IgG anti-oxLDL antibodies than normoglycemic patients,19 supporting the protective role of these antibodies. Our data indicate elevated levels in individuals with disorders of carbohydrate metabolism, ie, the type 2 DM and IGT groups, when compared with normoglycemic subjects. However, the sample studied by Garrido-Sanchez et al comprised hospitalized patients scheduled to undergo percutaneous coronary intervention, whereas coronary heart disease was an exclusion criterion in the present study.
In each study group, anti-oxLDL antibodies were found to be increased in obese subjects when compared with their nonobese counterparts, although there were no significant differences in age or other metabolic parameters in the same group. The difference in oxLDL concentrations between the obese and nonobese subgroups was significant in the control group (P<0.001) and in the IGT group (P<0.01) as shown in Figure 1, but was not significant in the type 2 DM group. This may be due to the weight loss often found in patients with diabetes.
The findings of the current study show a positive correlation between oxLDL levels and oxLDL antibody levels, as shown in Figure 2. Human anti-oxLDL antibodies play an important role in regulation of oxLDL levels. Supporting their proposed protective effect, these antibodies have been found in children and in healthy adults.20
BMI was found to be significantly increased in the type 2 DM group (P<0.05) and in the IGT group (P<0.001) compared with controls. Obesity is widely considered to be a strong risk factor for type 2 diabetes and its cardiovascular complications and is an independent risk factor for cardiovascular disease itself.30 Overweight and obesity are associated with higher lipoprotein oxidation, both abdominal and general obesity were shown to be independently associated with circulating oxLDL, weight reduction may potentially reduce oxLDL,31 especially in patients with metabolic syndrome.32 Previous studies have suggested a correlation between anti-oxLDL autoantibodies and anthropometric variables, making anti-oxLDL concentration a potential biochemical indicator of risk of metabolic syndrome.33 Our results show a significant positive correlation between BMI and oxLDL antibodies. This may explain the increased prevalence of type 2 DM and cardiovascular complications in obese subjects reported in many previous studies. The strong link between oxLDL concentration and risk of cardiovascular disease is well established, including its vital role in formation of foam cells. In the present study, a positive correlation between anti-oxLDL antibodies and BMI was found in all study groups, as shown in Figure 3. Weight loss recommendations in previous studies may also be beneficial for reduction of oxLDL antibodies, the immune complexes formed, and the ensuing complications of diabetes.
This study has several limitations. Our subjects were adult Saudi males, so we do not know if our findings are relevant in other ethnic populations, children, or women. Different procedures and kits are available for measuring oxLDL and oxLDL antibodies, and our results represent parameters measured according to the protocols described in the methodology section. The sample size was relatively small, and did not include measurement of cardiovascular endpoints. Large studies addressing clinical outcomes are needed to confirm our findings.
Conclusion
Increased levels of anti-oxLDL antibodies are associated with obesity and are positively correlated with BMI. Anti-oxLDL antibodies are increased in individuals with IGT or type 2 DM when compared with subjects who have normal glucose tolerance.
Acknowledgments
This research project was supported and funded by a grant (43109012) from the Institute of Scientific Research and Revival of Islamic Culture, Umm Al-Qura University, Makkah, Kingdom of Saudi Arabia. It represents a collaboration between researchers from Umm Al-Qura University and University of Gezira, Sudan.
Footnotes
Author contributions
ATB: biochemical laboratory investigations, statistical analysis, preparation of tables and figures, and editing, styling, writing, and revising of the main manuscript text. OME: design of the study, and writing and revising of the main manuscript text. AAA: design of the study, clinical selection, diagnosis and classification of cases, and writing and revising of the main manuscript text. AMA: design of the study, interpretation of biochemical results, writing and revising of the main manuscript text. BEA: follow-up of clinical cases, statistical analysis, and writing and revising of the main manuscript text. HYZ: design of the study and revising of the main manuscript text. SHF: biochemical laboratory investigations, editing, styling, and revising the manuscript. EMN: design of the study, interpretation of biochemical laboratory investigations, preparation of tables and figures, and editing, styling, writing, and revising of the main manuscript text. All authors read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zuliani G, Morieri ML, Volpato S, et al. Determinants and clinical significance of plasma oxidized LDLs in older individuals. A 9 years follow-up study. Atherosclerosis. 2013;226(1):201–207. doi: 10.1016/j.atherosclerosis.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Virella G, Lopes-Virella MF. The pathogenic role of the adaptive immune response to modified LDL in diabetes. Front Endocrinol (Lausanne) 2012;3:76. doi: 10.3389/fendo.2012.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perrin-Cocon L, Diaz O, Andre P, Lotteau V. Modified lipoproteins provide lipids that modulate dendritic cell immune function. Biochimie. 2013;95(1):103–108. doi: 10.1016/j.biochi.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan M, Aviram M, Hayek T. Oxidative stress and macrophage foam cell formation during diabetes mellitus-induced atherogenesis: role of insulin therapy. Pharmacol Ther. 2012;136(2):175–185. doi: 10.1016/j.pharmthera.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 5.El-Bassiouni EA, Helmy MH, El-Zoghby SM, El-Nabi Kamel MA, Hosny RM. Relationship between level of circulating modified LDL and the extent of coronary artery disease in type 2 diabetic patients. Br J Biomed Sci. 2007;64(3):109–116. doi: 10.1080/09674845.2007.11732768. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Mohamed AS, Zhou SH. Oxidized low density lipoprotein, stem cells, and atherosclerosis. Lipids Health Dis. 2012;11:85. doi: 10.1186/1476-511X-11-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wegner M, Piorunska-Stolzmann M, Araszkiewicz A, et al. Does oxidized LDL contribute to atherosclerotic plaque formation and microvascular complications in patients with type 1 diabetes? Clin Biochem. 2012;45(18):1620–1623. doi: 10.1016/j.clinbiochem.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Shimada K, Mokuno H, Matsunaga E, et al. Predictive value of circulating oxidized LDL for cardiac events in type 2 diabetic patients with coronary artery disease. Diabetes Care. 2004;27(3):843–844. doi: 10.2337/diacare.27.3.843. [DOI] [PubMed] [Google Scholar]
- 9.Garrido-Sanchez L, Cardona F, Garcia-Fuentes E, et al. Anti-oxidized low-density lipoprotein antibody levels are associated with the development of type 2 diabetes mellitus. Eur J Clin Invest. 2008;38(9):615–621. doi: 10.1111/j.1365-2362.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X, Yang Z, Chandrakala AN, Pressley D, Parthasarathy S. Oxidized low density lipoproteins – do we know enough about them? Cardiovasc Drugs Ther. 2011;25(5):367–377. doi: 10.1007/s10557-011-6326-4. [DOI] [PubMed] [Google Scholar]
- 11.Parthasarathy S, Raghavamenon A, Garelnabi MO, Santanam N. Oxidized low-density lipoprotein. Methods Mol Biol. 2010;610:403–417. doi: 10.1007/978-1-60327-029-8_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopes-Virella MF, Virella G. Pathogenic role of modified LDL antibodies and immune complexes in atherosclerosis. J Atheroscler Thromb. 2013;20(10):743–754. doi: 10.5551/jat.19281. [DOI] [PubMed] [Google Scholar]
- 13.Virella G, Carter RE, Saad A, Crosswell EG, Game BA, Lopes-Virella MF. Distribution of IgM and IgG antibodies to oxidized LDL in immune complexes isolated from patients with type 1 diabetes and its relationship with nephropathy. Clin Immunol. 2008;127(3):394–400. doi: 10.1016/j.clim.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopes-Virella MF, Virella G. Clinical significance of the humoral immune response to modified LDL. Clin Immunol. 2010;134(1):55–65. doi: 10.1016/j.clim.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt KJ, Baker N, Cleary P, et al. Oxidized LDL and AGE-LDL in circulating immune complexes strongly predict progression of carotid artery IMT in type 1 diabetes. Atherosclerosis. 2013;231(2):315–322. doi: 10.1016/j.atherosclerosis.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crisby M, Henareh L, Agewall S. Relationship between oxidized LDL, IgM, and IgG Autoantibodies to oxLDL levels with recurrent cardiovascular events in Swedish patients with previous myocardial infarction. Angiology. 2013 Nov 27; doi: 10.1177/0003319713512720. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 17.Lopes-Virella MF, Baker NL, Hunt KJ, Lachin J, Nathan D, Virella G. Oxidized LDL immune complexes and coronary artery calcification in type 1 diabetes. Atherosclerosis. 2011;214(2):462–467. doi: 10.1016/j.atherosclerosis.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos AO, Fonseca FA, Fischer SM, et al. High circulating autoantibodies against human oxidized low-density lipoprotein are related to stable and lower titers to unstable clinical situation. Clin Chim Acta. 2009;406(1–2):113–118. doi: 10.1016/j.cca.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Garrido-Sanchez L, Garcia-Pinilla JM, Jimenez-Navarro M, et al. Reduced levels of anti-MDA LDL antibodies in patients with carbohydrate metabolism disorders. Clin Lab. 2011;57(11–12):901–907. [PubMed] [Google Scholar]
- 20.Shoenfeld Y, Wu R, Dearing LD, Matsuura E. Are anti-oxidized low-density lipoprotein antibodies pathogenic or protective? Circulation. 2004;110(17):2552–2558. doi: 10.1161/01.CIR.0000143225.07377.EA. [DOI] [PubMed] [Google Scholar]
- 21.Rathmann W, Giani G. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(10):2568–2569. doi: 10.2337/diacare.27.10.2568. [DOI] [PubMed] [Google Scholar]
- 22.Du M, Wu M, Fu D, et al. Effects of modified LDL and HDL on retinal pigment epithelial cells: a role in diabetic retinopathy? Diabetologia. 2013;56(10):2318–2328. doi: 10.1007/s00125-013-2986-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hogan P, Dall T, Nikolov P. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26(3):917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 24.Rajkovic N, Zamaklar M, Lalic K, et al. OP3: oxidized LDL as residual lipid risk marker in type 2 diabetes. Diabetes Metab. 2012;38(Suppl 5):S98–S99. [Google Scholar]
- 25.Cicero AF, Reggi A, Tartagni E, Grandi E, D’Addato S, Borghi C, Brisighella Heart Study Dietary determinants of oxidized-low-density lipoprotein antibodies in a sample of pharmacologically untreated non-smoker subjects: data from the Brisighella Heart Study. Adv Clin Exp Med. 2013;22(1):69–76. [PubMed] [Google Scholar]
- 26.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 27.Holvoet P, Macy E, Landeloos M, et al. Analytical performance and diagnostic accuracy of immunometric assays for the measurement of circulating oxidized LDL. Clin Chem. 2006;52(4):760–764. doi: 10.1373/clinchem.2005.064337. [DOI] [PubMed] [Google Scholar]
- 28.Craig WY, Poulin SE, Nelson CP, Ritchie RF. ELISA of IgG antibody to oxidized low-density lipoprotein: effects of blocking buffer and method of data expression. Clin Chem. 1994;40(6):882–888. [PubMed] [Google Scholar]
- 29.Matsumoto H, Uchino M, Kato M. Evaluation of haemoglobin A1c measurement by an enzymatic method using an automated analyser that has an on-board haemolysis system. Ann Clin Biochem. 2013;50(Pt 5):443–449. doi: 10.1177/0004563213476859. [DOI] [PubMed] [Google Scholar]
- 30.Luca AC, Iordache C. Obesity – a risk factor for cardiovascular diseases. Rev Med Chir Soc Med Nat Iasi. 2013;117(1):65–71. [PubMed] [Google Scholar]
- 31.Beck J, Ferrucci L, Sun K, et al. Circulating oxidized low-density lipoproteins are associated with overweight, obesity, and low serum carotenoids in older community-dwelling women. Nutrition. 2008;24(10):964–968. doi: 10.1016/j.nut.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tumova E, Sun W, Jones PH, Vrablik M, Ballantyne CM, Hoogeveen RC. The impact of rapid weight loss on oxidative stress markers and the expression of the metabolic syndrome in obese individuals. J Obes. 2013;2013:729515. doi: 10.1155/2013/729515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanches LB, da Silva IT, Paz AF, et al. Anti-oxLDL autoantibodies and their correlation with lipid profile and nutritional status in adolescents. J Pediatr (Rio J) 2008;84(3):258–263. doi: 10.2223/JPED.1805. [DOI] [PubMed] [Google Scholar]