Summary
This study characterizes unique nicotine metabolic profiles for five ethnic/racial groups with variable lung cancer risk, low nicotine and cotinine N-glucuronidation in AAs, low C-oxidation in Japanese, but no difference in C-oxidation among Whites, AAs and LAs.
Abstract
Nicotine metabolism influences smoking behavior and differences in metabolism probably contribute to ethnic variability in lung cancer risk. We report here on the proportion of nicotine metabolism by cytochrome P450 2A6-catalyzed C-oxidation, UDP-glucuronosyl transferase 2B10 (UGT2B10)-catalyzed N-glucuronidation and flavin monooxygenase 3-catalyzed N-oxidation in five ethnic/racial groups and the role of UGT2B10 genotype on the metabolic patterns observed. Nicotine and its metabolites were quantified in urine from African American (AA, n = 364), Native Hawaiian (NH, n = 311), White (n = 437), Latino (LA, n = 453) and Japanese American (JA, n = 674) smokers. Total nicotine equivalents, the sum of nicotine and six metabolites, and nicotine metabolism phenotypes were calculated. The relationship of UGT2B10 genotype to nicotine metabolic pathways was determined for each group; geometric means were computed and adjusted for age, sex, creatinine, and body mass index. Nicotine metabolism patterns were unique across the groups, C-oxidation was lowest in JA and NH (P < 0.0001), and N-glucuronidation lowest in AA (P < 0.0001). There was no difference in C-oxidation among Whites and AA and LA. Nicotine and cotinine glucuronide ratios were 2- and 3-fold lower in AA compared with Whites. Two UGT variants, a missense mutation (Asp67Tyr, rs61750900) and a splice variant (rs116294140) accounted for 33% of the variation in glucuronidation. In AA, the splice variant accounted for the majority of the reduced nicotine glucuronidation. UGT2B10 variant allele carriers had increased levels of C-oxidation (P = 0.0099). Our data indicate that the relative importance of nicotine metabolic pathways varies by ethnicity, and all pathways should be considered when characterizing the role of nicotine metabolism on smoking behavior and cancer risk.
Introduction
Cigarette smoking is the leading cause of lung cancer and is responsible for the majority of cancer deaths in the USA (1). However, lung cancer risk due to smoking differs by ethnic/racial groups (2). Even after accounting for cigarettes per day (CPD) and years of smoking, African Americans (AAs) and Native Hawaiians (NHs) have a greater lung cancer risk than Whites, whereas Latino Americans (LAs) and Japanese Americans (JAs) have a lower risk (2). Nicotine is the primary addictive agent in tobacco and nicotine metabolism is associated with smoking behaviors (3). Ethnic differences in nicotine metabolism may affect cancer risk by influencing smoking intensity and therefore carcinogen exposure per cigarette, or by affecting carcinogen activation and detoxification, because enzymes that metabolize nicotine also metabolize tobacco carcinogens (4–7). A complete understanding of nicotine metabolism by ethnic group is critical to determining the underlying mechanisms contributing to ethnic differences in lung cancer risk.
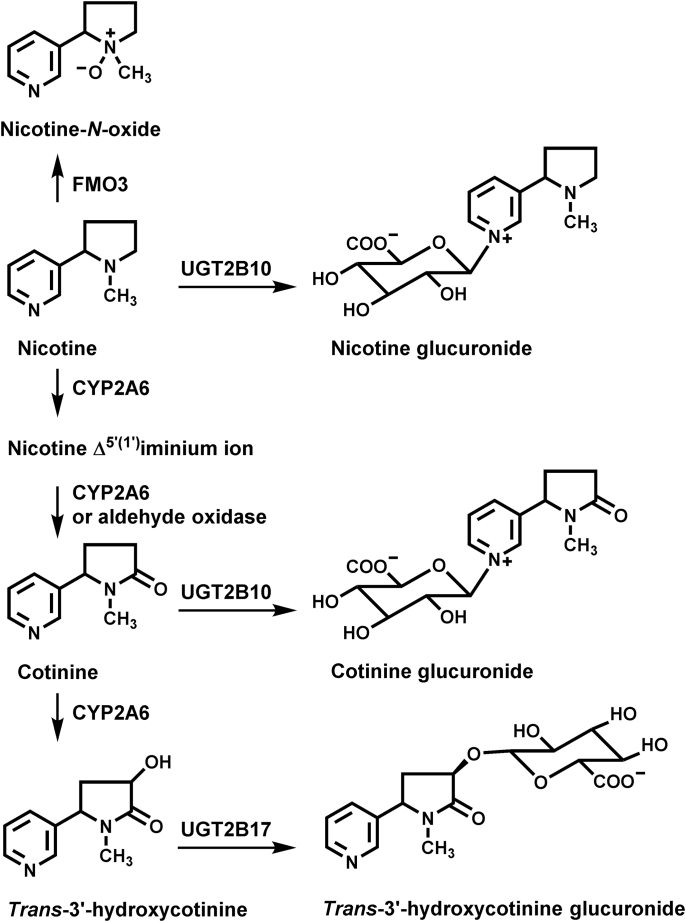
Nicotine is metabolized primarily by three enzymes (8,9): cytochrome P450 2A6 (CYP2A6), UDP-glucuronosyl transferase 2B10 (UGT2B10) and flavin monooxygenase 3 (FMO3, Figure 1). In most smokers, CYP2A6-catalyzed nicotine C-oxidation is the predominant pathway, accounting for >75% of nicotine metabolism (8). Nicotine N-glucuronidation and N-oxidation each typically account for <10% of metabolism. However, in some smokers nicotine glucuronide comprises as much as 40% of the excreted nicotine dose (10). CYP2A6-catalyzed C-oxidation generates nicotine Δ5′(1′) iminium ion, which is further metabolized by CYP2A6 or aldehyde oxidase to cotinine (8,11). Cotinine is then converted to trans 3′-hydroxycotinine (3-HCOT), a reaction also catalyzed by CYP2A6. Both cotinine and 3-HCOT are further metabolized by glucuronidation. UGT2B17 and to a lesser extent UGT2B7 catalyze 3-HCOT O-glucuronidation (12). UGT2B10 is the primary if not the only catalyst of nicotine and cotinine N-glucuronidation (9,13), although in vitro UGT1A4 also catalyzes this reaction (14).
Fig. 1.
Nicotine metabolic pathways.
Total nicotine equivalent (TNE), the sum of nicotine and its metabolites in urine, is an excellent biomarker of total cigarette smoke exposure (15,16). We reported previously that the TNE (after adjustment for CPD) excreted by JAs smokers were significantly lower than the levels excreted by Whites (17). Exposure to the tobacco specific lung carcinogen, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone was also lower in JAs, consistent with their lower lung cancer risk. In the same study, we reported intermediate levels of nicotine equivalents and metabolite of NNK, NNAL in NHs (18). These data in NHs do not support an increase in carcinogen exposure as an explanation for the increased cancer risk of NHs compared with Whites. A large study of over 3000 smokers reported that TNE excreted per cigarette was 20% higher in AAs compared with Whites (19). This is consistent with an early report that AAs take in more nicotine per cigarette than do Whites (20) and the relative risks of lung cancer in these two groups.
Smokers modulate their tobacco consumption to achieve brain nicotine concentrations that maintain the desired effects (21). Therefore, decreased nicotine metabolism may contribute to decreased smoking due to the increased half-life of nicotine (22). Both CYP2A6 activity and genotype are associated with CPD in Japanese and smokers of European ancestry (3,17,23,24). Recently, a predictive nicotine metabolism model based on CYP2A6 haplotype was used to demonstrate a similar association between nicotine C-oxidation and CPD in AA nicotine-dependent smokers (25). There is some evidence that other pathways of nicotine metabolism may also influence tobacco consumption. Genetic variation in FMO3 is associated with differences in CPD (26), and a UGT2B10 missense mutation (Asp67Tyr) relatively common in Whites may also influence cigarette consumption (27).
The extent of nicotine N-glucuronidation by AAs is significantly lower than by Whites. This was reported >15 years ago in a nicotine infusion study (28) and was more recently confirmed in AAs using the nicotine patch (9). Both studies reported lower cotinine N-glucuronidation in AAs smokers compared with Whites. The only loss of function UGT2B10 variant identified at the time of the patch study was the Asp67Tyr variant. This variant did not explain the lower cotinine and nicotine N-glucuronidation in AAs (9). More recently, a UGT2B10 splice variant was identified, which has an allele frequency of >30% in AAs (29). We report here the effect of the splice variant and the Asp67Tyr variant on nicotine N-glucuronidation, and the effect of these variants on phenotypes of CYP2A6 and FMO3 activity in five ethnic groups. Nicotine and six metabolites were quantified in all groups and the proportion of nicotine metabolized by C-oxidation, N-glucuronidation and N-oxidation determined for each.
Materials and methods
Study population and data collection
This study comprises a subgroup of Multiethnic Cohort study (MEC) participants who were current smokers at the time of biospecimen collection. The MEC, a prospective study established to investigate the association of lifestyle and genetic factors with chronic diseases (30) is comprised of 215 251 men and women between the ages of 45 and 75 years at baseline, primarily from five ethnic/racial groups: AAs, JAs, LA, NHs and Whites. Between 1993 and 1996, potential participants were identified in Hawaii and California (primarily Los Angeles County) through drivers’ license files, voter registration lists, and Health Care Financing Administration files. Each participant completed a mailed, self-administered questionnaire regarding demographic, dietary, lifestyle, and other exposure factors. The Institutional Review Boards at the Universities of Southern California and Hawaii approved the study protocol.
Approximately 10 years after cohort entry, 2393 current smokers with no cancer diagnosis participated in the biospecimen subcohort, providing a blood sample and overnight or first morning urine, completing an epidemiologic questionnaire, smoking history questionnaire and medication record. Urine was kept on ice, then aliquots were stored in a –80°C freezer until analysis.
Genotyping
Blood lymphocyte DNA was genotyped using the Ilumina Human1M-Duo BeadChip; SHAPEIT (31) and IMPUTE2 (32) were used to impute SNPs from the Thousand Genomes Project (33), as described previously (34). Samples were genotyped for UGT2B17 copy number variant (TaqMan UGT2B17 assay, Hs03185327_cn) with 7900HT Fast Real-Time System (Life Technologies, Foster City, CA). Copy number was determined using Life Technologies CopyCaller v2.0 software. Counts with <0.50 confidence interval were excluded, ~5% blind duplicates were included for quality control. Hardy Weinberg Equilibrium was met for all five populations (P > 0.05).
The exclusion criteria used were: no UGT2B10 genotype data available, call rates ≤98%, genetic relatedness or conflicting or indeterminate sex. In addition, samples with TNE <1.27 nmol/ml (sum of four times the limit of quantization (LOQ) for nicotine, cotinine, 3-HCOT and nicotine N-oxide) were excluded. After all exclusions 2239 subjects were retained for analysis.
Nicotine and nicotine metabolite analysis
The urine samples diluted 1:10 were stored at –20°C until analysis. Diluted urine (10 μl), 400 µl 100mM ammonium acetate, pH 5.0, and methyl-d3 internal standards (1ng each) were added to paired 96-well plates, one for the analysis of free nicotine, cotinine and 3-HCOT and the second for total (free + glucuronide) nicotine, cotinine and 3-HCOT. One plate was incubated overnight at 37°C with β-glucuronidase (500 units). Samples from both plates were loaded on Oasis MCX 2mg solid phase extraction 96-well plates (Waters) conditioned previously with successive additions of 600 μl 2% ammonium hydroxide/methanol, 200 μl water and 200 μl 0.5% formic acid. Columns were then washed with 200 μl 0.5% formic acid and 400 μl methanol. Nicotine, cotinine and 3-HCOT were eluted with 50 µl 2% ammonium hydroxide:methanol. The eluent was acidified with formic acid, dried under a stream of nitrogen and the capped 96-well plate stored at –20°C until liquid chromatography–tandem mass spectrometry analysis. Samples were analyzed independently for nicotine N-oxide, the same method described earlier was used with no β-glucuronidase treatment and 0.125ng methyl-d3-nicotine N-oxide.
Liquid chromatography–tandem mass spectrometry analysis was on an Agilent 1100 capillary HPLC system interfaced to a Finnigan TSQ Vantage mass spectrometer with positive ESI (Thermo Electron, San Jose, CA). Samples, re-suspended in 25μl 100mM ammonium acetate:methanol, were injected twice on an Atlantis HILIC column (300 µm × 100 mm Waters Corporation). For the first injection, nicotine and nicotine N-oxide were eluted (t R, 2.99 and 3.01min) with acetonitrile: water:formic acid and TFA (86/12.6/1.4/0.01). In a second injection, cotinine (t R, 3.43min) and 3-HCOT (t R, 3.35 min) were eluted with acetonitrile:water:formic acid (95/3.5/1.5). The flow rate was 20 µl/min. Mass transitions were: d0-nicotine (m/z 163→130 and m/z 163→117), d3-nicotine (m/z 166→130 and m/z 166→117), d0-cotinine (m/z 177→98 and m/z 177→80), d3-cotinine (m/z 180→101 and m/z 180→80), d0-3-HCOT (m/z 193→134 and m/z 193→80), d3-3-HCOT (m/z 196→134 and m/z 196→80), d0-nicotine N-oxide (m/z 179→130 and m/z 179→117), d3-nicotine N-oxide (m/z 182→130 and m/z 182→117).
The LOQ for nicotine, cotinine and 3-HCOT (1 μl urine) were 13ng/ml, 20ng/ml and 18ng/ml. Positive control samples were included in each plate, the mean values (n = 50) were: 3-HCOT, 3019±127; total 3-HCOT, 3690±182; cotinine, 563±41; total cotinine, 1529±78; nicotine, 395±37; total nicotine, 571±45 and nicotine N-oxide, 266±17. For each plate five samples were reanalyzed on the next plate, resulting in 115 duplicate analyses for a range of concentrations. The average percent differences for the duplicates were: 3-HCOT 6.8%, total 3-HCOT 8.7%, cotinine 6.0%, total cotinine 5.4%, nicotine 9.7%, total nicotine 11.1% and nicotine N-oxide 7.5%. The coefficient of variation for 22 undiluted samples from 10 blind replicates provided with the MEC samples were: nicotine, 16.7%; cotinine, 10.1% and 3-HCOT, 11.4%.
Statistical methods
Metabolite concentrations were in nmol/ml. The glucuronide conjugates were calculated from the difference between total and free analytes. No samples included in the analysis had cotinine, nicotine or 3-HCOT values below the LOQ, nicotine N-oxide values below the LOQ (1.2ng/ml), were reported as half the LOQ. The dependent variables of interest were: TNE (sum of total cotinine, total nicotine, total 3-HCOT and nicotine N-oxide); proportion of cotinine glucuronidation (cotinine glucuronide/total cotinine); cotinine glucuronidation ratio (cotinine glucuronide/cotinine); proportion of nicotine glucuronidation (nicotine glucuronide/total nicotine); nicotine glucuronidation ratio (nicotine glucuronide/nicotine); nicotine glucuronidation/TNE; 3-HCOT glucuronidation (3-HCOT glucuronide/total 3-HCOT); nicotine N-oxide/TNE; nicotine N-oxide/nicotine; and three CYP2A6 phenotypes: (i) total 3-HCOT/total cotinine; (ii) total 3-HCOT/cotinine and (iii) the sum of total 3-HCOT and total cotinine divided by TNE.
Pearson’s partial correlation coefficients, adjusting for age, sex, race/ethnicity and creatinine levels (natural log), were computed for nicotine and cotinine glucuronidation, CPD and TNE. All metabolites, except the proportion of cotinine and nicotine glucuronidation and cotinine glucuronidation/TNE, were transformed by the natural log. When the metabolite was transformed, values presented are back-transformed to their natural scale.
To examine the difference of nicotine metabolites across UGT2B10 genetic variants, (coded as 0, 1 or 2) and across ethnic/racial groups, covariate-adjusted arithmetic and geometric (when applicable) means were computed for each ethnic/racial group at the mean covariate vector. We combined the genotypes of two variants to create a genetic score for glucuronidation. A genetic score of 0 was assigned to those who were homozygous wildtype for both rs61750900 (GG) and rs116294140 (AA), a genetic score of 1 to those who were heterozygous for either genotype (rs61750900 GT or rs116294140 CA) and a score of 2 to those who carried rs61750900 TT or rs116294140 CC, or both rs61750900 GT and rs116294140 CA. Multivariable linear models were adjusted for the following predictors: age at time of urine collection (continuous), sex, race, creatinine (natural log) and body mass index (BMI) (natural log). Among subjects in this analysis, 11 were missing BMI values and 42 missing values for CPD at time of urine collection. The missing values were imputed by the Markov Chain Monte–Carlo method based on age cohort entry, race/ethnicity, time between cohort entry and time of blood draw, and BMI at baseline and at blood draw (for missing BMI) or CPD at baseline and at blood draw and smoking duration (for missing CPD). Ten datasets were created for the imputed missing values, and the mean value was used to replace the missing value. To identify additional covariates that predicted cotinine glucuronidation levels, we used stepwise regression with four variables forced into the model: age at time of urine collection (continuous), sex, race, and creatinine (natural log). Variables that were allowed to compete in the stepwise regression analysis were ones that provided additional information on smoking (e.g. smoking duration) or nicotine intake [e.g. TNE or CYP2A6 activity (total 3-HCOT/cotinine)], BMI and dietary or lifestyle factors that could modify nicotine metabolism (e.g. alcohol, soy, cruciferous vegetable intake). Here, the cumulative R 2 value was used to assess the percentage of variation of cotinine or nicotine glucuronidation accounted for by the independent variables.
Results
A total of 2239 individuals from five ethnic/racial groups were analyzed for UGT2B10 genotype and nicotine metabolism phenotypes, 53% were female and the median CPD was 10, although CPD varied by group (Table I, Supplementary Table S1, available at Carcinogenesis Online). The lowest median CPD was reported by LAs (CPD = 7.07) and was almost 3-fold lower than the median reported by Whites, the group reporting the highest CPD (CPD = 20, Table I). The TNE excreted varied by ethnic group; however, the magnitude of the variation was less than for CPD. The ranking of the groups by TNE, high to low, differed from the ranking by CPD. The median TNE excreted by AAs, 44.5 nmol/ml was the highest among the five groups. However, AAs reported smoking a median of 10 CPD, a lower value than the 12 CPD reported by JAs, the group with the lowest median TNE, 27.3 nmol/ml. The median TNE excreted by LAs and NHs were not significantly different (32.2 versus 30.3 nmol/ml); despite a more than 2-fold difference in CPD.
Table I.
Cigarettes per day and urinary nicotine and nicotine metabolite concentrations by racial/ethnic groupa
| AAs (n = 364) | NHs (n = 311) | Whites (n = 437) | Latinos (n = 453) | JAs (n = 674) | |
|---|---|---|---|---|---|
| Median (25th–75th%) | Median (25th–75th%) | Median (25th–75th%) | Median (25th–75th%) | Median (25th–75th%) | |
| Total nicotine | 5.44 (2.9–11.0) | 6.19 (3.32–11.4)* | 5.42 (3.04–8.80) | 4.41 (1.92–7.72)* | 6.38 (3.54–11.8)* |
| Nicotine glucuronide/ total nicotine | 0.26 (0.12–0.42)b,* | 0.30 (0.18–0.42)* | 0.34 (0.21–0.48) | 0.37 (0.25–0.54)c* | 0.32 (0.21–0.44)d |
| Total cotinine | 10.7 (6.19–15.4) | 9.85 (5.62–14.1) | 10.7 (5.54–17.2) | 9.34 (4.49–14.8)* | 7.78 (4.34–12.8)* |
| Cotinine glucuronide/ total cotinine | 0.48 (0.28–0.61)b,* | 0.55 (0.45–0.64)* | 0.58 (0.48–0.69) | 0.60 (0.49–0.69)e | 0.51 (0.41–0.60)* |
| Total 3-HCOT | 22.7 (11.5–44.4)* | 10.6 (5.61–19.9)* | 16.9 (8.77–30.1) | 16.4 (7.71–27.8) | 6.98 (2.37–15.6)* |
| 3-HCOT glucuronide/ total 3HCOT | 0.27 (0.21–0.35)b | 0.19 (0.14–0.25)* | 0.24 (0.18–0.30) | 0.23 (0.17–0.29) | 0.18 (0.12–0.24)f* |
| Nicotine N-oxide | 2.65 (1.37–4.89)* | 1.88 (0.96–3.56) | 1.76 (0.90–3.04) | 1.49 (0.64–3.07)* | 2.16 (1.14–3.6)* |
| TNEs | 44.4 (27.1–74.0)* | 30.3 (19.4–46.8)* | 36.3 (21.90–61.5) | 32.2 (20.8–53.6) | 27.3 (15.8–43.4)* |
| Products of C-oxidation | 0.81 (0.72–0.87) | 0.73 (0.64–0.81)* | 0.79 (0.71–0.86) | 0.81 (0.72–0.88) | 0.66 (0.47–0.79)* |
| CPD | 10.0 (5–15)* | 15.0 (9–20)* | 20.0 (10–20) | 7.07 (4.0–12)* | 12.0 (9–20)* |
aMetabolite values are expressed in nmol/ml, 3-HCOT, trans 3′-hydroxycotinine, TNE is the sum of total nicotine, total cotinine, total 3-HCOT and nicotine N-oxide, products of C-oxidation are the sum of total cotinine and total 3-HCOT divided by TNE.
b n = 363.
c n = 450.
d n = 673
e n = 452.
f n = 666.
*P-value for comparison with Whites (P < 0.05).
The median concentrations for total nicotine, total cotinine, total 3-HCOT and nicotine N-oxide excreted by the five ethnic/racial groups are reported in Table I. The medians of the proportion of nicotine, cotinine and 3-HCOT excreted are also presented. AAs had the lowest proportion of nicotine and cotinine excreted as glucuronide conjugates and JAs and NHs the lowest 3-HCOT glucuronidation.
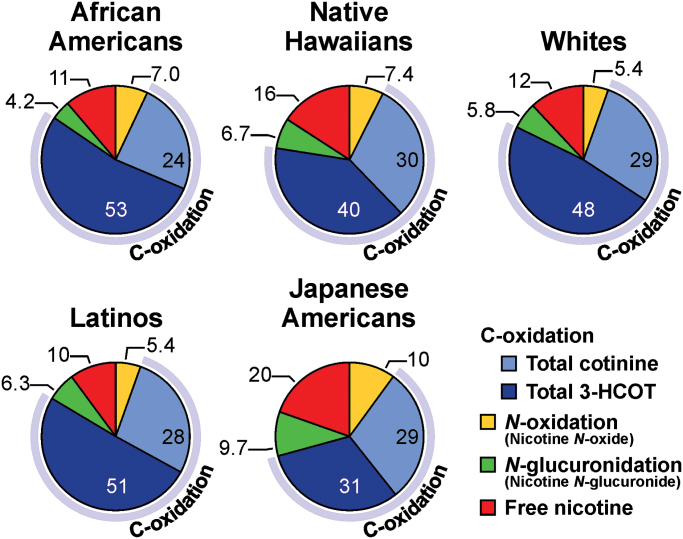
The unique patterns of nicotine metabolism across the five ethnic groups are illustrated in Figure 2. The pie graphs in this figure represent the percentage of TNE excreted as products of each of the three nicotine metabolism pathways (Figure 1), N-oxidation, N-glucuronidation and C-oxidation (the sum of cotinine, 3-HCOT and their respective glucuronide conjugates). Among the five groups, JAs excreted the lowest levels of nicotine C-oxidation products and the greatest amount of unchanged nicotine, nicotine glucuronide and N-oxide. Among AAs, Whites and LAs, there was no significant difference in the proportion of nicotine metabolized by C-oxidation (Table I, Figure 2). The extent of nicotine C-oxidation by NHs was less than that by these three groups but greater than by JAs. The percentage of unmetabolized nicotine excreted by NHs was less than for JAs but greater than for the other groups. Nicotine N-oxidation levels were higher in NHs relative to Latinos and Whites, but similar to the levels in AAs. Nicotine N-glucuronidation levels were lowest in AAs, accounting for 4.2% of nicotine metabolism, compared with 9.7% for JAs. The proportion of nicotine metabolized by N-glucuronidation ranged from 6.7% to 5.8% of TNE in the other three ethnic groups.
Fig. 2.
Proportion of nicotine metabolized by C-oxidation, N-glucuronidation and N-oxidation in five ethnic/racial groups (values are the molar percent of nicotine and six metabolites excreted in urine). The values for each slice of the pie are the mean percentage of the compound relative to TNE. See Table I for the median (25th−75th) concentrations of total nicotine, total cotinine, total 3-HCOT, nicotine N-oxide and TNE, the proportion of nicotine excreted as a glucuronide and the proportion of TNE excreted as products of C-oxidation.
UGT2B10 genotype and nicotine metabolism pathways
The observed ethnic specific patterns of nicotine metabolism are in part driven by genetic variations in the enzymes that catalyze C-oxidation, N-glucuronidation and N-oxidation; CYP2A6, UGT2B10 and FMO3. The effect of genetic variation in these enzymes may directly or indirectly influence the relative contribution of a particular pathway to nicotine metabolism. The influence of two UGT2B10 SNPs (rs116294140 and rs61750900) on nicotine metabolism by each pathway was determined in the five ethnic groups (Table II). The rs61750900 SNP codes for a missense mutation and the resulting Asp67Tyr UGT2B10 variant enzyme significantly reduced nicotine and cotinine glucuronidation (27,35). The SNP rs116294140 codes for a splice variant. The splice variant is common in AAs with a frequency of 34% and rare in Whites (0.23%), whereas the Asp67Tyr variant is most common in Whites (8.5%) and uncommon in JAs (0.3%, Supplementary Table S1, available at Carcinogenesis Online). As expected, individuals homozygous for either the splice variant (CC) or the Asp67Tyr missense mutation (GG) excreted little or no nicotine glucuronide (<0.2% of TNE, Table II). Neither UGT2B10 SNP affected the proportion of TNE excreted as nicotine N-oxide. However, AAs carriers of the UGT2B10 splice variant excreted a larger proportion of TNE as the products of nicotine C-oxidation: 82.6% compared with 75.7% for the CC and AA genotypes, respectively.
Table II.
Geometric means of nicotine metabolite ratios stratified by UGT2B10 genotype
| UGT 2B10 genotype | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp67Tyr variant (rs6175900) | Splice variant (rs116294140) | Both variants | |||||||||
| Alla | Alla | AAs | All | ||||||||
| N | Means (95% CI) | N | Means (95% CI) | N | Means (95% CI) | Genetic scoreb | N | Means (95% CI) | |||
| Nicotine metabolism pathways | |||||||||||
| N-glucuronidation | |||||||||||
| Nicotine glucuronide/TNE | GG | 2028 | 0.039 (0.037–0.042) | AA | 1853 | 0.042 (0.038–0.045) | 156 | 0.03 (0.024–0.039) | 0 | 1664 | 0.047 (0.043–0.051) |
| GT | 204 | 0.014 (0.01–0.021) | CA | 333 | 0.025 (0.016–0.04) | 166 | 0.02 (0.013–0.02) | 1 | 503 | 0.021 (0.018–0.024) | |
| TTc | 2 | 0.000 | CCc | 48 | 0.002 (0.001–0.003) | 41 | 0.00 (0.001–0.003) | 2c | 67 | 0.000 (0-0) | |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| N-oxidation | |||||||||||
| Nicotine N-oxide/TNE | GG | 2032 | 0.054 (0.052–0.056) | AA | 1858 | 0.053 (0.051–0.055) | 157 | 0.052 (0.046–0.058) | 0 | 1668 | 0.054 (0.052–0.056) |
| GT | 205 | 0.052 (0.044–0.061) | CA | 333 | 0.050 (0.041–0.063) | 166 | 0.050 (0.044–0.056) | 1 | 504 | 0.052 (0.048–0.055) | |
| TTc | 2 | 0.043 (0.041–0.044) | CCc | 48 | 0.060 (0.049–0.075) | 41 | 0.056 (0.045–0.07) | 2c | 65 | 0.070 (0.046–0.106) | |
| P-value | 0.5978 | 0.2584 | 0.6361 | 0.2553 | |||||||
| C-oxidationd | |||||||||||
| (Total 3-HCOT+total cotinine)/TNE | GG | 2032 | 0.726 (0.718–0.734) | AA | 1858 | 0.722 (0.713–0.73) | 157 | 0.757 (0.729–0.784) | 0 | 1668 | 0.720 (0.711–0.729) |
| GT | 205 | 0.743 (0.703–0.782) | CA | 333 | 0.746 (0.695–0.797) | 166 | 0.792 (0.765–0.819) | 1 | 504 | 0.748 (0.731–0.764) | |
| TTc | 2 | 0.913 (0.894–0.932) | CCc | 48 | 0.777 (0.727–0.828) | 41 | 0.826 (0.774–0.879) | 2c | 67 | 0.758 (0.66–0.856) | |
| P-value | 0.4112 | 0.5023 | 0.0334 | 0.0116 | |||||||
| Measures of enzyme activity | |||||||||||
| UGT2B10 | |||||||||||
| Nicotine glucuronide/ nicotine | GG | 2028 | 0.377 (0.344–0.414) | AA | 1853 | 0.39 (0.352–0.429) | 156 | 0.31 (0.225–0.421) | 0 | 1664 | 0.45 (0.402–0.496) |
| GT | 204 | 0.126 (0.08–0.201) | CA | 333 | 0.23 (0.128–0.411) | 166 | 0.19 (0.143–0.264) | 1 | 503 | 0.19 (0.155–0.224) | |
| TTc | 2 | 0.00 | CCc | 48 | 0.01 (0.008–0.027) | 41 | 0.02 (0.01–0.036) | 2c | 67 | 0.00 (0–0.001) | |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| Cotinine glucuronide/ cotinine | GG | 2031 | 1.12 (1.07–1.17) | AA | 1857 | 1.28 (1.22–1.38) | 157 | 1.10 (0.97–1.24)b | 0 | 1668 | 1.38 (1.33–1.44) |
| GT | 205 | 0.54 (0.43–0.68) | CA | 333 | 0.63 (0.5–0.80) | 166 | 0.48 (0.42–0.54) | 1 | 504 | 0.68 (0.63–0.73) | |
| TTc | 1 | 0.01 | CCc | 47 | 0.02 (0.02–0.03) | 40 | 0.02 (0.02–0.03) | 2c | 65 | 0.01 (0.005–0.013) | |
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
| CYP2A6 | |||||||||||
| Total 3-HCOT/total cotinine | GG | 2032 | 1.38 (1.34–1.43) | AA | 1858 | 1.33 (1.29–1.38) | 157 | 1.65 (1.469–1.853) | 0 | 1668 | 1.29 (1.24–1.34) |
| GT | 205 | 1.84 (1.56–2.18) | CA | 333 | 1.63 (1.31–2.02) | 166 | 2.24 (2.003–2.513) | 1 | 504 | 1.78 (1.66–1.90) | |
| TTc | 2 | 4.06 (2.82–5.30) | CCc | 48 | 3.76 (3.03–4.67) | 41 | 4.75 (3.794–5.952) | 2c | 67 | 3.09 (2.04–4.69) | |
| P-value | 0.0011 | 0.0006 | <0.0001 | <0.0001 | |||||||
| Total 3-HCOT/cotinine | GG | 2031 | 3.21 (3.10–3.32) | AA | 1857 | 3.23 (3.11–3.35) | 157 | 3.78 (3.35–4.26) | 0 | 1668 | 3.23 (3.10–3.37) |
| GT | 205 | 3.35 (2.82–3.98) | CA | 333 | 2.90 (2.32–3.63) | 166 | 3.64 (3.23–4.09) | 1 | 504 | 3.19 (2.97–3.43) | |
| TTc | 1 | 5.35 | CCc | 47 | 3.72 (2.97–4.66) | 40 | 4.52 (3.57–5.72) | 2c | 65 | 3.11 (2.01–4.81) | |
| P-value | 0.631 | 0.6264 | 0.2706 | 0.9452 | |||||||
| FMO3 | |||||||||||
| Nicotine-N-oxide/nicotine | GG | 2028 | 0.54 (0.52–0.56) | AA | 1853 | 0.52 (0.50–0.54) | 156 | 0.55 (0.48–0.63) | 0 | 1664 | 0.53 (0.51–0.56) |
| GT | 204 | 0.54 (0.45–0.65) | CA | 333 | 0.50 (0.39–0.64) | 166 | 0.67 (0.59–0.76) | 1 | 503 | 0.51 (0.47–0.55) | |
| TTc | 2 | 1.03 (0.69–1.38) | CCc | 48 | 0.66 (0.52–0.85) | 41 | 0.77 (0.6–0.97) | 2c | 67 | 0.71 (0.44–1.14) | |
| P-value | 0.933 | 0.7811 | 0.0235 | 0.287 | |||||||
aIncudes all five ethnic groups, values are adjusted for age, sex, creatinine (natural log), BMI (natural log), and race.
bGenetic score 0, rs61750900 GG and rs116294140 AA; score 1, rs61750900 GT or rs116294140 CA; score 2, rs61750900 TT or rs116294140 CC, or both rs61750900 GT and rs116294140 CA
cIf n < 10 the mean (minimum-maximum) are presented, not the geometric means and 95% CIs.
dArithmetic means are presented.
A genetic score for decreased nicotine glucuronidation was defined. A score of 2 was assigned to homozygous carriers of either the splice variant or Asp67Tyr and to individuals heterozygous for both variants. A score of 1 was assigned to individuals that carried one allele of either variant and a score of 0 assigned if they carried neither variant. Individuals with a genetic score of 2 excreted no nicotine glucuronide. The proportion of nicotine metabolized by C-oxidation increased as the genetic score increased (Table II).
UGT2B10 catalyzes both nicotine and cotinine glucuronidation and either the ratio of cotinine glucuronide to cotinine or nicotine glucuronide to nicotine have been used as phenotypic measures of UGT2B10 activity. In this multiethnic cohort, the nicotine and cotinine glucuronide ratios were well correlated (P < 0.0001). The Pearson’s partial coefficient, r, was 0.33 for all subjects, but varied by ethnic group from 0.44 for AAs to 0.22 for JAs. Likewise, the proportions of nicotine and cotinine glucuronidated were well correlated; 0.42 for all subjects, 0.53 for AAs, 0.30 for Whites and 0.36–0.38 for the other three groups (P < 0.0001, for all). The primary, if not exclusive, role of UGT2B10 as the catalyst of nicotine and cotinine N-glucuronidation was confirmed by the effect of UGT2B10 genotype on both glucuronide ratios (Table II). The extent of either cotinine or nicotine glucuronidation was significantly different by genotype. Individuals who are homozygous for the variant allele for either SNP (GG, rs61750900 or CC, rs116294140) do not glucuronidate cotinine or nicotine to a significant extent. The levels of glucuronidation by individuals heterozygous for the splice variant (CA) or with a genetic score of 1 were half those of homozygous wild-type individuals, genetic score 0 (Table II). The 95% confidence intervals for the cotinine glucuronide ratio was much smaller than for the nicotine glucuronide ratio supporting the use of the former as a more robust measure of UGT2B10 activity. Therefore, cotinine glucuronidation ratios were used to investigate variation in UGT2B10 activity by ethnic group.
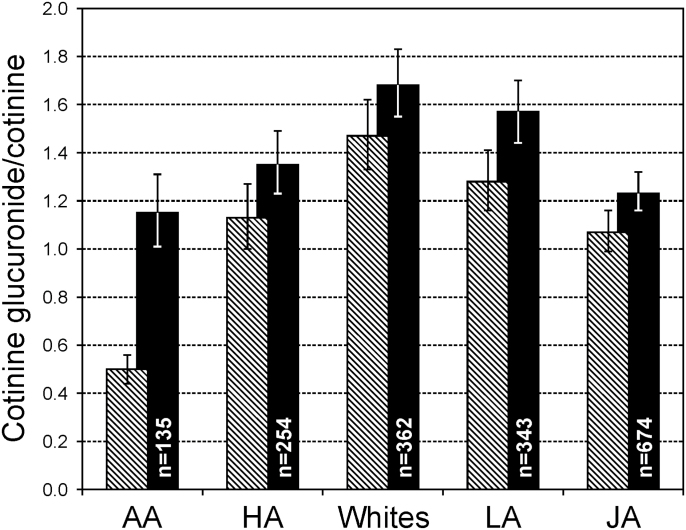
The relative levels of UGT2B10 activity measured by either the cotinine glucuronide/cotinine ratio or the proportion of cotinine glucuronidated were as follows: AAs < JAs < NHs < LAs = Whites (Supplementary Table S2, available at Carcinogenesis Online, and Figure 3, hatched bars). The cotinine glucuronide to cotinine ratio in AAs was one third the ratio in Whites, 0.50 versus 1.47. This ratio for AAs who did not carry the UGT2B10 splice variant was 1.10 (Table II). When the cotinine glucuronidation ratio was stratified by UGT2B10 genetic score, the ratio still differed significantly by ethnic group when the genetic score was 1 or 0 (Supplementary Table S3, available at Carcinogenesis Online); however, the magnitude of the difference was much smaller (Figure 3, solid bars). The ratio in AAs that carried neither the splice variant nor the Asp67Tyr variant (genetic score 0) was 1.15 compared with the ratio of 1.68 in Whites. The ratio in Latinos was not significantly different from Whites, whereas the ratio for JAs and NHs Americans were lower, 1.23 and 1.35, respectively.
Fig. 3.
Cotinine glucuronidation stratified by ethnic/racial group, hatched bars all subjects, Dark bars include only individuals with UGT2B10 genetic score 0, i.e. who carry no variant allele for either the Asp67Tyr or the splice variant.
Because cotinine is both a product of CYP2A6-catalyzed nicotine metabolism and a substrate for CYP2A6, the effect of CYP2A6 phenotype (total 3-HCOT/cotinine) on the cotinine glucuronidation phenotypes was determined (Supplementary Table S4, available at Carcinogenesis Online). Within the lowest tertiles of total 3-HCOT/cotinine, there is no difference in cotinine glucuronidation between Whites and JAs or NHs. AAs at any tertile have lower levels of cotinine glucuronidation.
We found that the associations of age, race, sex and creatinine levels with cotinine glucuronidation explained only 11.6% of the variance (Table III). Age was positively associated with cotinine glucuronidation (P = 0.04). The male sex, compared with the female sex (P < 0.0001), AA (P < 0.0001), NH (P = 0.007) and JA (P = 0.04) ethnicities versus White ethnicity were negatively associated with cotinine glucuronidation. Using stepwise regression to identify other determinants of cotinine glucuronidation, BMI (natural log) and CYP2A6 activity (natural log) were positively associated with cotinine glucuronidation (P’s<0.0001). The inclusion of these two variables resulted in a 3.6% increase of the variance for cotinine glucuronidation from the base model. In addition, the C allele for rs116294140 and T allele for rs61750900 were found to be negatively associated with cotinine glucuronidation (P’s<0.0001). Rs116294140 and rs61750900 explained an additional 26.2% and 7.2% of the variance, respectively.
Table III.
Determinants of cotinine glucuronidationa
| Independent variables | Cumulative R 2 | % Variance explained |
|---|---|---|
| Age | ||
| Sex (males versus females) | ||
| Black versus Whites | ||
| NH versus Whites | ||
| JA versus Whites | ||
| Latinos versus Whites | ||
| Creatinine | ||
| 0.116 | NA | |
| After stepwiseb | ||
| + BMI (kg/m2) (natural log) | 0.121 | 0.50% |
| + CYP2A6 phenotype (natural log) | 0.15 | 3.10% |
| + rs116294140 CA versus AA | 0.412 | 26.20% |
| rs116294140 CC versus AA | ||
| + rs61750900 GT versus GG | 0.484 | 7.20% |
| rs61750900 TT versus GG |
aCotinine glucuronide/cotinine.
bAdditional predictor identified through stepwise regression. Variables allowed to compete in the stepwise regression: Measures of nicotine metabolism (CYP2A6 activity and TNEs), smoking quantity (cigarettes per day) and intakes of cruciferous vegetable, total fruits, caffeine, green leafy vegetables, total vegetables, alcohol, processed meats and soy.
Effect of UGT2B10 genotype on FMO3 and CYP2A6 phenotypes
The ratio of nicotine N-oxide to nicotine may be used to phenotype FMO3 activity. However, in AAs this ratio was significantly higher in carriers of the UGT2B10 splice variant (Table II), or those with a genetic score of 1 or 2, compared with homozygous wild-type individuals (Supplementary Table S3, available at Carcinogenesis Online). Consistent with these data, the median nicotine N-oxide to nicotine ratio was higher for AAs relative to Whites (0.63 versus 0.51, P = 0.0005, Supplementary Table S2, available at Carcinogenesis Online).
Because UGT2B10 activity influences cotinine levels, the effect of UGT2B10 genotype on two nicotine metabolite ratios used to phenotype CYP2A6 activity, the ratio of total 3-HCOT to total cotinine and the ratio of total 3-HCOT to cotinine was determined. The ratio of 3-HCOT to total cotinine was significantly increased in carriers of variant UGT2B10 alleles (Table II). This was true among all ethnic groups, although which variant contributed to the observed increase was dependent on the frequency of that variant in a particular group (Supplementary Table S3, available at Carcinogenesis Online). When all ethnic groups were combined, the 3-HCOT to total cotinine ratio was significantly higher in individuals with a genetic score of 2 compared with those with a score of zero (3.09 versus 1.29, P < 0.0001, Table II). The total 3-HCOT/cotinine ratio was not significantly affected by the nicotine glucuronidation genetic score in the group as a whole or in any of the ethnic groups (Supplementary Table S3, available at Carcinogenesis Online).
UGT2B17 and nicotine metabolism
UGT2 B17 is a catalyst of the O-glucuronidation of 3-HCOT (12), but individuals who are homozygous null for UGT2B17 still excreted 3-HCOT glucuronide, albeit ~50% less than individuals who have two copies of this gene. The geometric means (95% CI) of the 3-HCOT glucuronide to total 3-HCOT for these two groups were 0.198 (0.185–0.213, n = 856) and 0.357 (0.313–0.406, n = 494). JAs have a highest frequency of the UGT2B17 null allele (90%) and the lowest proportion of 3-HCOT excreted as its glucuronide conjugate (Table I). However, among individuals who carry two copies of UGT2B17 there is no difference in 3-HCOT glucuronidation by ethnicity (P = 0.253). These data support the UGT2B17 deletion as the primary contributor to ethnic/racial differences in 3-HCOT glucuronidation. The CYP2A6 phenotype, 3-HCOT/cotinine did not vary by UGT2B17 copy number (P = 0.761).
Discussion
Lung cancer induction in smokers begins with carcinogen exposure. The pathway to metastatic cancer involves many steps from carcinogen activation to DNA adduct formation and the activation of critical genes, and is influenced by inflammation, co-carcinogens and tumor promoters (36). The variable risk of lung cancer among different ethnic/racial groups may be due to variability at any point in this pathway. However, to access the contribution of these downstream events to cancer risk requires an accurate measure of tobacco exposure. TNE, the sum of nicotine and its major metabolites has been developed as an objective quantitative biomarker of smoking exposure (15,16). In this study, we report significant differences in TNE across five ethnic/racial groups. We observed a striking difference between reported CPD and TNE for AAs and JAs, the groups with the highest and lowest risk of lung cancer, respectively. The median reported CPD of these two groups are both low (10 and 12) and quite similar, but the median TNE level in AAs are 1.6 times higher than the level in JAs. The TNE levels and the lung cancer risk for Whites are intermediate. These data provide evidence that some of the variability in lung cancer risk at similar reported CPD in these three groups is due to the limitations of CPD as a measure of tobacco exposure.
Smoking behaviors and TNE levels per cigarette are influenced by individual differences in nicotine metabolism (3,17). Therefore, we characterized all pathways of nicotine metabolism in the five ethnic/racial groups. Due to our earlier study of reduced nicotine glucuronidation in AAs (9), we focused our analyses on the influence of UGT2B10 genotype on total nicotine metabolism. We report here that the relatively high frequency of a UGT2B10 splice variant (rs116294140) in AAs is the major cause of reduced nicotine glucuronidation in this population and that individuals with this UGT2B10 variant have significantly increased nicotine-C-oxidation. The reverse relationship is also true, that is if nicotine C-oxidation is low then nicotine N-glucuronidation increases. The proportion of nicotine metabolized by N-glucuronidation in JAs, who on average have low rates of C-oxidation, is twice that of AAs and 50% higher than Whites. Although this relationship is not surprising, accurate quantitation of all pathways of nicotine metabolism is critical to accessing the influence of a particular pathway on smoking behavior.
UGT2B10 catalyzes the N-glucuronidation of both nicotine and cotinine and smokers who are heterozygous for a UGT2B10 SNP that codes for an Asp67Tyr variant excrete decreased levels of both nicotine and cotinine glucuronides (13,27,37). The frequency of this SNP in AAs is about half of that in Whites. The most common UGT2B10 SNP in AAs among the >80 SNPs identified is a splice variant. The frequency of this variant in this study was 34% similar to the 37% reported previously (29). The effect of these two SNPs on nicotine N-glucuronidation was significant in all ethnic groups; smokers who were heterozygous for either variant excreted about 50% less nicotine glucuronide. The contribution of these two SNP to the observed ethnic/racial differences was significant. There is only a handful of missense variants in UGT2B10 identified in AAs with allele frequencies >1% (29). In a parallel GWAS study carried out in the MEC, we reported no association between cotinine glucuronidation and two SNPs, rs147368959 and rs111772923, which code for missense mutations and have allele frequencies of 4% and 7% in AAs (34). Some of the differences in N-glucuronidation among ethnic groups not explained by UGT2B10 coding region SNPs may be due to variation in enzyme expression. In addition, variation in other pathways of nicotine and cotinine metabolism will influence the UGT2B10 phenotypes.
UGT2B10 activity may be phenotyped by either a nicotine glucuronide or a cotinine glucuronide ratio. However, the latter is a more robust measure due to the longer half-life of cotinine compared with nicotine, which is partly a function of the kinetic parameters of their CYP2A6-catalyzed C-oxidation (38,39). Nicotine and cotinine glucuronidation ratios both varied by ethnic group, but if the groups are ranked (from low to high) for the two ratios the order differed (Supplementary Table S2, available at Carcinogenesis Online). Glucuronidation of either nicotine or cotinine was the lowest in AAs relative to the other four groups. However, cotinine glucuronidation was lower in JAs and NHs, than in Whites, but nicotine glucuronidation was higher in JAs and NHs, than in Whites. These apparently discordant data can be explained by the significantly lower CYP2A6 activity (17) in these two populations compared with Whites. That is when nicotine C-oxidation is low, nicotine N-glucuronidation increases to compensate; therefore, the higher nicotine glucuronidation in JAs and NHs is probably not a reflection of the genetics of UGT2B10. The lower cotinine glucuronidation in these two groups may also be explained by lower CYP2A6 activity. Zhu et al. (40) reported that plasma cotinine levels are dependent on the rate of metabolism to 3-HCOT, more than on the rate of formation from nicotine. Therefore, lower CYP2A6 activity will result in higher levels of cotinine and probably a lower ratio of cotinine glucuronide to cotinine. This prediction is consistent with the positive association of CYP2A6 activity with cotinine glucuronidation reported here (Table III).
UGT2B10 genotype influences the proportion of nicotine metabolized by C-oxidation. It also influences the total 3-HCOT/total cotinine ratio, which has been used to phenotype CYP2A6 (17). Therefore, individuals with low rates of nicotine glucuronidation may be misclassified for CYP2A6 phenotype. No effect of UGT2B10 genotype was observed on the total 3-HCOT/cotinine ratio, supporting this ratio as the better urinary phenotype for CYP2A6 activity. In an earlier much smaller study of predominantly Whites, we reported no impact of UGT2B10 genotype for the Asp67Tyr variant on the total 3-HCOT to cotinine ratio in plasma. However, in the same study, we reported significantly higher plasma cotinine in smokers heterozygous for the UGT2B10 Asp67Tyr, compared with wild-type individuals. The impact of UGT2B10 genotype on plasma cotinine may impact the 3-HCOT ratio in populations, such as AAs, with relatively high frequencies of UGT2B10 variants. In this study we did not see any difference in total nicotine C-oxidation or CYP2A6 phenotype ratio between AAs and Whites, suggesting the higher plasma cotinine levels consistently reported in AAs (20,41–43) may be primarily due to low cotinine glucuronidation and high nicotine consumption per cigarette, not low CYP2A6 activity.
In summary, we present essentially complete metabolic profiles of nicotine metabolism in five ethnic/racial groups having variable lung cancer risks. We identified a UGT2B10 splice variant as the primary cause of low levels of nicotine and cotinine N-glucuronidation in AAs and characterized the effect of variable nicotine glucuronidation on the other pathways of nicotine metabolism. The effect of this variant on nicotine glucuronidation probably does not directly contribute to lung cancer risk, and in a parallel GWAS study in this cohort, genetic variation in nicotine and cotinine glucuronidation was not significantly associated with TNE (34). However, the importance of the prevalence of the UGT2B10 splice variant in AAs extends beyond nicotine metabolism, to the metabolism of other drugs and carcinogens metabolized by N-glucuronidation (44,45).
Supplementary material
Supplementary Tables 1–4 can be found at http://carcin.oxfordjournals.org/
Funding
This study was funded by National Institutes of Health grant CA138338 and the liquid chromatography–tandem mass spectrometry analysis of nicotine and metabolites was carried out in the Analytical Biochemistry Core of the University of Minnesota Cancer Center supported in part by National Institutes of Health CA77598. The Multiethnic Cohort was funded by National Institutes of Health grant R37CA54281, R01CA63464, P01CA33619 and UM1CA164973.
Supplementary Material
Acknowledgements
We thank Nicole Thomson for carrying out the analysis of nicotine N-oxide.
Conflict of Interest Statement: None declared.
Glossary
Abbreviations:
- AA
African American
- BMI
body mass index
- CPD
cigarettes per day
- CYP2A6
cytochrome P450 2A6
- FMO3
flavin monooxygenase 3
- 3-HCOT
3′-hydroxycotinine
- JA
Japanese American
- LA
Latino American
- LOQ
limit of quantization
- MEC
Multiethnic Cohort study
- NH
Native Hawaiian
- TNE
total nicotine equivalent
- UGT2B10
UDP-glucuronosyl transferase 2B10.
References
- 1. Siegel R., et al. (2014). Cancer statistics, 2014. CA. Cancer J. Clin., 64, 9–29 [DOI] [PubMed] [Google Scholar]
- 2. Haiman C.A., et al. (2006). Ethnic and racial differences in the smoking-related risk of lung cancer. N. Engl. J. Med., 354, 333–342 [DOI] [PubMed] [Google Scholar]
- 3. Wassenaar C.A., et al. (2011). Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J. Natl. Cancer Inst., 103, 1342–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jalas J.R., et al. (2005). Cytochrome P450 enzymes as catalysts of metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a tobacco specific carcinogen. Chem. Res. Toxicol., 18, 95–110 [DOI] [PubMed] [Google Scholar]
- 5. Patten C.J., et al. (1997). Evidence for Cytochrome P450 2A6 and 3A4 as major catalysts for N′-nitrosonornicotine α-hydroxylation in human liver microsomes. Carcinogenesis, 18, 1623–1628 [DOI] [PubMed] [Google Scholar]
- 6. Wong H.L., et al. (2005). Cytochrome P450 2A-catalyzed metabolic activation of structurally similar carcinogenic nitrosamines: N′-nitrosonornicotine enantiomers, N-nitrosopiperidine, and N-nitrosopyrrolidine. Chem. Res. Toxicol., 18, 61–69 [DOI] [PubMed] [Google Scholar]
- 7. Chen G., et al. (2008). Identification of a prevalent functional missense polymorphism in the UGT2B10 gene and its association with UGT2B10 inactivation against tobacco-specific nitrosamines. Pharmacogenet. Genomics, 18, 181–191 [DOI] [PubMed] [Google Scholar]
- 8. Hukkanen J., et al. (2005). Metabolism and disposition kinetics of nicotine. Pharmacol. Rev., 57, 79–115 [DOI] [PubMed] [Google Scholar]
- 9. Berg J.Z., et al. (2010). Nicotine metabolism in African Americans and European Americans: variation in glucuronidation by ethnicity and UGT2B10 haplotype. J. Pharmacol. Exp. Ther., 332, 202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murphy S.E., et al. (2004). A comparison of urinary biomarkers of tobacco and carcinogen exposure in smokers. Cancer Epidemiol. Biomarkers Prev., 13, 1617–1623 [PubMed] [Google Scholar]
- 11. von Weymarn L.B., et al. (2012). CYP2A6- and CYP2A13-catalyzed metabolism of the nicotine Δ5′(1′)iminium ion. J. Pharmacol. Exp. Ther., 343, 307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen G., et al. (2012). Glucuronidation of trans-3′-hydroxycotinine by UGT2B17 and UGT2B10. Pharmacogenet. Genomics, 22, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen G., et al. (2007). Glucuronidation of nicotine and cotinine by UGT2B10: loss of function by the UGT2B10 Codon 67 (Asp>Tyr) polymorphism. Cancer Res., 67, 9024–9029 [DOI] [PubMed] [Google Scholar]
- 14. Kuehl G.E., et al. (2003). N-glucuronidation of nicotine and cotinine by human liver microsomes and heterologously expressed UDP-glucuronosyltransferases. Drug Metab. Dispos., 31, 1361–1368 [DOI] [PubMed] [Google Scholar]
- 15. Scherer G., et al. (2007). Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regul. Toxicol. Pharmacol., 47, 171–183 [DOI] [PubMed] [Google Scholar]
- 16. Wang J., et al. (2011). Is 24h nicotine equivalents a surrogate for smoke exposure based on its relationship with other biomarkers of exposure? Biomarkers, 16, 144–154 [DOI] [PubMed] [Google Scholar]
- 17. Derby K.S., et al. (2008). Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiol. Biomarkers Prev., 17, 3526–3535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Derby K.S., et al. (2009). Exposure to the carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in smokers from 3 populations with different risks of lung cancer. Int. J. Cancer, 125, 2418–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roethig H.J., et al. (2009). Population estimates for biomarkers of exposure to cigarette smoke in adult U.S. cigarette smokers. Nicotine Tob. Res., 11, 1216–1225 [DOI] [PubMed] [Google Scholar]
- 20. Perez-Stable E.J., et al. (1998). Nicotine metabolism and intake in black and white smokers. JAMA, 280, 152–156 [DOI] [PubMed] [Google Scholar]
- 21. Benowitz N.L. (2010). Nicotine addiction. N. Engl. J. Med., 362, 2295–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Benowitz N.L., et al. (2006). CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clin. Pharmacol. Ther., 80, 457–467 [DOI] [PubMed] [Google Scholar]
- 23. Fujieda M., et al. (2004). Evaluation of CYP2A6 genetic polymorphisms as determinants of smoking behavior and tobacco-related lung cancer risk in male Japanese smokers. Carcinogenesis, 25, 2451–2458 [DOI] [PubMed] [Google Scholar]
- 24. Kumasaka N., et al. (2012). Haplotypes with copy number and single nucleotide polymorphisms in CYP2A6 locus are associated with smoking quantity in a Japanese population. PLoS One, 7, e44507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bloom A.J., et al. (2012). Use of a predictive model derived from in vivo endophenotype measurements to demonstrate associations with a complex locus, CYP2A6. Hum. Mol. Genet., 21, 3050–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bloom A.J., et al. (2013). Effects upon in-vivo nicotine metabolism reveal functional variation in FMO3 associated with cigarette consumption. Pharmacogenet. Genomics, 23, 62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berg J.Z., et al. (2010). UGT2B10 genotype influences nicotine glucuronidation, oxidation, and consumption. Cancer Epidemiol. Biomarkers Prev., 19, 1423–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benowitz N.L., et al. (1999). Ethnic differences in N-glucuronidation of nicotine and cotinine. J. Pharmacol. Exp. Ther., 291, 1196–1203 [PubMed] [Google Scholar]
- 29. NHLBI Exome Sequencing Project (ESP). Exome Variant Server. http://evs.gs.washington.edu/EVS/ (18 July 2014, date last accessed).
- 30. Kolonel L.N., et al. (2000). A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am. J. Epidemiol., 151, 346–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Delaneau O., et al. (2012). A linear complexity phasing method for thousands of genomes. Nat. Methods, 9, 179–181 [DOI] [PubMed] [Google Scholar]
- 32. Howie B.N., et al. (2009). A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet., 5, e1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. 1000 Genomes Project Consortium (2010). A map of human genome variation from population-scale sequencing. Nature, 467, 1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel Y.M., et al. (2014) Common genetic variation explains a substantial fraction of nicotine and cotinine glucuronidation in multiple ethnic/racial populations. Cancer Epidemiol Biomarkers Prev., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen G., et al. (2010). Glucuronidation genotypes and nicotine metabolic phenotypes: importance of functional UGT2B10 and UGT2B17 polymorphisms. Cancer Res., 70, 7543–7552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hecht S.S., et al. (2014). Fifty years of tobacco carcinogenesis research: from mechanisms to early detection and prevention of lung cancer. Cancer Prev. Res. (Phila)., 7, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaivosaari S., et al. (2007). Nicotine glucuronidation and the human UDP-glucuronosyltransferase UGT2B10. Mol. Pharmacol., 72, 761–768 [DOI] [PubMed] [Google Scholar]
- 38. Murphy S.E., et al. (2005). Nicotine 5′-oxidation and methyl oxidation by P450 2A enzymes. Drug Metab. Dispos., 33, 1166–1173 [DOI] [PubMed] [Google Scholar]
- 39. Brown K.M., et al. (2005). Identification of N-(hydroxymethyl) norcotinine as a major product of cytochrome P450 2A6, but not cytochrome P450 2A13-catalyzed cotinine metabolism. Chem. Res. Toxicol., 18, 1792–1798 [DOI] [PubMed] [Google Scholar]
- 40. Zhu A.Z., et al. (2013). The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: the influence of genetics, race, and sex. Cancer Epidemiol. Biomarkers Prev., 22, 708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wagenknecht L.E., et al. (1990). Racial differences in serum cotinine levels among smokers in the Coronary Artery Risk Development in (Young) Adults study. Am. J. Public Health, 80, 1053–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Caraballo R.S., et al. (1998). Racial and ethnic differences in serum cotinine levels of cigarette smokers: Third National Health and Nutrition Examination Survey, 1988–1991. JAMA, 280, 135–139 [DOI] [PubMed] [Google Scholar]
- 43. Benowitz N.L., et al. (2009). Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am. J. Epidemiol., 169, 236–248 [DOI] [PubMed] [Google Scholar]
- 44. Kato Y., et al. (2013). Human UDP-glucuronosyltransferase (UGT) 2B10 in drug N-glucuronidation: substrate screening and comparison with UGT1A3 and UGT1A4. Drug Metab. Dispos., 41, 1389–1397 [DOI] [PubMed] [Google Scholar]
- 45. Chen G., et al. (2008). Glucuronidation of tobacco-specific nitrosamines by UGT2B10. Drug Metab. Dispos., 36, 824–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.