Abstract
Many gram-negative bacteria have specific outer membrane receptors for free heme, hemoproteins, and hemophores. Heme is a major iron source and is taken up intact, whereas hemoproteins and hemophores are not transported: the iron-containing molecule has to be stripped off at the cell surface, with only the heme moiety being taken up. The Serratia marcescens hemophore-specific outer membrane receptor HasR can transport either heme itself or heme bound to the hemophore HasA. This second mechanism is much more efficient and requires a higher TonB-ExbB-ExbD (TonB complex) concentration than does free or hemoglobin-bound heme uptake. This requirement for more of the TonB complex is associated with a higher energy requirement. Indeed, the sensitivity of heme-hemophore uptake to the protonophore carbonyl cyanide m-chlorophenyl hydrazone is higher than that of heme uptake from hemoglobin. We show that a higher TonB complex concentration is required for hemophore dissociation from the receptor. This dissociation is concomitant with heme uptake. We propose that increasing the TonB complex concentration drives more energy to the outer membrane receptor and speeds up the release of empty hemophores, which, if they remained on receptors, would inhibit heme transport.
Iron is an essential compound for cells. Its solubility in the presence of oxygen at a physiological pH is very low, and it is therefore biologically unavailable despite its abundance. Due to its high toxicity, levels of free iron are kept low in living organisms, and most iron is sequestered by carrier proteins (including lactoferrin, transferrin, and ferritins) or bound to protoporphyrins in heme and hemoproteins. Most bacteria have several mechanisms to acquire iron or heme from the various iron sources they may encounter in soil and hosts (3). Some gram-negative bacteria have specific receptors for the host iron and heme carrier proteins (26). They also excrete siderophores, which chelate ferric ions with an extremely high affinity (22), and hemophores, which scavenge heme (2, 20). Heme and siderophores still loaded with iron are transported as a whole into the cells. Ferriproteins, host hemoproteins, and hemophores are stripped of their iron loads at the cell surface, and only the prosthetic group is transported.
Despite the wide range of iron and heme sources, the gram-negative bacterial acquisition systems have common features. All of the systems are encoded by genes belonging to operons regulated by the repressor Fur loaded with iron (14). Uptake through the outer membrane is an energy-driven active transport process. It involves ligand-specific outer membrane receptors. The three-dimensional structures of four such receptors in Escherichia coli have been determined. They are monomeric proteins with a β-barrel domain inserted in the outer membrane and an N-terminal domain forming a cork that closes the channel through the β barrel (4, 8, 10-12). For all of these receptors, active transport is dependent on a protein complex comprising the inner membrane proteins ExbB, ExbD, and TonB (the TonB complex) (1). The periplasmic C-terminal part of TonB interacts with a short conserved region named the TonB box on the receptor cork domain, inducing multiple receptor conformational changes allowing substrate uptake (for reviews, see references 11 and 24). The two-dimensional structures of the other iron and heme outer membrane receptors have been predicted from sequence and functional analogies to fold similarly.
The Serratia marcescens hemophore-dependent heme acquisition system has been reconstituted in a heme synthesis mutant of E. coli to allow for exogenous heme utilization. This led to the identification of the has operon, encoding the hemophore-specific outer membrane receptor HasR, the hemophore HasA, and the specific inner membrane hemophore secretion proteins HasD and HasE (13). The last gene of the has operon, hasB, encodes a TonB homolog (23). The receptor has double functions: it promotes the uptake of both free and hemophore-bound heme. The second uptake mechanism is more efficient and allows growth at lower heme or hemoglobin concentrations. The HasR affinity for apo- and holo-HasA is about 5 nM, but preliminary results indicate that the HasR affinity for heme is much lower, at about 1 μM (F. Huché and N. Izadi, unpublished data). Purified, exogenously added hemophore similarly stimulates HasR-dependent heme and hemoglobin acquisition (13). The interaction between the hemophore and its receptor is of a high affinity, does not involve heme, is mediated by two distinct hemophore segments, and is TonB complex independent (18). In contrast, both HasR transport activities for free and hemophore-bound heme are dependent on the TonB complex. Heme-hemophore uptake must require additional as yet uncharacterized steps because heme has to be stripped off at the cell surface: only the heme moiety is transported through the receptor (21). In addition, the fate of the discharged hemophore, once heme has been taken up, is not understood. It may remain on the receptor, as apo- and holo-hemophores both bind to the receptor with the same affinity.
In previous work, we dissociated the two HasR activities by chance. We constructed a hasR gene lacking its own promoter and Fur box that was transcribed under the control of the arabinose promoter. In an E. coli heme auxotroph mutant carrying this gene integrated into the chromosome, direct heme uptake via HasR (at a high heme concentration) was dependent on arabinose induction. In contrast, heme-hemophore uptake was not possible under the same conditions, despite the presence of the inducer arabinose. Moreover, the exogenous addition of hemophore inhibited free heme acquisition. We showed that hemophore binding to the receptor is responsible for the inhibition of heme acquisition. When iron was restricted by the addition of dipyridyl or when iron repression was abolished by a fur mutation and arabinose was present, the same strain was able to use both free and hemophore-bound heme (18). Thus, some functions that are essential for heme-hemophore uptake but not for free heme uptake appear to be under iron-loaded Fur regulation. Most of the genes involved in iron and heme acquisition, including the has genes and the tonB, exbB, and exbD genes, belong to operons regulated by iron via Fur.
Here we show that TonB complex overexpression is required for heme-hemophore uptake and that the larger requirement for the TonB complex is associated with a higher energy requirement for heme-hemophore uptake. We demonstrate that the step which requires more of the TonB complex is the dissociation of the empty hemophore from the receptor.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli C600 (F− thr leu fhuA lacY thi supE), C600 ΔhemA::Km (13), and C600 tonB::Tn5 were from our laboratory collection. pUC18-tonB (previously named PtonB ECpuc) and pAM238-exbB-exbD (previously named pExbBDpam) were described previously (23). PR10K is a derivative of pBGS19 carrying hasR under the lacp promoter. pAM238, pUC18 cm, pBAD24, and pBGS19 were from our laboratory collection. C600 att λ::hasR and PBAD24-hasR were described previously (18). pSYC34PAM, pSYC150, and pSYC134-H32A-Y75A-H83A were also described previously (19). pNC1 was previously described (5) and was kindly provided by R. Kadner. An att λμ::hasR strain was P1 transduced into C600 ΔhemA::Km with selection for low (25 μg/ml) ampicillin resistance. The tonB trp::Tn10 mutant of strain H5073 (kindly given by K. Hantke) was P1 transduced into C600 ΔhemA::Km att λ::hasR.
Media.
Bovine hemin and bovine hemoglobin were obtained from Sigma Chemical Co. Heme was dissolved immediately before use in a minimal volume of 0.1 N NaOH, centrifuged, and diluted with the appropriate buffer to the desired concentration. Hemoglobin was dissolved in 100 mM NaCl. Hemin solutions were filter sterilized with 0.45-μm-pore-size Millipore filters for bacterial growth experiments. 2,3,4,5-[3H]l-leucine was from ICN Biomedicals. LBD medium contained 0.2 mM 2,2′-dipyridyl to chelate iron. δ-Aminolevulinic acid was added to Luria-Bertani (LB) medium (LBΔ) to a final concentration of 20 μg ml−1 for heme auxotroph growth. The protonophore carbonyl cyanide m-chlorophenyl hydrazone (CCCP) was obtained from Sigma Chemical Co. CCCP (used at 5 mM) was dissolved in 100% ethanol.
Extraction and manipulation of plasmids.
Standard methods were used for the isolation of plasmids, cloning, restriction map analysis, and transformation (25).
Electrophoresis and immunological techniques.
Proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie blue staining. An anti-HasR rabbit polyclonal serum was used for immunodetection at a dilution of 1/5,000. Anti-ExbD and anti-TonB antibodies were kindly provided by Peter Howard and Volkmar Braun and were used at a dilution of 1/2,000.
Expression and purification of HasA proteins.
Apo-HasA wild-type and H32A-Y75A-H83A mutant proteins were obtained from culture supernatants of strain POP3(pSYC34PAM) and POP3(pSYC150, pSYC134-H32A-Y75A-H83A) grown at 30°C in M9 Gly medium. The supernatants were collected, concentrated by 65% ammonium sulfate precipitation, and then extensively dialyzed against TN buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl). The heme content of the proteins was determined from the absorbance at their Soret band wavelength. Less than 0.5% (mol/mol) of the purified proteins were loaded with heme. The purity of the protein preparations as estimated from SDS gels was >99%.
Radiolabeled apo-HasA preparation from culture supernatants.
Twenty milliliters of a culture of strain C600(pSYC34PAM) grown to an optical density at 600 nm (OD600) of 1.2 in a solution containing M9 Gly medium, 50 μg of spectinomycin ml−1, 0.4 mM (each) threonine and leucine, 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (to induce the hasADE operon, which is under lacp control in the plasmid), and 5 mCi of [3H]l-leucine was centrifuged for 10 min at 8,000 × g at 4°C. The supernatant was collected, and the proteins were precipitated with ammonium sulfate and dialyzed against TN buffer to remove free [3H]l-leucine. The final apo-HasA concentration was 10 mg/ml, and the [3H]apo-HasA-specific activity was 800,000 cpm/μg. Radiolabeled apo-HasA was loaded with heme by mixing 1 ml of 5 × 10−7 M apo-HasA in TN buffer with 50 μl of 10−5 M heme in TN buffer and incubating the mixture for 15 min at room temperature.
Heme and hemophore utilization as porphyrin source.
Cultures of C600 ΔhemA::Km att λ::hasR tonB trp::Tn10 carrying various plasmids were grown in LB medium supplemented with 20 μg of δ-aminolevulinic acid/ml and appropriate antibiotics to an OD600 of 1. Aliquots of 100 μl were plated on LB agar-0.02% arabinose as indicated in Table 1. All experiments were repeated more than six times.
TABLE 1.
Growth of C600 ΔhemA strains with heme or hemophore as the sole protoporphyrin source
| Strain | Halo radius (mm)a
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Heme
|
Holo-HasA
|
Heme plus HasA H32A-Y75A-H83A mutant protein
|
||||||
| 10 μM | 1 μM | 0.1 μM | 10 μM | 1 μM | 0.1 μM | 10 μM | 1 μM | |
| C600 ΔhemA | 0 | 0 | 0 | 0 | 0 | 0 | NT | NT |
| C600 ΔhemA attλ::hasR | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| C600 ΔhemA attλ::hasR tonB trp::Tn10 | 0 | 0 | 0 | 0 | 0 | 0 | NT | NT |
| C600 ΔhemA attλ::hasR tonB trp::Tn10 (pUC18-tonB, pAM238-exbB-exbD) | 4.5 | 2.5 | 0 | 8 | 4 | 2 | 0 | 0 |
Growth was measured around 5-mm wells filled with 50 μl of the indicated solution. 0, no growth; NT, not tested.
Recycling of hemoglobin and holo-hemophore.
C600(pBAD24 + pNC1) and C600(pBAD24-hasR + pNC1) and their isogenic tonB::Tn5 derivatives C600 tonB::Tn5(pBAD24 + pNC1) and C600 tonB::Tn5(pBAD24-hasR + pNC1) were grown exponentially in 0.4% M9 Gly medium with 0.2% Casamino Acids to an OD600 of 0.1, induced with 0.002% l-arabinose, and grown to an OD600 of 1. The bacteria were harvested, washed, and resuspended to an OD600 of 10 in 0.4% M9 Glu medium with 0.2% Casamino Acids containing various concentrations of CCCP. The incubation was continued for 40 min at 37°C (6). Aliquots of 900 μl were mixed with 100 μl of either hemoglobin or holo-HasA to final concentrations of 4 × 10−6 and 3.5 × 10−6 M, respectively. These concentrations are roughly 10 times higher than the concentration of HasA binding sites on HasR (3 × 10−7 M) at an OD600 of 10. They were chosen to ensure that only a small proportion (10%) of extracellular hemoproteins could bind to HasR-producing cells. The mixtures were incubated for 180 min at 37°C on a rotary shaker, and the cells were removed by centrifugation. The heme content of each supernatant was determined from the UV-visible absorption spectra by using culture supernatants containing CCCP at the same various concentrations, but no hemoproteins, as blanks. tonB mutants do not transport free or hemoprotein-bound heme, and the drop in heme content of the supernatant after incubation with tonB mutant strains is a measure of hemoprotein binding to HasR-producing cells. Ten percent of the holo-HasA was bound to C600 tonB::Tn5(pBAD24-hasR) (data not shown). Hemoglobin did not bind to either tonB+ or tonB mutant HasR-producing strains, suggesting that heme is acquired from hemoglobin by diffusion to HasR without a stable complex formation (data not shown). The absorption at 407 nm of the supernatant from the control cultures (which do not produce HasR) was identical to that of the initial hemoprotein solutions. Recycling was calculated as the difference between the decrease in absorption at 407 nm of the supernatant after incubation with strain C600(pBAD24 + pNC1) and that with strain C600(pBAD24-hasR + pNC1). For HasA, a 10% absorption decrease due to binding was subtracted for each point.
Liquid-phase radiolabeled HasA binding assay.
C600 att λ::hasR(pUC18, pAM238) and its control strain without hasR, C600(pUC18, pAM238); the isogenic tonB mutant C600 att λ::hasR tonB trp::Tn10(pUC18, pAM238) and its control strain without hasR, C600 tonB trp::Tn10(pUC18, pAM238); and the isogenic strain overexpressing TonB-ExbB-ExbD, C600 att λ::hasR tonB trp::Tn10(pUC18-tonB, pAM238-exbB-exbD), and its control strain without hasR, C600 tonB trp::Tn10(pUC18-tonB, pAM238-exbB-exbD), were grown to an OD600 of 1 in LB broth-0.02% arabinose, harvested, and washed in TN buffer, and 1 OD600 of the HasR-producing strains was mixed with 9 OD600 of the control strains (which lack the hasR gene) in a 900-μl volume such that the bacterial density corresponded to an OD600 of 10 in all experiments. These samples were mixed with 100 μl of various concentrations (3, 8, and 30 nM) of radioactive apo- and holo-HasA in TN buffer. HasA was allowed to bind to cells for 15 min at 4°C on a rotary shaker. All of the binding experiments were performed at 4°C to minimize heme uptake. The cells were removed by centrifugation in a microcentrifuge for 10 min at 8,000 × g at 4°C, and 400-μl aliquots of the supernatants were mixed with 4 ml of Fluoran scintillation liquid and counted in a Beckman liquid scintillation counter. For each HasA concentration, the radioactivity associated with HasR-producing cells was measured as the decrease in unbound radioactivity present in the supernatant. Specific binding was calculated as the difference between the unbound radioactivity of the control strain (which does not produce HasR) and that of the preparations of cells expressing HasR. Binding to the control strain was <5% of the binding to cells expressing HasR.
Displacement of radiolabeled apo-HasA by cold apo- and holo-HasA.
Strains C600 att λ::hasR(pUC18, pAM238), C600 att λ::hasR tonB trp::Tn10(pUC18, pAM238), and C600 att λ::hasR tonB trp::Tn10(pUC18-tonB, pAM238-exbB-exbD) were grown as described above. After the binding of 30 nM radiolabeled protein, as described above, at 4°C followed by washing in TN buffer at 4°C, the cells were resuspended in 1 ml of M9-0.4% Glu medium containing various amounts of wild-type unlabeled apo- and holo-hemophores at concentrations from 0 to 1 μM, a mutant unlabeled protein (HasA H32A-Y75A-H83A) at concentrations of 0 and 1 μM, hemin alone at a concentration of 10 μM, or a mixture of free hemin (10 μM) and mutant protein (1 μM). Samples were incubated for a further 2 h at 30°C. The cells were removed by centrifugation in a microcentrifuge for 10 min at 8,000 × g at 4°C, and 400-μl aliquots of the supernatants were mixed with 4 ml of Fluoran scintillation liquid and counted in a Beckman liquid scintillation counter to measure unbound radioactivity. All binding reactions were performed in duplicate. All of these experiments were repeated three times.
RESULTS
Dissociation of HasR-mediated utilization of heme from that of hemophore-bound heme.
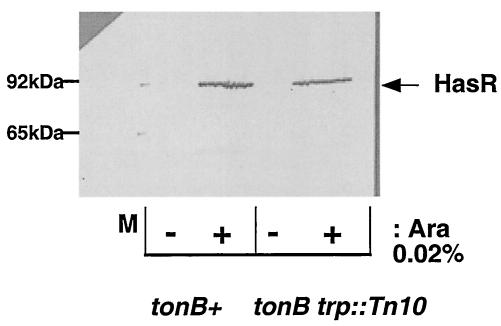
Strain C600 ΔhemA::Km att λ::hasR carries the hasR gene under the control of arabinose (18). The amounts of HasR produced are dependent on arabinose induction, as assessed by immunodetection with anti-HasR antibodies (Fig. 1). An arabinose concentration of 0.02% was used for the growth tests. Strain C600 ΔhemA::Km att λ::hasR is a heme auxotroph and was tested for the ability to use exogenous heme and thus to bypass the heme synthesis mutation and grow aerobically. The strain was plated on LB agar with 0.02% arabinose. Heme at concentrations from 0.1 to 10 μM was placed in wells in the agar. The strain growth was dependent on the porphyrin source concentration. The strain grew strongly around wells containing 10 μM heme and poorly around wells containing 1 μM heme. There was no growth at 0.1 μM heme (Table 1). The introduction of a tonB mutation into C600 ΔhemA::Km att λ::hasR abolished heme utilization without affecting HasR production (Table 1 and Fig. 1). This demonstrates that heme uptake by this strain is TonB dependent, which is consistent with previous results. No growth was observed around wells containing hemophore loaded with 50% heme (holo-hemophore), even at 10 μM holo-hemophore, the highest concentration tested (Table 1). Thus, by using heme instead of hemoglobin and a test based on growth around wells containing various concentrations of heme and hemophore, we obtained the same paradoxical finding as in our previous work: there is a dissociation between free and hemophore-bound heme uptake via HasR in iron-rich medium (18).
FIG. 1.
HasR immunodetection. Cells were grown in LB medium with 0.02% arabinose at 30°C to an OD600 of 1. The cell pellets were washed, and the proteins precipitated with trichloroacetic acid. A 0.2 OD equivalent was loaded in each lane of an SDS-10% PAGE gel. The relevant genotypes are indicated on the figure. HasR was immunodetected with a polyclonal anti-HasR serum used at a dilution of 1/5,000.
HasR-mediated heme-hemophore utilization requires larger amounts of TonB-ExbB-ExbD than does HasR-mediated heme uptake.
Strain C600 ΔhemA::Km att λ::hasR can use holo-hemophore as a heme source on iron-chelated medium (18). The hemophore is the most efficiently used heme source. Possibly genes required for heme-hemophore utilization are induced in iron-chelated medium.
TonB-ExbB-ExbD expression is repressed threefold by the iron-loaded Fur repressor (15). We therefore tested whether these three proteins have to be induced to allow heme-hemophore utilization.
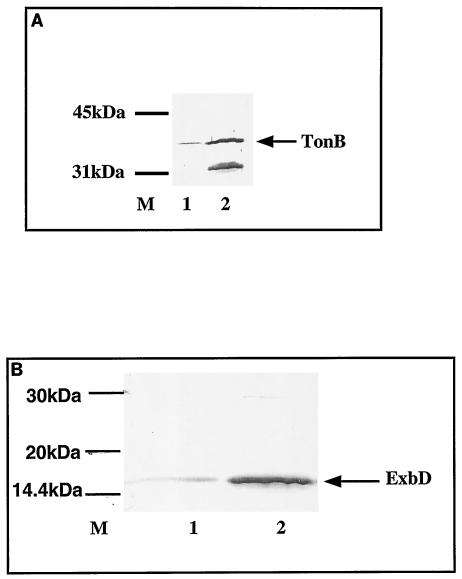
tonB and exbB-exbD mutations carried on compatible plasmids under the lacp promoter and without their Fur-regulated promoters (pUC18-tonB and pAM238-exbB-exbD) were introduced together into C600 ΔhemA::Km att λ::hasR tonB trp::Tn10. The strains were analyzed by immunoblotting with anti-TonB and anti-ExbD antibodies. Their presence led to an approximately 15-fold overproduction of TonB and ExbD proteins, as measured by immunoblot scanning (Fig. 2). There was no change in HasR levels (data not shown). The amount of TonB that was immunodetected in total membrane preparations was lower (about 50% lower [data not shown]) in cells overexpressing TonB alone than in those overexpressing all three proteins. To avoid alterations in the protein complex stoichiometry, we performed the following experiments with strains that overexpressed all three TonB-complex proteins.
FIG. 2.
TonB and ExbD immunodetection. SDS-PAGE of samples and immunodetection of whole-cell extracts were performed. Cells were grown in LB medium at 30°C to an OD600 of 1. The cell pellets were washed, and the proteins were precipitated with trichloroacetic acid. A 0.5 OD equivalent was loaded in each lane of an SDS-12% PAGE gel. (A) Anti-TonB polyclonal serum. (B) Anti-ExbD polyclonal serum. Both sera were used at a dilution of 1/2,000. Lanes: 1, C600ΔhemA att λ::hasR; 2, C600ΔhemA att λ::hasR tonB trp::Tn10(pUC18-tonB, pAM238-exbB-exbD); M, molecular size marker.
Heme and heme-hemophore utilization were tested as described above. Strain C600 ΔhemA::Km att λ::hasR tonB trp::Tn10(pUC18-tonB, pAM238-exbB-exbD) grew around wells containing 0.1 μM holo-hemophore (Table 1). Thus, hemophore-heme was acquired in the presence of larger amounts of TonB-ExbB-ExbD than those allowing heme acquisition. At this higher concentration of the TonB complex, hemophore was the best heme source, as it is in iron-chelated medium.
More energy is required for heme-hemophore uptake than for heme-hemoglobin uptake.
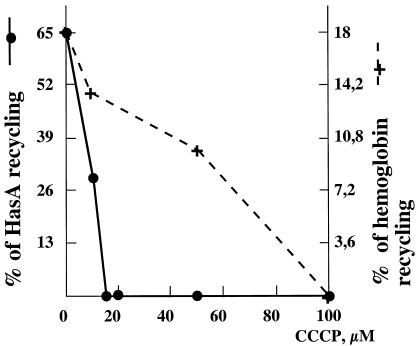
The TonB complex is involved in the transduction of energy from the proton motive force (pmf) to the outer membrane receptors. We tested whether the requirement for a higher TonB complex concentration for heme-hemophore uptake corresponds to a requirement for a higher membrane potential for heme-hemophore than for heme uptake by comparing the relative pmf dependence of heme and heme-hemophore uptake. Hemoprotein recycling into an apo-protein by HasR-producing strains is a simple way to measure heme uptake. The heme status of hemoproteins is easily measured by absorption spectrometry at the Soret band (around 407 nm); free heme gives a large absorption peak and the concentration is harder to quantify. Bovine hemoglobin is found mostly in the methemoglobin form, which has a weak affinity for heme (N. Izadi, personal communication). It behaves like free heme in the growth test described above, i.e., it does not require larger amounts of the TonB complex to be used as a heme source by HasR-producing strains (18). Thus, we compared holo-hemophore and hemoglobin recycling by HasR-producing cells in the presence of a series of concentrations of CCCP, a protonophore which dissipates the pmf. We used a strain carrying pNC1, overproducing ExbB, ExbD, and TonB (data not shown), and pBAD24-hasR, which produces 10 times more HasR than C600 att λ::hasR when induced with arabinose (data not shown). The amounts of HasR produced by this plasmid were identical in tonB+ and tonB mutant backgrounds, as determined by Western blotting (data not shown). We first confirmed that, as expected, strain C600 ΔhemA::Km(pBAD24-hasR) used bovine hemoglobin at 10 μM but did not use the heme provided by the hemophore and that strain C600 ΔhemA::Km(pBAD24-hasR, pNC1) grew around wells containing 0.1 μM holo-hemophore. Thus, HasR expressed from pBAD-hasR gives the same phenotype as that described above for att λ::hasR (data not shown). We used isogenic hemA+ strains to avoid having to add δ-aminolevulinic acid to the cultures, as this partially derepresses heme synthesis and leads to heme excretion out of the cells. C600(pBAD24, pNC1) and C600(pBAD24-hasR, pNC1) and the corresponding isogenic tonB::Tn5 strains were prepared, preincubated with various concentrations of CCCP, and mixed with either hemoglobin or holo-HasA as described in Materials and Methods. Recycling was calculated as indicated in Materials and Methods. In the absence of CCCP, 65% of the holo-HasA was recycled to the apo form. Similar experiments indicated that 18% of the hemoglobin was recycled after incubation with C600(pBAD24-hasR, pNC1) in the absence of CCCP. Thus, hemophore recycling was more efficient than hemoglobin recycling. This was probably due to the weaker affinity of the receptor for heme than for HasA. Nevertheless, the hemoglobin unloading range was HasR and TonB dependent, reproducible, and large enough to allow CCCP inhibition experiments. Hemophore recycling was inhibited 50% by 10 μM CCCP and entirely blocked by 15 μM CCCP (Fig. 3). Hemoglobin recycling was blocked only at 100 μM CCCP. Thus, holo-hemophore recycling is much more sensitive than hemoglobin recycling to pmf depletion (Fig. 3).
FIG. 3.
Effect of CCCP concentration on hemophore and hemoglobin recycling. Supernatants from cultures of C600(pBAD24, pNC1) and C600(pBAD24-hasR, pNC1) treated with various CCCP concentrations and controls incubated with either hemophore (•) or hemoglobin (+) were harvested. The heme content was determined from the UV-visible absorption spectra for culture supernatants containing CCCP at the same concentrations but no hemoproteins, which were used as blanks. For each sample, hemoprotein binding to HasR-producing strains was 10% for HasA and not detectable (<1%) for hemoglobin (in CCCP-treated cultures or tonB mutant derivatives). Recycling was calculated as the difference between the decrease in the absorption of the supernatant at 407 nm after incubation with strain C600(pBAD24, pNC1) and that with strain C600(pBAD24-hasR, pNC1). For HasA, a 10% absorption decrease due to binding was subtracted for each point.
Overproduction of TonB-ExbB-ExbD does not alter the affinities of apo- and holo-HasA for HasR-producing strains.
Apo- and holo-hemophores were previously shown to have the same affinity for HasR-producing cells. However, their relative affinities were measured without varying the TonB-ExbB-ExbD level.
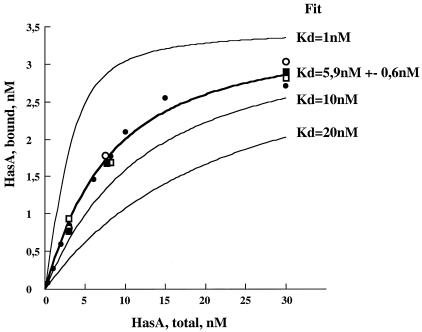
To test whether the overproduction of TonB-ExbB-ExbD promoted the displacement of empty hemophores by enhancing the affinity of HasR for the holo-hemophore, we measured the affinity of radiolabeled apo- and holo-hemophore for binding to whole cells expressing HasR. HasR is under the control of arabinose in these strains, and preliminary experiments were done with C600 att λ::hasR to determine the total numbers of HasA binding sites at various arabinose concentrations. An arabinose concentration of 0.02%, giving a HasA binding site concentration of 3 nM at an OD of 1, was chosen for the following binding experiments. Strains C600 att λ::hasR tonB trp::Tn10(pUC18-tonB, pAM238-exbB-exbD) and C600 att λ::hasR tonB trp::Tn10(pUC18, pAM238) and the control strains lacking hasR, C600 tonB trp::Tn10(pUC18-tonB, pAM238-exbB-exbD) and C600 tonB trp::Tn10(pUC18 and pAM238), were exponentially grown in LB medium with 0.02% arabinose and were resuspended at various OD600 values to determine the total number of HasA sites. Strains expressing HasR (the tonB mutant and the strain overproducing TonB) had the same binding site concentration (3 nM at an OD600 of 1) for apo- and holo-HasA. Aliquots of exponentially growing cells adjusted to an OD of 1 were prepared and mixed with various amounts of radiolabeled proteins as described in Materials and Methods. The specific binding was saturable (Fig. 4) and similar for apo- (not shown) and holo-HasA proteins and was similar for the tonB mutant and the strain overproducing TonB and expressing HasR. These findings were consistent with the published binding affinities of apo- and holo-HasA in strain POP3(pR10K) (shown for comparison in Fig. 4 [closed circles]) (19). Theoretical binding curves with Kds of 1, 10, and 20 nM, together with the fit of the POP3(pR10K) data (thick line; Kd = 5.9 nM), showed that the apparent Kds were not significantly different for the different strains. Thus, TonB complex overexpression did not modify the apparent affinity of the hemophore for its receptor at 4°C. Yet hemophore dissociation could be an energy-consuming process that occurs only in metabolically active cells. A Kd estimation in the nanomolar range could not be determined for energized cells because of the drop in the heme concentration resulting from heme uptake.
FIG. 4.
In vivo interaction of holo-HasA with HasR in various strain backgrounds. The data reported are the amounts of holo-HasA bound to the cells versus the total holo-HasA concentration (see Materials and Methods for details). •, POP3(pR10K); □, C600 att λ::hasR(pAM238, pUC18); ○, C600 att λ::hasR tonB trp::Tn10(pAM238, pUC18); ▪, C600 att λ::hasR tonB trp::Tn10(pAM238exbB-exbD, pUC18-tonB). The thick line represents the fit with the POP3(pR10K) data. The data were fit with Kaleidagraph software using a simple binding equation with one class of sites: the fitted parameters were Kd = 5.9 ± 0.6 nM, and the total receptor concentration was 3.5 ± 0.1 nM (R = 0.998). The thin lines represent theoretical binding curves with the same total receptor concentration and Kds of 1, 10, and 20 nM.
Hemophore dissociation is dependent on TonB-ExbB-ExbD overproduction.
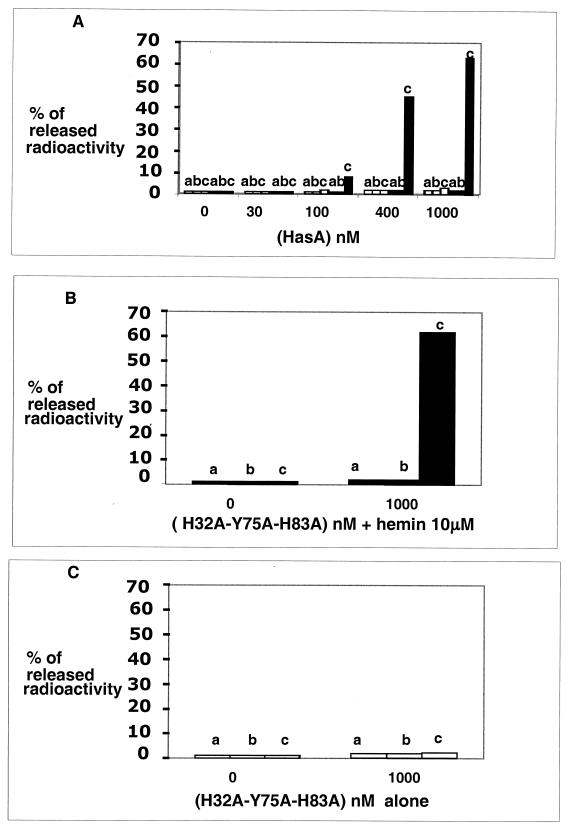
Since we did not see any difference in affinities at 4°C, we studied hemophore dissociation at 30°C in metabolically active cells. Radiolabeled apo-HasA or holo-HasA (30 nM) was allowed to bind to the various strains for 15 min at 4°C. The cells were centrifuged and washed once in buffer to remove unbound proteins. The samples were resuspended in M9 medium with 0.4% glucose and incubated at 30°C. Samples were taken after 30 min and after 2 and 12 h, and the released radioactivity was counted. Using radiolabeled apo- and holo-HasA, we could not detect apo- or holo-hemophore dissociation in any of these strains (data not shown). Possibly, in the absence of unlabeled competitors, rebinding occurs. Thus, the dissociation of radiolabeled apo-HasA was tested in the presence of unlabeled competitors as follows. Radiolabeled apo-HasA was allowed to bind to the various strains for 15 min at 4°C as described above. The cells were centrifuged and washed once in buffer to remove unbound proteins. The samples were resuspended in M9 medium with 0.4% glucose containing various concentrations (from 10 nM to 1 μM) of unlabeled apo-HasA or holo-HasA and then incubated at 30°C. Samples were taken after 30 min and after 2 and 12 h, and the released radioactivity was counted. Less than 3% of the radioactivity was released in the presence of unlabeled apo-HasA. In the presence of holo-HasA, only the strain overexpressing the TonB-ExbB-ExbD complex released radioactivity. The release was concentration dependent and was 63% at the highest holo-HasA concentration (1 μM) (Fig. 5A). This maximum level was reached after 2 h and did not increase with further incubation (data not shown). These results show that hemophore displacement is TonB-ExbB-ExbD dependent and occurs only when this complex is overproduced. No radioactivity release was observed (<1% in 2 h) when the cells were incubated with holo-HasA in buffer or M9 medium without any carbon source or at 4°C. This suggests that the displacement is energy driven.
FIG. 5.
Radiolabeled apo-HasA release. The dissociation of [3H]apo-HasA from C600 att λ::hasR(pUC18, pAM238), C600 att λ::hasR tonB trp::Tn10(pUC18, pAM238), and C600 att λ::hasR tonB trp::Tn10(pUC18-tonB, pAM238-exbB-exbD) was measured. After 15 min of binding of 30 nM [3H]apo-HasA and washing to remove unbound proteins at 4°C, various concentrations of unlabeled apo- and holo-HasA were added and incubation was continued for 2 h at 30°C. The radioactivity released is reported as a percentage of the total specific radioactive binding. Values are means of three different experiments. The standard deviations were <1% in all experiments. (A) Radiolabeled apo-HasA displacement according to the concentration of unlabeled apo-HasA (white bars) or holo-HasA (black bars) added. Strains: a, C600 att λ::hasR(pUC18, pAM238); b, C600 att λ::hasR tonB trp::Tn10(pUC18, pAM238); c, C600 att λ::hasR tonB trp::Tn10(pUC18-tonB, pAM238-exbB-exbD). (B) Radiolabeled apo-HasA displacement by hemin (10 μM) in the presence and absence of the unlabeled mutant protein, H32A-Y75A-H83A. Strains a, b, and c are the same as for panel A. Values are means of three different experiments. The standard deviations were <1% in all experiments. (C) Radiolabeled apo-HasA displacement by the unlabeled H32A-Y75A-H83A mutant alone. Strains a, b, and c are the same as for panel A.
Since displacement was observed only with the holo-hemophore, it may be concomitant with heme uptake or transfer to the receptor. We thus tested whether free heme could induce a hemophore release. Free heme alone did not allow hemophore displacement (Fig. 5B). This could be due to rebinding of the labeled protein in the absence of unlabeled competitors. To test whether free heme could promote hemophore turnover, we mixed it with a protein that is unable to bind heme but that still binds to the receptor with a high affinity. The H32A-Y75A-H83A mutant is such a HasA mutant protein, which has no detectable affinity for heme (Kd > 10−5 M) but binds to HasR as well as the wild-type HasA protein (19). In the presence of unlabeled H32A-Y75A-H83A protein alone, <3% of the radioactivity was released (Fig. 5C). In the presence of heme and the mutant protein, the radioactivity was released only from the strain overexpressing the TonB-ExbB-ExbD complex (Fig. 5B); 62% of the bound radioactivity was released (Fig. 5B) in this case. The H32A-Y75A-H83A mutant does not bind heme, showing that free heme is as efficient as the holo-hemophore for inducing hemophore release from the receptor. Heme could induce hemophore release either by reaching the receptor directly or through binding to the hemophore first, followed by a transfer to the receptor.
Inhibition of heme assimilation by the mutant hemophore HasA H32A-Y75A-H83A.
To test whether heme can bind to a receptor that is already loaded with hemophore and displace it, we tested heme uptake in the presence of an exogenously added mutant hemophore. Strain C600 ΔhemA::Km att λ::hasR tonB trp::Tn10(pUC18-tonB, pAM238-exbB-exbD) grew around wells containing 10 μM heme. This growth was entirely inhibited by the addition of 1 μM mutant HasA protein into the wells (Table 1). This suggests that heme cannot reach its binding site on a receptor that is loaded with hemophore and cannot drive empty mutant hemophore release.
DISCUSSION
HasR has a double function in heme acquisition: it acts as both a free and a hemophore-bound heme receptor. Expressing HasR in an iron-rich medium allowed us to dissociate these two HasR functions: free heme acquisition was possible but the acquisition of heme from the hemophore was not.
Here we describe an investigation of functions that can restore hemophore utilization in an iron-rich medium. The overexpression of TonB, ExbB, and ExbD (the TonB complex) on multicopy plasmids under lacp promoter control (which leads to a 15-fold overproduction of the complex) restored hemophore utilization as the best heme source. This indicates that an uninduced TonB complex in iron-rich medium is limiting for hemophore-heme uptake, but not for direct heme uptake via HasR. Kadner and Heller also described the TonB complex as limiting and showed that two substrates (ferrichrome and vitamin B12) with different outer membrane TonB-dependent receptors compete for TonB (16). Assaying the TonB components in iron-rich and chelated media indicated that the number of copies of each protein was roughly multiplied by 3 in the presence of 200 μM iron chelator dipyridyl (15).
The TonB-ExbB-ExbD complex couples outer membrane transport to the pmf, which provides energy for ligand transport. We investigated hemoprotein recycling by using hemoglobin and hemophore. Hemophore unloading was more sensitive than hemoglobin unloading to CCCP treatment (there was a 50% inhibition at 10 μM CCCP for hemophore and at 50 μM CCCP for hemoglobin). Since increasing the CCCP concentration decreases the pmf, this strongly suggests that the two processes differ in terms of energy requirements.
We then investigated which steps require a higher TonB complex concentration. TonB is not required for apo- and holo-hemophore binding to the receptor. Both apo- and holo-HasA bind with the same affinity and compete when mixed prior to binding, indicating identical or overlapping binding sites. Yet the overexpression of the TonB complex could modify the relative holo- and apo-hemophore affinities for the receptor. We compared the affinities of both hemophores and they were similar to those measured with tonB mutant or tonB+ strains and strains overexpressing TonB, ExbB, and ExbD. Thus, the overexpression of the TonB complex does not promote hemophore-heme uptake by enhancing the affinity of the holo-hemophore. This suggested that the interactions of apo- and holo-HasA with HasR differ only in energized cells. However, since heme permease mutants are not available, hemophore affinities could only be measured at 4°C. At temperatures above 25°C, heme uptake in tonB+ strains will complicate the Kd estimation. To overcome this limitation, we designed experiments to measure hemophore dissociation from energized cells expressing HasR.
Using radiolabeled apo-HasA bound to HasR-producing cells, we measured the radioactive HasA release in the presence of an excess of unlabeled competitors. Neither wild-type apo-HasA nor the mutant protein H32A-Y75A-H83A, which does not bind heme, could displace the bound radioactive protein. Only holo-HasA displaced radiolabeled apo-HasA in a concentration-dependent way. This occurred only in cells overexpressing TonB, ExbB, and ExbD. This dissociation did not occur at 4°C, but it did occur at 30°C and in the presence of a carbon source, suggesting that it is energy driven.
Thus, the overexpression of the TonB complex strongly increases the rate of hemophore dissociation from the receptor. This dissociation is not driven by apo-HasA, indicating that it is concomitant with either heme uptake or heme transfer from the hemophore to the receptor.
Free heme in the presence of the mutant protein H32A-Y75A-H83A, which does not bind heme, as an unlabeled competitor was also able to dissociate the bound radioactive protein, but only in cells overexpressing TonB, ExbB, and ExbD. This clearly shows that the hemophore bound to the receptor can be loaded with heme and is released during the heme transport process. However, the empty hemophore release could be due to either the heme transport itself or heme transfer from the hemophore to the receptor.
The mutant hemophore H32A-Y75A-H83A inhibits heme uptake even when the TonB-ExbB-ExbD complex is overproduced. This suggests that the mutant hemophore, and presumably the wild-type hemophore as well, when bound to the receptor could prevent the direct access of free heme to its site on the receptor. Thus, it is still not established whether hemophore release is induced by a heme transfer from the hemophore to the receptor or during the heme uptake step. Studies of transferrin-iron uptake in Neisseria have led to similar conclusions by showing that transferrin binds with similar high affinities to both tonB+ and tonB mutants and that TonB-driven energy is required for transferrin release. However, it remains unclear whether iron uptake is required for apo-transferrin release (9).
How the TonB complex in the inner membrane causes hemophore release at the cell surface is unclear. Most likely, it unfolds the bound HasA through a HasR-mediated conformational change which decreases the affinity of HasA for HasR, allowing the release of empty hemophores. This event is concomitant with heme uptake and must occur at a speed that is sufficient to allow loaded hemophores to replace ejected empty hemophore molecules. Otherwise, the system is blocked: apo-hemophores remain bound to HasR and most likely do not extract heme from surrounding extracellular hemophores. On the other hand, free heme uptake is still possible at a low TonB complex concentration, as there is no inhibitory state. It is currently believed that the TonB complex functions as a motor that uses the pmf through a proton channel comparable to the MotA/MotB flagellar motor (7, 27). Here we show that heme-hemophore use consumes more energy than heme-hemoglobin uptake. We propose that increasing the TonB complex concentration facilitates the use of the pmf to increase the speed of hemophore turnover. A recent publication reported that TonB-dependent phenotypes are variably sensitive to cellular TonB levels (17). It is possible that the various levels of TonB requirements do not only reflect the sensitivity of each assay, but also mechanistic needs.
Acknowledgments
We gratefully acknowledge Laurent Debarbieux and Jean Marc Ghigo for helpful discussions. We thank Rachel Binet and Julie Deleule for help with plasmid constructions, Volkmar Braun for anti-TonB antibodies, and Robert Kadner for the pNC1 plasmid.
REFERENCES
- 1.Ahmer, B. M., M. G. Thomas, R. A. Larsen, and K. Postle. 1995. Characterization of the exbBD operon of Escherichia coli and the role of ExbB and ExbD in TonB function and stability. J. Bacteriol. 177:4742-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnoux, P., R. Haser, N. Izadi, A. Lecroisey, M. Delepierre, C. Wandersman, and M. Czjzek. 1999. The crystal structure of HasA, a hemophore secreted by Serratia marcescens. Nat. Struct. Biol. 6:516-520. [DOI] [PubMed] [Google Scholar]
- 3.Braun, V., and H. Killmann. 1999. Bacterial solutions to the iron-supply problem. Trends Biochem. Sci. 24:104-109. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan, S., B. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitkar, R. Chakraborty, D. van der Helm, and D. Deisenhofer. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol 6:56-63. [DOI] [PubMed] [Google Scholar]
- 5.Cadieux, N., and R. Kadner. 1999. Site-directed disulfide bonding reveals an interaction site between energy-coupling protein TonB and BtuB, the outer membrane cobalamin transporter. Proc. Natl. Acad. Sci. USA 96:10673-10678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao, Z., P. Warfel, S. Newton, and P. Klebba. 2003. Spectroscopic observations of ferric enterobactin transport. J. Biol. Chem. 278:1022-1028. [DOI] [PubMed] [Google Scholar]
- 7.Cascales, E., R. Lloubes, and J. Sturgis. 2001. The TolQ-TolR proteins energize TolA and share homologies with the flagellar motor proteins MotA-MotB. Mol. Microbiol. 42:795-807. [DOI] [PubMed] [Google Scholar]
- 8.Chimento, D., A. Mohanty, R. Kadner, and M. Wiener. 2003. Substrate-induced transmembrane signaling in the cobalamin transporter BtuB. Nat. Struct. Biol. 10:394-401. [DOI] [PubMed] [Google Scholar]
- 9.Cornelissen, C. 2003. Transferrin-iron uptake by gram-negative bacteria. Front. Biosci. 8:836-847. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715-1719. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson, A. D., and J. Deisenhofer. 2002. TonB-dependent receptors—structural perspectives. Biochim. Biophys. Acta 1565:318-332. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson, A. D., E. Hofmann, J. W. Coulton, K. Diederichs, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 282:2215-2220. [DOI] [PubMed] [Google Scholar]
- 13.Ghigo, J. M., S. Létoffé, and C. Wandersman. 1997. A new type of hemophore-dependent heme acquisition system of Serratia marcescens reconstituted in Escherichia coli. J. Bacteriol. 179:3572-3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hantke, K. 2001. Iron and metal regulation in bacteria. Curr. Opin. Microbiol. 4:172-177. [DOI] [PubMed] [Google Scholar]
- 15.Higgs, P. I., R. A. Larsen, and K. Postle. 2002. Quantification of known components of the Escherichia coli TonB energy transduction system: TonB, ExbB, ExbD and FepA. Mol. Microbiol. 44:271-281. [DOI] [PubMed] [Google Scholar]
- 16.Kadner, R. J., and K. J. Heller. 1995. Mutual inhibition of cobalamin and siderophore uptake systems suggests their competition for TonB function. J. Bacteriol. 177:4829-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen, R. A., G. J. Chen, and K. Postle. 2003. Performance of standard phenotypic assays for TonB activity, as evaluated by varying the level of functional, wild-type TonB. J. Bacteriol. 185:4699-4706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Létoffé, S., L. Debarbieux, N. Izadi, P. Delepelaire, and C. Wandersman. 2003. Ligand delivery by haem carrier proteins: the binding of Serratia marcescens haemophore to its outer membrane receptor is mediated by two distinct peptide regions. Mol. Microbiol. 50:77-89. [DOI] [PubMed] [Google Scholar]
- 19.Létoffé, S., C. Deniau, N. Wolff, E. Dassa, P. Delepelaire, A. Lecroisey, and C. Wandersman. 2001. Haemophore-mediated bacterial haem transport: evidence for a common or overlapping site for haem-free and haem-loaded haemophore on its specific outer membrane receptor. Mol. Microbiol. 41:439-450. [DOI] [PubMed] [Google Scholar]
- 20.Létoffé, S., J. M. Ghigo, and C. Wandersman. 1994. Iron acquisition from heme and hemoglobin by Serratia marcescens extracellular protein. Proc. Natl. Acad. Sci. USA 91:9876-9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Létoffé, S., F. Nato, M. E. Goldberg, and C. Wandersman. 1999. Interactions of HasA, a bacterial haemophore, with haemoglobin and with its outer membrane receptor HasR. Mol. Microbiol. 33:546-555. [DOI] [PubMed] [Google Scholar]
- 22.Neilands, J. B. 1995. Siderophores: structure and function of microbial iron transport compounds. J. Biol. Chem. 270:26723-26726. [DOI] [PubMed] [Google Scholar]
- 23.Paquelin, A., J. M. Ghigo, S. Bertin, and C. Wandersman. 2001. Characterization of HasB, a Serratia marcescens TonB-like protein specifically involved in the haemophore-dependent haem acquisition system. Mol. Microbiol. 42:995-1005. [DOI] [PubMed] [Google Scholar]
- 24.Postle, K., and R. J. Kadner. 2003. Touch and go: tying TonB to transport. Mol. Microbiol. 49:869-882. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook, J., E. F. Fritsch, and T. E. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 26.Wandersman, C., and I. Stojiljkovic. 2000. Bacterial heme sources: role of hemophores and receptors. Curr. Opin. Microbiol. 3:215-220. [DOI] [PubMed] [Google Scholar]
- 27.Zhai, Y., W. Heijne, and M. J. Saier. 2003. Molecular modeling of the bacterial outer membrane receptor energizer, ExbBD/TonB, based on homology with the flagellar motor, MotAB. Biochim. Biophys. Acta 1614:201-210. [DOI] [PubMed] [Google Scholar]