Abstract
Sixteen different variants (KPC-2 to KPC-17) in the KPC family have been reported, and most current studies are focusing on KPC-2 and KPC-3. The KPC-15 variant, which isolated from Klebsiella pneumoniae in a Chinese hospital, was a recently discovered KPC enzyme. To compare the characteristics of KPC-15 and KPC-2, the variants were determined by susceptibility testing, PCR amplification and sequencing, and study of kinetic parameters. The strain harboring the KPC-15 showed resistance to 18 conventional antimicrobial agents, especially to cabapenem antibiotics, and the strain involving the KPC-2 also indicated resistance to cabapenem antibiotics, but both strains were susceptible to polymyxin B and colistin. The conjugation experiments showed that the changes of MIC values to the antibiotics were due to the transferred plasmids. The differences of amino acids were characterised at sites of 119 leucine and 146 lysine with KPC-15 and KPC-2. The minimum evolution tree indicated the KPC alleles evolution, and showed that the KPC-15 appeared to be homogenous with KPC-4 closely. Steady-state kinetic parameters showed the catalytic efficiency of KPC-15 was higher than that of KPC-2 for all tested antibiotics in this study. The catalytic efficiency of KPC-15 caused resistance to β-lactam antibiotics was higher than that of KPC-2. Meanwhile, an evolutionary transformation changed KPC from an efficient carbapenemase to its variants (KPC-15) with better ceftazidimase catalytic efficiency, and the old antibiotics polymyxin B and colistin might play a role in the therapy for multi-resistant strains.
Introduction
The recent emergence and spread of acquired carbapenem-resistance in gram-negative bacteria, which resulting in more clinical failures in infection control, have been increasingly reported. Carbapenem-resistant Enterobacteriaceae are usually resistant not only to β-lactam antibiotics but also to most other classes of antimicrobial agents [1].
Resistance to carbapenems in gram-negative bacteria is frequently observed due to the high expression of AmpC β-lactamases, which are associated with the loss of outer membrane proteins [2], the increased expression of efflux pumps systems [3], and with changes in the affinity of penicillin-binding proteins (PBPs) for carbapenems [4]. Carbapenem resistance can primarily be mediated by carbapenem-hydrolysing enzymes, such as metallo-β-lactamases (MBLs), Guiana extended-spectrum (GES) and Klebsiella pneumoniae carbapenemases (KPCs) [5]–[7].
KPC was first discovered in a K. pneumoniae clinical isolate collected from North Carolina in 2001 [8]. Thereafter, an amino acid variant of KPC-1, which is termed KPC-2, is discovered in Klebsiella spp. [9], [10] and Salmonella enterica [11]. However, a recent correction of the bla KPC-1 sequence have indicated that the sequences of bla KPC-1 and bla KPC-2 are identical, so, KPC-1 and KPC-2 are the same enzymes [8]. Mutations in the bla KPC-2 gene have resulted in two variants, bla KPC-3 [12] and bla KPC-4 [13]. Since then, KPC have been detected in several members of Enterobacteriaceae and discovered in American, Asian, European and Near East regions [7], [14]–[20].
Currently, sixteen different variants (KPC-2 to KPC-17) in the KPC family have been reported, and most current studies are focusing on KPC-2 and KPC-3 [21]–[23]. In the study, we characterised phenotypic and enzymatic comparative analysis for the KPC-2 enzyme and novel KPC-15 variant which was discovered recently by us [24].
Materials and Methods
The ethics committee approval
This study was approved by the the Ethics Committee of Municipal Hospital of Taizhou University, and the written informed consents obtained from each of the participants. The right of research objects was protected, and we agreed this study was conducted in our hospital.
Bacterial strains
The two strains of K. pneumoniae, which were numbered Kp1769 and Kp1241, were isolated from samples of sputum and blood, respectively. The strain Kp1241 was isolated from the hepatobiliary unit in June 2012, and the strain Kp1769 was isolated from the ICU in September 2011 in respiration unit in the Taizhou Municipal Hospital of Taizhou University. The strain Kp1769, which was confirmed involving the bla KPC-2 gene, was used to compare the characteristics of the kinetic parameters with the recently discovered bla KPC-15 variant in this study. These two strains were assigned to K. pneumoniae using a Vitek GNI+ card (bioMérieux, France), and species identification was confirmed by a sequence analysis of the 16 S-23 S rRNA (Midi Labs, USA).
Antimicrobial susceptibility testing and conjugation experiments
The MICs of 20 antimicrobial agents, including imipenem, meropenem, ertapenem, ampicillin, aztreonam, cefazolin, cefepime, ceftazidime, ceftriaxone, ciprofloxacin, levofloxacin, amikacin, tobramycin, gentamicin, cephalosporin, ampicillin/sulbactam, piperacillin/tazobactam, cefoperazone/sulbactam, polymyxin B and colistin in the strains of E. coli J53AzR, Kp1241, Kp1769 and transconjugants, were determined using the Micro- Scan (USA) broth dilution method with plates. Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853 were used as the controls. The results were interpreted according to CLSI (2012) [25].
According to a previously reported protocol [26], conjugation experiments were performed in lysogeny broth (LB) with the strain E. coli J53AzR (resistance to sodium azide) as the recipient and with the strains Kp1241 and Kp1769 as the donor strains. Donor and recipient cells in logarithmic phase (0.5 mL of each) were added to 4 mL of fresh LB, which was followed by incubation at 35°C for 18–24 h without shaking. The transconjugants were selected on trypticase soy agar (TSA) plates containing sodium azide (0.3 g/L) and imipenem (0.02 g/L) following incubation for 18–24 h at 35°C.
PCR amplification and sequencing
PCR was performed for the amplification and sequencing of pre-bla KPC-15, the bla KPC-15 variant, and its surrounding genes (bla TEM, tnpA and tnpR) in K. pneumonae using the primers shown in Table 1. PCRs were also performed for the amplification of the transformant conjugated with E. coli J53AzR. The primers were synthesised by Sangon Biological Technology (Shanghai, China). The PCR conditions for pre-bla KPC-15 and the bla KPC-15 variant were as follows: 3 min at 94°C and 30 cycles of 1 min at 94°C, 1 min at 52°C, and 1 min at 72°C, followed by an elongation step for 10 min at 72°C, which produced a band of ca. 1,431 bp for bla KPC-15, encompassing the entire KPC coding region. The same PCR conditions were used to amplify bla TEM, tnpA (ISKpn8 and ISKpn6-like transposases) and tnpR genes. The primers of tnpA1, tnpA2 and tnpA3 were used to amplify the ISKpn8 transposon, and the tnpA primer was used to amplify the ISKpn6-like transposon. The positive PCR products were also sequenced by Sangon Biological Technology (Shanghai, China). Then, the sequencing results were assembled using the contigExpress Program software and the sequences were obtained for the genetic environment of the bla KPC-15 variant from the strain Kp1241.
Table 1. Sequences of primers utilised to determine the bla KPC genetic environment in this study.
| Primer | Sequence (5′-3′) | Productsize (bp) | reference |
| pre-KPC-15 | For, ACTGGATGGAGGCGGATAA | 700 | The study (based on GenBank accession no. AY395881) |
| Rev, CACAGCGGCAGCAAGAAA | |||
| pre-KPC-15 (sequencing) | For, TAAGCCCTCCCGTATCGTAG | The study (based on GenBank accession no. AY395881) | |
| Rev, TATCCGCCTCCATCCAGT | |||
| KPC-15 | For, CGAGCAACTATGGATGAACG | 1,431 | The study (based on GenBank accession no. AY395881) |
| Rev, GTATCTGTGAGGGCGAAGG | |||
| KPC-15 (sequencing) | For, CGGAACCATTCGCTAAACTCG | The study (based on GenBank accession no. AY395881) | |
| Rev, CGCCAACTCCTTCAGCAACA | |||
| tnpA1 | For, ATCCGCATCGGGAAAGCC | 1144 | The study (based on GenBank accession no. JX500679) |
| Rev, CTGCTCGTCGGCAAAGGA | |||
| tnpA2 | For, GCCAAGACACTGCTGCCTAA | 1082 | The study (based on GenBank accession no. JX500679) |
| Rev, TTGACGGAAGCGAAACACG | |||
| tnpA3 | For, CAAGTTTCTGTCGGCCTTCA | 1434 | The study (based on GenBank accession no. JX500679) |
| Rev, CACGCCTTTGCTCCTGGGT | |||
| tnpA | For, ATACGCCATTCGCCTCAG | 1320 | The study (based on GenBank accession no. JX500679) |
| Rev, GTCGGCAAGGTGGTCTCA | |||
| tnpR | For, TCTTCGCAACACGCACCA | 1834 | The study (based on GenBank accession no. JX500680) |
| Rev, ACCTCGCCGTGGAAATAG | |||
| TEM | For, CTGTCTATTTCGTTCATCC | 1061 | The study (based on GenBank accession no. X64523) |
| Rev, CTCAGTATTGCCCGCTCC |
Plasmids were isolated from the strain Kp1241 using an Axygen kit (USA) in accordance with the manufacturer’s instructions. Plasmids were then separated by electrophoresis through a 0.6% agarose gel in the presence of 1× TBE buffer at a constant voltage of 90 V for 90 min. These plasmid DNA fragments served as samples for the amplification of bla KPC-15 gene by PCR, where the initial position of the bla KPC-15 gene plasmid was determined. The estimated sizes of plasmid DNAs were determined according to reference [26]. Based on these data, we mapped the genetic sequence of the bla KPC-15 upstream and downstream regions and determinded the start sites of transcription and the promoter regions of relevant genes.
β-lactamase preparation and the kinetic parameters for a comparison of KPC-15 and KPC-2
Cultures of K. pneumoniae harboring bla KPC-15 and bla KPC-2 were grown overnight at 37°C in 100 ml of trypticase soy broth with amoxicillin (100 mg/mL). Bacterial suspensions were disrupted by sonication (20 s at 20 KHz×4 times) and centrifuged (30 min, 20,000 g, 4°C). The supernatants, which contained the crude enzyme extracts, were collected. Then, the crude enzyme extracts were mixed with 50 mM Tris-Cl, pH 7.4, with 1 mM magnesium sulphate, and lysed with 40 mg/L lysozyme. Next, 1.0 U/ml benzonase nuclease was added to digest nucleic acids, and a 2.0 mM concentration of EDTA was added to complete the periplasmic fractionation. The lysed cells were centrifuged at 12,000 rpm for 10 min to remove the cellular debris. The supernatant was further enriched and purified for β-lactamase by using preparative isoelectric focusing as described previously [27], [28]. The β-lactamase purification step was performed in accordance with reference [29].
Steady-state kinetic parameters were determined using a diode array spectrophotometer (Agilent, USA). Each assay was performed with 10 mM PBS, pH 7.4, at 25°C. In all assays, the enzyme was maintained at 10 nM, whereas substrate concentrations were varied from 50 to 200 µM. Kinetic assays were performed under steady-state conditions to determine the kms and kcats of the antimicrobial agents: imipenem, meropenem, ceftazidime, cefotaxime, cefepime, aztreonam and nitrocefin for the enzyme according to a previously established model represented in the kinetic parameter equation [30], [31] and in reference [29]. Concentrations varied from 25 to 250 µM for imipenem; 50 to 250 µM for meropenem; 150 to 500 µM for ceftazidime; 100 to 500 µM for cefotaxime; 250 µM to 1.5 mM for cefepime; and 50 mM to 500 mM for aztreonam. A final concentration of 100 µM nitrocefin was used as the reporter substrate.
Results
Antimicrobial susceptibility testing and conjugation experiment
The MICs of 20 antimicrobial agents for the strains Kp1241 and Kp1769 were depicted in Table 2. The results suggested that the strain Kp1241 was resistant to 18 antimicrobial agents, and particularly exhibited high MIC values for imipenem, meropenem, and ertapenem at 16, 16, and 32 µg/mL, respectively; however, the strains Kp1769 showed MIC values for imipenem, meropenem, and ertapenem at 2, 2, and 4 µg/mL, respectively. And the strain Kp1241 remained susceptible to polymyxin B and colistin, so it was as the strain Kp1769. By the conjugation experiments, the transconjugant of strain Kp1241 exhibited a phenotype of resistance to imipenem, meropenem, ertapenem, cephalosporin, aminoglycosides, ampicillin/sulbactam, piperacillin/tazobactam and cefoperazone/sulbactam, but, the transconjugant of strain Kp1769 indicated intermedium values to imipenem, meropenem, and ertapenem. Hence, the results of conjugation experiments showed that the changes of MIC values of the antibiotics were due to the transferred plasmids.
Table 2. The susceptibility for strains of E. coli J53AzR, Kp1241, Kp1769, and transconjugants (J53AzR-Kp1241 and J53AzR-Kp1769).
| Antimicrobial agent | MIC (µg/mL) | ||||
| E.coli J53AzR | Kp1241 | Kp1769 | J53AzR-Kp1241 | J53AzR-Kp1769 | |
| Ciprofloxacin | 0.0025 | 16 | 0.25 | 4 | 0.0625 |
| Levofloxacin | 0.0025 | 16 | 0.25 | 8 | 0.625 |
| Amikacin | 0.25 | 128 | 2 | 32 | 1 |
| Tobramycin | 0.025 | 64 | 1 | 32 | 0.5 |
| Gentamicin | 0.25 | 32 | 1 | 16 | 0.5 |
| Ampicillin | 0.050 | 64 | 128 | 32 | 32 |
| Aztreonam | 0.50 | 128 | 2 | 32 | 1 |
| Cefotetan | 0.0125 | 128 | 64 | 32 | 16 |
| Cefazolin | 0.50 | 128 | 64 | 64 | 16 |
| Cefepime | 0.0125 | 128 | 1 | 32 | 0.625 |
| Ceftazidime | 0.050 | 16 | 2 | 8 | 1 |
| Ceftriaxone | 0.025 | 128 | 16 | 32 | 4 |
| Imipenem | 0.25 | 16 | 2 | 8 | 1 |
| Meropenem | 0.25 | 16 | 2 | 8 | 1 |
| Ertapenem | 0.50 | 32 | 4 | 16 | 2 |
| Ampicillin/Sulbactam | 0.050 | 128 | 64 | 32 | 16 |
| Piperacillin/Tazobactam | 0.025 | 256 | 16 | 64 | 4 |
| Cefoperazone/Sulbactam | 0.0125 | 128 | 2 | 32 | 0.5 |
| Polymyxin B | 0.006 | 0.5 | 0.25 | 0.125 | 0.0625 |
| Colistin | 0.0125 | 1 | 0.5 | 0.25 | 0.125 |
Clinical breakpoints of MICs for the antimicrobial agents see the reference [25].
Amino acid variations, the minimum evolution tree of amino acid sequences and the kinetic parameters for KPC-15 and other KPC enzymes
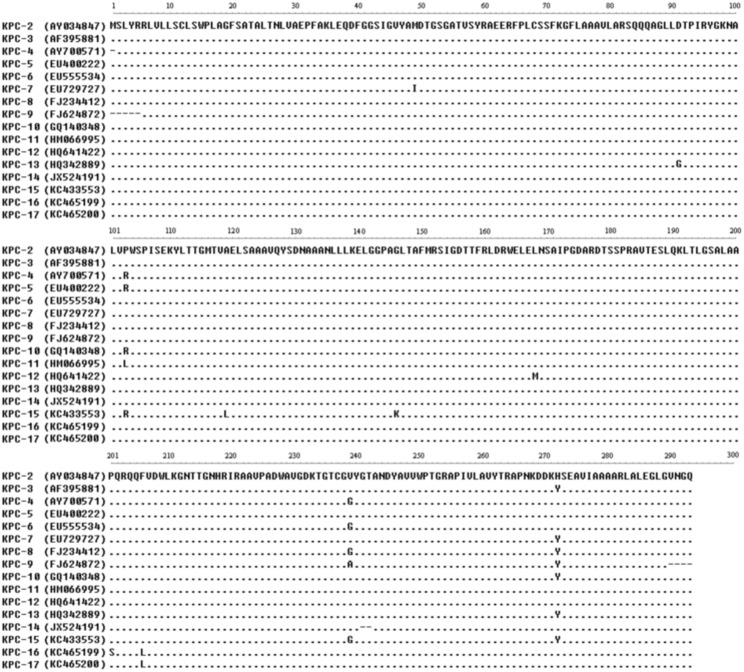
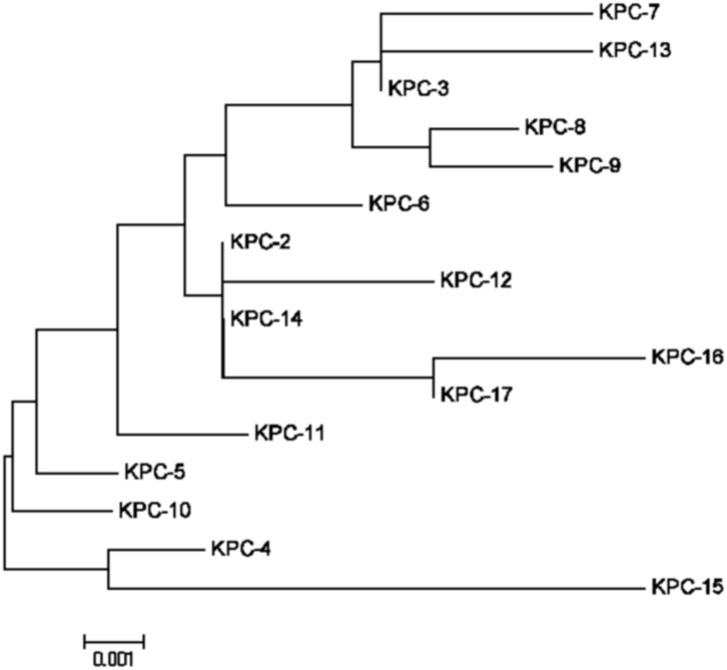
Amino acid variations between novel bla KPC-15 and other bla KPC genes were listed in Figure 1, and basing on the data of Figure 1, we drew the minimum evolution tree of amino acid sequences for KPC-2 to KPC-17 (Figure 2) using MEGA 5.05 software. The tree obviously indicated the KPC alleles evolution, and showed that the KPC-15 appeared to be homogenous with KPC-4 closely.
Figure 1. Comparison of amino acid sequence alignments of KPC carbapenemases.
KPC-15 was recently identified as a carbapenemase in Klebsiella pneumonae from the Taizhou Municipal Hospital of China. The data within parentheses are GenBank accession numbers.
Figure 2. Minimum Evolution tree of amino acid sequences for KPC-2 to KPC-17.
KPC-15 carbapenemase was our newly discovered and appeared to be homogenous with KPC-4 closely. The amino acid sequences of KPCs based on the data of Figure 1. This comparison was designed and analysed using MEGA 5.05 software.
Steady-state kinetic parameters were measured five times and averaged as shown in Table 3. Consistent with the MIC data, the catalytic efficiency (kcat/km ratio) of the KPC-15 enzyme was lowest for ceftazidime, at 0.0142 µM−1 s−1, and was higher at 5.26-fold compared with that of the KPC-2 enzyme, at 0.0027 µM−1 s−1. However, the catalytic efficiency of the KPC-15 enzyme was highest for nitrocefin, at 9.2 µM−1 s−1, and was a little higher at 1.10-fold compared with that of the KPC-2 enzyme, at 8.4 µM−1 s−1. The catalytic efficiency of the KPC-15 enzyme was higher at 2.96-fold for imipenem, with a value of 2.81 µM−1 s−1, compared with that of the KPC-2 enzyme, at 0.95 µM−1 s−1. Additionally, the catalytic efficiency of the KPC-15 enzyme was also higher at 2.72-fold for meropenem, with a value of 0.68 µM−1 s−1, compared with that of the KPC-2 enzyme, at 0.25 µM−1 s−1; the catalytic efficiency of KPC-15 enzyme was higher at 2.19-fold and 2.43-fold for cefotaxime and aztreonam, with values of 0.92 and 0.34 µM−1 s−1, respectively, compared with those values of the KPC-2 enzyme, at 0.42 and 0.14 µM−1 s−1, respectively, but higher at 2.01-fold for cefazolin, with a value of 5.62 µM−1 s−1, compared with that of the KPC-2 enzyme, at 2.8 µM−1 s−1. The quantitative values of the initial rate versus substrate concentration (v0/[s]) for KPC-15 and KPC-2 enzymes listed in Table S1. In general, the catalytic efficiency of KPC-15 was higher than that of KPC-2 for all tested antibiotics in this study (Table 3).
Table 3. Kinetic parameters for KPC-15 and KPC-2 enzymes.
| Substrate | KPC-15 | KPC-2 | |||||
| km (µM) | kcat (s−1) | kcat/km (µM−1s−1) | km (µM) | kcat (s−1) | kcat/km (µM−1s−1) | ||
| Imipene | 69±3 | 194±2 | 2.81±0.54 | 21±2 | 20±1 | 0.95±0.65 | |
| Meropenem | 19.6±2.1 | 13.4±0.95 | 0.68±0.73 | 16.2±3.1 | 4.1±0.99 | 0.25±0.061 | |
| Ceftazidime | 192±8 | 2.73±0.91 | 0.0142±0.00074 | 217±6 | 0.59±0.83 | 0.0027±0.00056 | |
| Cefotaxime | 101±7 | 93±9 | 0.92±0.18 | 124±6 | 52±6 | 0.42±0.13 | |
| Aztreonam | 373±4 | 126±6 | 0.34±0.053 | 389±5 | 53±2 | 0.14±0.077 | |
| Cefazolin | 21±2 | 118±2 | 5.62±0.87 | 16±1 | 44±2 | 2.8±1.0 | |
| Nitrocefin | 5.2±1.1 | 47.6±3.2 | 9.2±0.96 | 6.5±2.4 | 54.7±4.1 | 8.4±0.34 | |
PCR sequencing analysis and genetic organisation
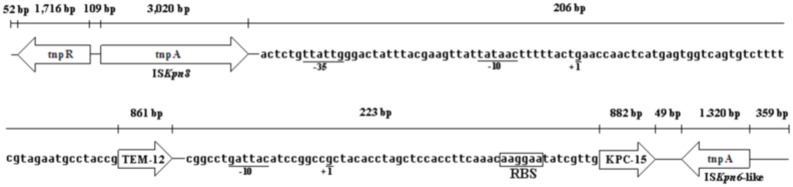
The analysis of the 8,997 bp nucleotide sequence for Kp1241 was shown in Figure 3. There were 5 different genes in the sequence, including the genes tnpR, bla TEM-12 and bla KPC-15, and the transposons ISKpn8 and ISKpn6-like [24]. The sites of the transcriptional promoters for the bla TEM-12 and bla KPC-15 genes were indicated as +1 and the −10 and −35 regions were also shown; however, there were no obvious transcriptional promoters in tnpR, ISKpn8 and ISKpn6-like transposons. Between the tnpR gene and the ISKpn8 transposon, there was a 109 bp nucleotide interval; the ISKpn8 transposon nucleotide sequence was 3,020 bp. Between the ISKpn8 transposon and the bla TEM-12 gene, there was a 206 bp nucleotide interval, which included the site of the transcriptional promoter for the bla TEM-12 gene, +1 (g, start codon), −10 region (tataac) and −35 region (ttattg). Between the bla TEM-12 and bla KPC-15 genes, there was a 223 bp nucleotide interval, which included the site of the transcriptional promoter for the bla KPC-15 gene, +1 (g, start codon), −10 region (gattac) and RBS (aaggaa, the ribosome binding site), but no obvious −35 region was shown in the bla KPC-15 gene. Between the bla KPC-15 gene and the ISKpn6-like transposon, there was a 249 bp nucleotide interval, and the ISKpn6-like transposon had a 1,320 bp nucleotide sequence.
Figure 3. Promoter elements of the bla KPC-15 and bla TEM genes in 8.997 kb-length nucleotide sequence.
The sequence provided the upstream and downstream regions of bla KPC-15 structural genes. The tnpA gene, which was upstream of the bla KPC-15 (3,020 bp) gene, was homologous to a putative ISKpn8 transposon, and the downstream region of the bla KPC-15 gene (1,320 bp) was homologous to a putative ISKpn6-like transposon. The nucleotides upstream of the bla KPC-15 and bla TEM-12 gene translational start codons were shown in the box. The putative −10 promoter elements of the bla KPC-15 gene were shown as gattaa, labeled as −10 below, and there were no obvious −35 promoter elements to be discovered in the promoter region. The putative −10 and −35 promoter elements of the bla TEM-12 gene were shown as tataac and ttattg, labeled as −10 and −35 below the promoter region. The start sites of transcription were indicated as G by +1 residue. RBS was the abbreviation of the ribosome binding site.
Discussion
In recent years, many reports have described the presence of KPC β-lactamases (particularly KPC-2), and discovered they can cause resistance to carbapenem or reduce susceptibility to carbapenem in strains of the family of Enterobacteriaceae from different areas in China [5], [16], [32]. It seems that KPC enzymes are becoming widely disseminated in China, and plasmids of different sizes may harbor the bla KPC-2 gene [33]. The genetic environments of the bla KPC gene have been characterised in some studies, and various transposon elements seems to be responsible for the rapid spread of bla KPC [33]–[35].
In this study, the susceptibility tests indicated that the strain Kp1241 showed resistance to quinolones, aminoglycosides, and the beta-lactam antibiotics, but susceptibility to polymyxin B and colistin with MICs of 0.5 and 1 µg/mL (Table 2), respectively. This result suggested that the old antibiotics, polymyxin B and colistin, might play an important role in the treatment of the strain Kp1241 [36].
Comparing the amino acids with other KPCs, KPC-15 showed more than two sites of amino acid changes (A119L and G146 K), and emerged to be homogenous with KPC-4 closly (Figures 1 and 2). The changes of the amino acids would influence the kinetic properties of the KPC enzymes. In spite of different values for the kms and kcats, the catalytic efficiency (kcat/km) of KPC-15 was higher than that of KPC-2 for all tested antibiotics (Table 3). These data implied that the effect of KPC-15 caused resistance to β-lactam antibiotics (including imipenem, meropenem, ceftazidime, cefotaxime, aztreonam and cefazolin) was higher than that of KPC-2. Moreover, an evolutionary transformation changed KPC from an efficient carbapenemase to its variants (KPC-15) with better ceftazidimase catalytic efficiency. Because of the multi-resistance to quinolones or to aminoglycosides antibiotics, in addition to carbapenemase, the strain Kp1241 might involve other genes, such as quinolone or aminoglycoside genes.
According to the literature, there is a distinct genetic environment with the chimera of several transposon-associated elements in China, such as Tn3, ISKpn8 and an ISKpn6-like element on plasmid pKP048 [33]. With conjugation and gene mapping studies, we acquired three plasmids of different lengths. The bla KPC-15 variant is on a ca. 73-kb plasmid and carries ISKpn8 and an ISKpn6-like element determinant [24]. The transformant was also much less resistant than the original isolate, it was most likely due to the lack of any permeability lesion. The genetic environment of the bla KPC-15 gene in the plasmid showed a structural and context similarity to those characteristics found in plasmid pKP048 [33], except that a truncated bla TEM-12 gene and the bla KPC-15 variant were inserted and located between ISKpn8 and the novel bla KPC-15 variant [24]. The nucleotides upstream of the bla KPC-15 translational start codon were also shown in Figure 3. The start codon was indicated as G by the +1 residue, and the putative −10 promoter elements were shown as gattaa and labeled as −10 below. These data were consistent with a study by Yigit et al. [8]; however, no obvious −35 region was discovered in the promoter region, which was different from previous report [8]. Only a 233 bp sequence was discovered between the bla TEM-12 gene and the bla KPC-15 variant in this study (Figure 3).
Conclusions
The study suggested that old antibiotics (polymyxin B and colistin) might play a role in therapy. The novel KPC-15 had more than two sites of amino acid changes (A119L and G146 K), and was homogenous with KPC-4 closly. The catalytic efficiency of KPC-15 causing resistance to β-lactam antibiotics was higher than that of KPC-2. Moreover, an evolutionary transformation changed KPC from an efficient carbapenemase to its variants (KPC-15) with better ceftazidimase catalytic efficiency.
Supporting Information
The quantitative values of the initial rate versus substrate concentration (v0/[s]) for KPC-15 and KPC-2 enzymes.
(DOC)
Acknowledgments
The authors would like to thank professor Sen Zhang, from the Peking Union Hospital, for critical reading of the manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. Data is available from GenBank using accession number KC433553.
Funding Statement
This study was supported by grants from the Zhejiang Natural Science Foundation (Y2100248), the Foundation of the Department of Science and Technology of Zhejiang Province (2014C33153), the Foundation of the Zhejiang Health Department (2014KYB313), the Foundation of the Taizhou Science and Technology Bureau (1102KY15, 1201KY22 and 1301KY36), and the Foundation of the Jiaojiang Science and Technology Bureau of Taizhou (83041 and 112071), China. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Centers for Disease Control and Prevention (CDC) (2009) Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb Mort Wkly Rep 58: 256–260. [PubMed] [Google Scholar]
- 2. Cao VB, Arlet G, Ericsson BM, Tammelin A, Courvalin P, et al. (2000) Emergence of imipenem resistance in Klebsiella pneumoniae owing to combination of plasmid- mediated CMY-4 and permeability alteration. J Antimicrob Chemother 46: 895–900. [DOI] [PubMed] [Google Scholar]
- 3. Wieczorek P, Sacha P, Hauschild T, Ζórawski M, Krawczyk M, et al. (2008) Multidrug resistant Acinetobacter baumannii-the role of AdeABC (RND family) efflux pump in resistance to antibiotics. Folia Histochem Cytobiol 46: 257–267. [DOI] [PubMed] [Google Scholar]
- 4. Neuwirth C, Siébor E, Duez JM, Péchinot A, Kazmierczak A (1995) Imipenem resistance in clinical isolates of Proteus mirabilis associated with alterations in penicillin-binding proteins. J Antimicrob Chemother 36: 335–342. [DOI] [PubMed] [Google Scholar]
- 5. Nordmann P, Cuzon G, Naas T (2009) The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis 9: 228–236. [DOI] [PubMed] [Google Scholar]
- 6. Poirel L, Héritier C, Tolun V, Nordmann P (2004) Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae . Antimicrob Agents Chemother 48: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Walther-Rasmussen J, Høiby N (2007) Class A carbapenemases. J Antimicrob Chemother 60: 470–482. [DOI] [PubMed] [Google Scholar]
- 8. Yigit H, Queenan AM, Anderson GJ, Domenech-Sanchez A, Biddle JW, et al. (2001) Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae . Antimicrob Agents Chemother 45: 1151–1161 (Erratum 52: 809).. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith Moland E, Hanson ND, Herrera VL, Black JA, Lockhart TJ, et al. (2003) Plasmid mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 51: 711–714. [DOI] [PubMed] [Google Scholar]
- 10. Yigit H, Queenan AM, Rasheed JK, Biddle JW, Domenech-Sanchez A, et al. (2003) Carbapenem-resistant strain of Klebsiella oxytoca harboring carbapenem-hydrolyzing beta-lactamase KPC-2. Antimicrob Agents Chemother 47: 3881–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miriagou V, Tzouvelekis LS, Rossiter S, Tzelepi E, Angulo FJ, et al. (2003) Imipenem resistance in a Salmonella clinical strain due to plasmid-mediated class A carbapenemase KPC-2. Antimicrob Agents Chemother 47: 1297–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woodford N, Tierno PM, Jr Young K, Tysall L, Palepou MF, et al. (2004) Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta- lactamase, KPC-3, in a New York Medical Center. Antimicrob Agents Chemother 48: 4793–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palepou MF, Woodford N, Hope R, Colman M, et al. (2005) Novel class A carbapenemase, KPC-4, in an Enterobacter isolate from Scotland, abstr. 1134–01–20. Prog. Abstr. 15th Eur. Cong. Clin Microboil Infect Dis, Copenhagen, Denmark.
- 14. Maltezo HC (2009) Metallo-β-lactamases in Gram-negative bacteria: introducing the era of multi-resistance? Int J Antimicrob Agents 33: 405e.1–405e.7. [DOI] [PubMed] [Google Scholar]
- 16. Queenan AM, Bush K (2007) Carbapenemases: the versatile β-lactamases. Clin Microbiol Rev 20: 440–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sacha P, Zórawski M, Hauschild T, Wieczorek P, Jaworowska J, et al. (2007) The presence of bla IMP genes on plasmids DNA isolated from multidrug-resistant Pseudomonas aeruginosa strains at University Hospital in Bialystok (Poland)-first report. Folia Histochem Cytobiol 45: 405–408. [PubMed] [Google Scholar]
- 18. Deshpande LM, Rhomberg PR, Sader HS, Jones RN (2006) Emergence of serine carbapenemases (KPC and SME) among clinical strains of Enterobacteriaceae isolated in the United States Medical Centers: report from the MYSTIC Program (1999–2005). Diagn Microbiol Infect Dis 56: 367–372. [DOI] [PubMed] [Google Scholar]
- 19. Sacha P, Wieczorek P, Hauschild T, Zórawski M, Olszañska D, et al. (2008) Metallo-β-lactamases of Pseudomonas aeruginosa – a novel mechanism resistance to β-lactam antibiotics. Folia Histochem Cytobiol 46: 137–142. [DOI] [PubMed] [Google Scholar]
- 20. Hawkey PM, Jones AM (2009) The changing epidemiology of resistance. J Antimicrob Chemother 64: i3–i10. [DOI] [PubMed] [Google Scholar]
- 21. Wolter DJ, Kurpiel PM, Woodford N, Palepou M-FI, Goering RV, et al. (2009) Phenotypic and enzymatic comparative analysis of the novel KPC variant KPC-5 and its evolutionary variants, KPC-2 and KPC-4. Antimicrob Agents Chemother 53: 557–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Robledo IE, Aquino EE, Santé MI, Santana JL, DM Otero, et al. (2010) Detection of KPC in Acinetobacter spp. in Puerto Rico. Antimicrob Agents Chemother 54: 1354–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitchel B, Rasheed JK, Patel JB, Srinivasan A, Navon-Venezia S, et al. (2009) Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob Agents Chemother 53: 3365–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang D, Hou W, Chen J, Mou Y, Yang L, et al. (2014) Characterization of the bla KPC-2 and bla KPC-3 genes and the novel bla KPC-15 gene in Klebsiella pneumoniae . J Med Microbiol 63: 981–987. [DOI] [PubMed] [Google Scholar]
- 25.CLSI (2012) Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement. Clin Lab Stand Inst M100–S22, 31.
- 26. Wang M, Tran JH, Jacoby GA, Zhang Y, Wang F, et al. (2003) Plasmid-mediated quinolone resistance in clinical isolates of Escherichia coli from Shanghai, China. Antimicrob Agents Chemother 47: 2242–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin S, Thomas M, Shlaes DM, Rudin SD, Knox JR, et al. (1998) Kinetic analysis of an inhibitor-resistant variant of the OHIO-1 β-lactamase, an SHV-family class A enzyme. Biochem J 333: 395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thomson JM, Distler AM, Bonomo RA (2007) Overcoming resistance to β-lactamase inhibitors: comparing sulbactam to novel inhibitors against clavulanate resistant SHV enzymes with substitutions at Ambler position 244. Biochemistry 46: 11361–11368. [DOI] [PubMed] [Google Scholar]
- 29. Papp-Wallac KM, Bethel CR, Distler AM, Kasuboski C, Taracila M, et al. (2010) Inhibitor resistance in the KPC-2 β-actamase: a preeminent property of this class A β-lactamase. Antimicrob Agents Chemother 54: 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frere JM, Dormans C, Duyckaerts C, De Graeve J (1982) Interaction of β- iodopenicillanate with the β-lactamases of Streptomyces albus G and Actinomadura R39. Biochem J 207: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matagne A, Ledent P, Monnaie D, Felici A, Jamin M, et al. (1995) Kinetic study of interaction between BRL 42715, β-lactamases, and D-alanyl-D-alanine peptidases. Antimicrob Agents Chemother 39: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mendes RE, Bell JM, Turnidge JD, Yang Q, Yu Y, et al. (2008) Carbapenem-resistant isolates of Klebsiella pneumoniae in China and detection of a conjugative plasmid (bla KPC-2 plus qnrB4) and a bla IMP-4 gene. Antimicrob Agents Chemother 52: 798–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shen P, Wei Z, Jiang Y, Du X, Ji S, et al. (2009) Novel genetic environment of the carbapenem-hydrolyzing beta-lactamase KPC-2 among Enterobacteriaceae in China. Antimicrob Agents Chemother 53: 4333–4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naas T, Nordmann P, Vedel G, Poyart C (2005) Plasmid-mediated carbapenem -hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 49: 4423–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu Q, Zhang YB, Han LZ, Sun JY, Ni YX (2009) Plasmid mediated 16 S rRNA methylases in aminoglycoside-resistant Enterobacteriaceae isolates in Shanghai, China. Antimicrob Agents Chemother 53: 271–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zavascki AP, Goldani LZ, Li J, Nation RL (2007) Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother 60: 1206–1215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The quantitative values of the initial rate versus substrate concentration (v0/[s]) for KPC-15 and KPC-2 enzymes.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. Data is available from GenBank using accession number KC433553.