Abstract
Microarray-based analysis of the transcriptional profiles of the genetically distinct Staphylococcus aureus strains COL, GP268, and Newman indicate that a total of 251 open reading frames (ORFs) are influenced by σB activity. While σB was found to positively control 198 genes by a factor of ≥2 in at least two of the three genetic lineages analyzed, 53 ORFs were repressed in the presence of σB. Gene products that were found to be influenced by σB are putatively involved in all manner of cellular processes, including cell envelope biosynthesis and turnover, intermediary metabolism, and signaling pathways. Most of the genes and/or operons identified as upregulated by σB were preceded by a nucleotide sequence that resembled the σB consensus promoter sequence of Bacillus subtilis. A conspicuous number of virulence-associated genes were identified as regulated by σB activity, with many adhesins upregulated and prominently represented in this group, while transcription of various exoproteins and toxins were repressed. The data presented here suggest that the σB of S. aureus controls a large regulon and is an important modulator of virulence gene expression that is likely to act conversely to RNAIII, the effector molecule of the agr locus. We propose that this alternative transcription factor may be of importance for the invading pathogen to fine-tune its virulence factor production in response to changing host environments.
Transcription of DNA into RNA is catalyzed by RNA polymerase. In bacteria, one RNA polymerase generates nearly all cellular RNAs, including ribosomal, transfer, and mRNA. This enzyme consists of six subunits, α2ββ′ωσ, with α2ββ′ω forming the catalytically competent RNA polymerase core enzyme (E). The core is capable of elongation and termination of transcription, but it is unable to initiate transcription at specific promoter sequences. The σ subunit, which when bound to E forms the holoenzyme (E-σ), directs the multisubunit complex to specific promoter elements and allows efficient initiation of transcription (reviewed in references 5 and 6). Therefore, σ factors provide an elegant mechanism in eubacteria to allow simultaneous transcription of a variety of genetically unlinked genes, provided all of these genes share the same promoter specificities.
In addition to the housekeeping sigma subunit, σ70 or σA, most bacteria produce one or more additional σ subunits, termed alternative σ factors, which direct the respective E-σ complex to distinct classes of promoters that contain alternative σ factor-specific sequences. Alternative σ factors have been shown in various bacteria to be of importance for survival under extreme conditions (7, 14, 23, 31, 38, 44, 49, 60, 68, 73, 78, 79, 80) and to influence virulence and pathogenicity (8, 13, 32, 35, 37, 42, 51, 57, 61, 71, 75, 78, 81).
At least six alternative σ factors are produced by the enteric bacterium Escherichia coli (reviewed in reference 6). Genomic sequence analysis suggests that many alternative σ factors also exist in a number of other pathogenic species such as Treponema palladium (4 alternative σ factors) (21), Vibro cholerae (7 alternative σ factors) (29), Mycobacterium tuberculosis (12 alternative σ factors) (12), and Pseudomonas aeruginosa (23 alternative σ factors) (76). Two alternative σ factors, σB and σH, have been identified in Staphylococcus aureus (43, 82). σH has only recently been characterized as a bona fide S. aureus sigma factor, which is involved in the transcriptional regulation of DNA competence factors (56).
In contrast to σH, the S. aureus alternative transcription factor σB has been studied intensively. It has been shown to be involved in the general stress response (7, 24, 26, 34, 43, 44). σB also directly or indirectly influences the expression of a variety of genes (25, 44, 84), including many associated with virulence, such as α-hemolysin (26, 34, 84), clumping factor (58, 60), coagulase (55, 60) fibronectin-binding protein A (58), lipases (44, 84), proteases (34, 36, 84), and thermonuclease (44, 84). In addition, σB has been shown to influence the expression of several global virulence factor regulators, including SarA (4, 15, 25, 52), SarS (also known as SarH1) (76), and RNAIII (4, 34). However, no effect of σB on S. aureus pathogenicity has been demonstrated in any in vivo model analyzed to date (7, 34, 60).
Besides its function in regulating virulence determinants, σB is likely to play a role in mediating antibiotic resistance. Inactivation of the gene coding for σB, sigB, in the homogeneously methicillin-resistant strain COL increases its susceptibility to methicillin (82) while mutations within the rsbU-defective strain BB255, leading to σB hyperproduction, are associated with an increase in glycopeptide resistance (3). Moreover, σB was shown to affect pigmentation (26, 44), to increase resistance to hydrogen peroxide (26, 44) and UV (26), and to promote microcolony formation (1) and biofilm production (67).
The genetic organization of the S. aureus sigB operon (43, 82) closely resembles that of the distal part of the well-characterized homologous operon of the soil-borne gram-positive bacterium Bacillus subtilis (reviewed in references 28 and 65). DNA microarray technology-based analysis of the general stress response in B. subtilis identified 127 genes controlled by σB (66), and heat shock studies suggest that the σB regulon of this organism comprises up to 200 genes (reviewed in references 27 and 30). Because S. aureus σB seems to be a pleotrophic regulator that plays a role in a number of clinically relevant processes, a number of investigators have begun characterizing the σB regulon. Proteomic approaches have identified 27 S. aureus cytoplasmic proteins and one extracellular protein to be under the positive control of σB, and 11 proteins were found to be repressed by the factor (25, 84), indicating that the σB regulon of this pathogen is likely to comprise a much higher number of genes than known to date.
In this study, we present DNA microarray-based data from three distinct genetic backgrounds that suggest that the S. aureus σB influences the expression of at least 251 genes. Of these, 198 genes are positively controlled by σB while 53 genes are repressed in the presence of the alternative σ factor.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
Strains and plasmids used in this study are listed in Table 1. S. aureus was routinely cultured on sheep blood agar or Luria-Bertani (LB) medium with rotary agitation at 200 rpm at 35°C. Exogenous glucose was not added to the growth medium. When included, antibiotics were used at the following concentrations: ampicillin, 50 mg liter−1; chloramphenicol, 40 mg liter−1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant genotype and phenotypea | Reference or source |
|---|---|---|
| Strains | ||
| E. coli XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB laclq ZΔM15 Tn10(Tcr)] | Stratagene |
| S. aureus | ||
| BB255 | rsbU, low σB activity | 2 |
| COL | mec, highly Mcr clinical isolate, Mcr Tcr | 41 |
| Newman | Clinical isolate, high level of clumping factor (ATCC 25904) | 17 |
| IK181 | BB255 ΔrsbUVW-sigB, Emr | 44 |
| IK183 | COL ΔrsbUVW-sigB, Emr Mcr Tcr | 44 |
| IK184 | Newman ΔrsbUVW-sigB, Emr | 44 |
| GP268 | BB255 rsbU+, Tcr | 26 |
| Plasmids | ||
| pAC7 | Cmr, expression plasmid containing the PBAD promoter and the araC gene | 70 |
| pAC7-sigB | Cmr, 767-bp PCR fragment of the sigB ORF from strain COL into pAC7 | This study |
| pSB40N | Apr, promoter probe plasmid | 39 |
| pSA0455p | Apr, 360-bp PCR fragment covering the promoter region of the COL homologue of ORF N315-SA0455 into pSB40N | This study |
Abbreviations are as follows: Apr, ampicillin resistant; Cmr, chloramphenicol resistant; Emr, crythromycin resistant; Mcr, methicillin resistant; Tcr, tetracycline resistant.
Sampling, RNA isolation, and transcriptional profiling.
Overnight cultures of S. aureus were diluted 1:100 into fresh prewarmed LB medium and grown as described above. For experiment 1, cultures were grown to an optical density at 600 nm (OD600) of 2, at which time RNA samples were prepared as described below. For experiment 2, cultures were grown for 9 h and sample volumes corresponding to 1010 cells were removed after 1, 3, 5, and 8 h of growth. For RNA isolation, samples were centrifuged at 7,000 × g at 4°C for 5 min, the culture supernatants were removed, and the cell sediments were snap-frozen in a dry ice-alcohol mixture. Frozen cells were resuspended in 5 ml of ice-cold acetone-alcohol (1:1) and incubated for 5 min on ice. After centrifugation at 7,000 × g and 4°C for 5 min, cells were washed with 5 ml of TE buffer (10 mM Tris, 1 mM EDTA [pH 8]) and resuspended on ice in 900 μl of TE. The cell suspensions were transferred to 2-ml lysing matrix B tubes (Bio 101, Vista, Calif.), and the tubes were shaken in an FP120 reciprocating shaker (Bio 101) two times at 6,000 rpm for 20 s. After centrifugation at 14,000 × g at 4°C for 5 min, the supernatants were used for RNA isolation with the RNeasy Midi system (QIAGEN, Inc., Valencia, Calif.) according to the manufacturer's recommendations. To remove any contaminating genomic DNA, approximately 125 μg of total RNA was treated with 20 U of DNase I (Amersham Biosciences, Piscataway, N.J.) at 37°C for 30 min. The RNA was then purified with an RNeasy mini column (QIAGEN) by following the manufacturer's cleanup protocol. The integrity of the RNA preparations was analyzed by electrophoresis in 1.2% agarose-0.66 M formaldehyde gels. Reverse transcription-PCR, cDNA fragmentation, cDNA terminal labeling, and hybridization of approximately 1.5 μg of labeled cDNA to custom-made Wyeth S. aureus GeneChips were carried out in accordance with the manufacturer's (Affymetrix Inc., Santa Clara, Calif.) instructions for antisense prokaryotic arrays. The GeneChip contains 7,723 qualifiers representing the consensus open reading frame (ORF) sequences of the genomes of N315, Mu50, COL, 8325, 252, and 476 as well as those of N315 intergenic regions greater than 50 bp in length (P. Dunman, E. Murphy, and S. Projan, unpublished data). GeneChip arrays were scanned with the GeneArray laser scanner (Agilent Technologies, Palo Alto, Calif.). Data for biological duplicates were normalized and analyzed by using the GeneSpring, version 5.1, gene expression software package (Silicon Genetics, Redwood City, Calif.). Genes that were considered upregulated in a σB-dependent manner were found to demonstrate a >2-fold increase in RNA titers under σB-producing conditions in comparison to isogenic non-σB-producing cells. In addition, these genes were considered present by Affymetrix algorithms in the σB-producing strains and demonstrated a significant difference in expression (t test, with a P cutoff of at least 0.05). Genes considered downregulated in a σB-dependent manner demonstrated at least a twofold reduction in RNA transcript titers in the wild-type as opposed to their isogenic σB mutant background and were both considered present by the Affymetrix criteria in mutant cells and where characterized as having significantly differing amounts of transcripts based on t tests with a P cutoff of at least 0.05.
Construction of plasmids pAC7-sigB and pSA0455p.
A DNA fragment constituting the sigB ORF of S. aureus COL was amplified by PCR with an upstream primer (5′-GATCATATGGCGAAAGAGTCGAAATCAGC-3′) containing an NdeI site (underlined) and a downstream primer (5′-GCGAAGCTTCAAATTCTATTGATGTGCTGC-3′) containing a HindIII site (underlined), with italic nucleotides corresponding to positions 2687 to 2709 and 3443 to 3463 of the sequence found under GenBank accession no. Y09929, respectively. The resulting PCR product was digested with NdeI and HindIII and cloned into plasmid pAC7 (70) to obtain pAC7-sigB, which was subsequently transformed by electroporation into E. coli XL1-Blue (Stratagene, La Jolla, Calif.). Sequence analysis and comparison confirmed the identity of the construct. For pSA0455p, a DNA fragment representing 360 bp of the N315-SA0455 promoter region of COL was generated by PCR with an upstream primer (5′-CGGATCCAGTAGTAGTGATTAGAAAAGAC-3′) containing a BamHI site (underlined) and a downstream primer (5′-CGGCTCGAGATAAACTGTTGCCAGGTTCTACG-3) containing an XhoI site (underlined), with italic nucleotides corresponding to positions 227569 to 227592 and 227895 to 227919, respectively, of the sequence found under GenBank accession no. AP003130. The PCR product was digested with BamHI and XhoI and cloned into promoter probe plasmid pSB40N (39) to obtain pSA0455p. Sequence analysis confirmed the identity of the insert. Plasmid pSA0455p was transformed into E. coli XL1-Blue containing either compatible plasmid, pAC7-sigB or pAC7.
High-resolution S1 nuclease mapping.
For RNA isolation from recombinant E. coli cultures, strains were grown at 37°C in LB supplemented with ampicillin and chloramphenicol to an OD600 of 0.3. At this growth stage, expression of S. aureus sigB was induced by adding 0.0002% (wt/vol) arabinose, and cultivation was continued for an additional 3 h. Isolation of total RNA and high-resolution S1 nuclease mapping were performed as described by Kormanec (40). A 450-bp DNA fragment covering the SA0455 promoter region was amplified by PCR from pSA0455p, with universal oligonucleotide primer −47 (5′-CGCCAGGGTTTTCCCAGTCACGAC-3′), labeled at the 5′ end with [γ-32P]ATP, and mut80 primer (5′-GGGTTCCGCGCACATTTCCCCG-3′). Forty micrograms of RNA was hybridized to 0.02 pmol of the 5′ end-labeled DNA fragment (approximately 3 × 106 cpm/pmol of probe) and treated with 100 U of S1 nuclease. The protected DNA fragment was analyzed on a DNA sequencing gel together with G+A and T+C sequencing ladders derived from the end-labeled probe (54).
RESULTS AND DISCUSSION
Identification of σB-regulated genes.
Proteomic approaches and computational analyses, based on the method described by Petersohn and colleagues (64), indicate that the σB regulon of S. aureus comprises many more genes than described to date, suggesting that the regulon might be as large as that of the well-characterized homologous regulon of B. subtilis (reviewed in references 27 and 30). In an effort to better define the S. aureus σB regulon, DNA microarray studies were performed in three genetically distinct backgrounds. DNA microarray technology is a powerful tool to analyze the transcription profiles of the whole genome, provided that all genes are represented on the respective GeneChip. There is increasing evidence that extensive variation in gene content exists among strains of many pathogenic bacterial species. A genomic comparison of 36 S. aureus strains of divergent clonal lineage identified a very large genetic variation to be present in this pathogen, with approximately 22% of the genome being dispensable (18). The custom-made Affymetrix S. aureus GeneChip used in this study includes probes that monitor the expression of virtually all ORFs from six S. aureus genomes, making it an ideal tool for the identification of almost all transcriptional changes that are caused by the alternative transcription factor σB.
Two different approaches were chosen to identify σB-dependent genes. In experiment 1, the transcriptional profiles of three genetically distinct S. aureus strains harboring an intact sigB operon (COL, Newman, and GP268) and their isogenic ΔrsbUVW-sigB mutants were analyzed. For this purpose, total bacterial RNA was obtained from cells that were grown to the late-exponential growth phase (OD600 = 2), a time point at which σB has been shown to be active (26). Comparison of the transcriptional profiles of the sigB+ strains to their respective isogenic sigB mutants identified 229 ORFs to be influenced by σB by a factor of more than 2 in at least two of the three genetic backgrounds analyzed (Tables 2 and 3). While the majority of ORFs were positively influenced by σB (Table 2), as expected for a σ factor, a number of ORFs that were repressed in the presence of σB were also identified (Table 3). Forty-six of the genes identified were previously shown to be influenced by σB in S. aureus. Additionally, 23 genes were previously identified to be regulated by σB in B. subtilis (30, 66). This high correlation indicates that the GeneChip method used accurately identified the genes belonging to the σB regulon of the strains analyzed.
TABLE 2.
Genes upregulated by σB
| N315 ORF no.a | N315 genea | N315 descriptiona | Fold changeb in strain:
|
σB consensusc,d | Reference(s) with reported σB dependencee for:
|
|||
|---|---|---|---|---|---|---|---|---|
| COL | Newman | GP268 | S. aureus | B. subtilisf | ||||
| N315-SA1984 | asp23 | Alkaline shock protein 23 | Up | Up | Up | Yes | 24, 26, 44, 55 | |
| CAB75732.1 | bbp | Bone sialoprotein-binding protein Bbp | 3.2 | 4.5 | 4.8 | ? | — | |
| N315-SA2008 | budB | α-acetolactate synthase | Up | Up | Up | Yesd | ||
| N315-SA0144 | cap5A | Capsular polysaccharide synthesis enzyme Cap5A | Up | Up | 12.8 | ? | ||
| N315-SA0145 | cap5B | Capsular polysaccharide synthesis enzyme Cap5B | Up | Up | 10.8 | ? | ||
| N315-SA0146 | cap5C | Capsular polysaccharide synthesis enzyme Cap8C | Up | Up | 8.6 | ? | ||
| N315-SA0147 | cap5D | Capsular polysaccharide synthesis enzyme Cap5D | Up | Up | 7.3 | ? | ||
| N315-SA0148 | cap5E | Capsular polysaccharide synthesis enzyme Cap8E | Up | Up | 7.5 | ? | ||
| N315-SA0149 | cap5F | Capsular polysaccharide synthesis enzyme Cap5F | Up | Up | 7.5 | ? | ||
| N315-SA0150 | cap5G | Capsular polysaccharide synthesis enzyme Cap5G | Up | Up | 6.8 | ? | ||
| N315-SA0151 | cap5H | Capsular polysaccharide synthesis enzyme Cap5H | Up | Up | 5.1 | ? | — | |
| N315-SA0152 | cap5I | Capsular polysaccharide synthesis enzyme Cap5I | Up | Up | 5.7 | ? | — | |
| N315-SA0153 | cap5J | Capsular polysaccharide synthesis enzyme Cap5J | Up | Up | 3.5 | ? | — | |
| N315-SA0155 | cap5L | Capsular polysaccharide synthesis enzyme Cap5L | Up | Up | 5.1 | ? | ||
| N315-SA0156 | cap5M | Capsular polysaccharide synthesis enzyme Cap5M | Up | Up | 4.5 | ? | ||
| N315-SA0157 | cap5N | Capsular polysaccharide synthesis enzyme Cap5N | 2.7 | Up | 5.2 | ? | ||
| N315-SA0158 | cap5O | Capsular polysaccharide synthesis enzyme Cap8O | 2.6 | Up | 4.2 | ? | ||
| CAA79304 | clfA | Clumping factor A | 35.7 | Up | 7.8 | Yes | 33, 60 | |
| N315-SA2336 | clpL | ATP-dependent Clp proteinase chain ClpL | 17.3 | 13.2 | Up | Yes | 25, 33 | |
| N315-SA2349 | crtM | Squalene desaturase | Up | Up | Up | Yesd | 26 | 66 (yisP) |
| N315-SA2348 | crtN | Squalene synthase | Up | Up | Up | Yesd | 26 | |
| N315-SA1452 | csbD | HP, σB-controlled gene product CsbD (Csb8) | 37.0 | Up | Up | Yes | 25, 33 | 30, 66 (ywmG) |
| COL-SA1872 | epiE | Epidermin immunity protein EpiE | Up | Up | Up | Yesd | — | |
| COL-SA1873 | epiF | Epidermin immunity protein EpiF | Up | Up | Up | Yes | — | |
| N315-SA1634 | epiG | Epidermin immunity protein EpiG | Up | Up | Up | Yesd | — | |
| N315-SA2260 | fabG | HP, similar to glucose l-dehydrogenase | Up | Up | Up | Yes | 30 (yxbG) | |
| N315-SA1901 | fabZ | (3R)-hydroxymyristoyl-[acyl carrier protein] dehydratase | 2.2 | 5.1 | 2.0 | Yesd | ||
| N315-SA2125 | hutG | HP, similar to formiminoglutamase | 3.7 | 14.6 | 2.9 | Yes | ||
| N315-SA1505 | lysP | Lysine-specific permease | 2.4 | 7.9 | 2.0 | ? | ||
| N315-SA1962 | mtlA | PTS system, mannitol-specific IIA component | 8.5 | 17.2 | Up | Yesd | ||
| N315-SA1963 | mtlD | Mannitol-l-phosphate 5-dehydrogenase | 8.2 | Up | Up | Yesd | ||
| N315-SA1902 | murA | UDP-N-acetylglucosamine l-carboxyvinyl transferase 1 | 2.2 | 5.1 | 2.0 | Yesd | ||
| N315-SA0547 | mvaK1 | Mevalonate kinase | 2.4 | 4.5 | 1.3 | Yes | ||
| N315-SA0548 | mvaD | Mevalonate diphosphate decarboxylase | 3.3 | 7.3 | 1.8 | Yesd | — | |
| N315-SA0549 | mvaK2 | Phosphomevalonate kinase | 3.7 | 10.6 | 2.2 | Yesd | ||
| N315-SA1987 | opuD | Glycine betaine transporter opuD homologue | Up | Up | Up | Yes | 26, 33 | |
| N315-SA1871 | rsbV | Anti-σB factor antagonist | Up | Up | Up | Yes | 26, 43, 82 | 30, 66 |
| N315-SA1870 | rsbW | Anti-σB factor | Up | Up | Up | Yesd | 26, 43, 82 | 30, 66 |
| N315-SA0573 | sarA | Staphylococcal accessory regulator A (Csb35) | 2.9 | 3.8 | 2.0 | Yes | 4, 15, 25, 55 | |
| N315-SA0108 | sarS | Staphylococcal accessory regulator A homologue S | 2.6 | 1.1 | 2.1 | Yes | 77 | — |
| N315-SA0099 | sbtA | HP, similar to transmembrane efflux pump protein | Up | Up | Up | ? | 66 (yusP) | |
| N315-SA1869 | sigB | Alternative transcription factor σB | Up | Up | Up | Yesd | 26, 43, 82 | 30, 66 |
| N315-SA0456 | spoVG | Stage V sporulation protein G homologue | 4.3 | 9.8 | 3.0 | Yesd | ||
| N315-SA1114 | truB | tRNA pseudouridine 5S synthase | 2.1 | Up | 2.3 | Yes | ||
| N315-SA2119 | ydaD | HP, similar to dehydrogenase (Csb28) | 4.8 | 33.1 | 16.9 | Yes | 25 | 30 (yhxD) |
| N315-SA0084 | HP, similar to Homo sapiens CG1-44 protein | Up | Up | 3.0 | Yes | — | ||
| N315-SA0098 | HP, similar to aminoacylase | Up | Up | Up | ? | |||
| N315-SA0102 | 67 kDa myosin-crossreactive streptococcal antigen homologue | Up | Up | Up | Yes | — | ||
| N315-SA0105 | HP | Up | Up | Up | ? | — | ||
| N315-SA0163 | HP, similar to cation efflux system membrane protein CzcD | Up | Up | Up | ? | |||
| N315-SA0164 | HP | Up | Up | Up | Yes | — | ||
| N315-SA0261 | HP, similar to rbs operon repressor RbsR | 2.5 | Up | Up | Yes | |||
| N315-SA0296 | Conserved HP | 7.6 | 20.5 | 3.9 | Yes | — | ||
| N315-SA0297 | HP, similar to ABC transporter ATP-binding protein | 6.3 | 13.1 | 2.8 | Yesd | — | ||
| N315-SA0317 | HP, similar to dihydroflavonol-4-reductase | 11.6 | 20.7 | 3.9 | Yes | — | ||
| N315-SA0326 | Conserved HP | 2.5 | 2.1 | 2.0 | Yes | |||
| N315-SA0327 | Conserved HP | 2.2 | 2.1 | 2.0 | Yesd | — | ||
| N315-SA0359 | Conserved HP | Up | Up | Up | Yes | 33 | ||
| N315-SA0360 | Conserved HP | Up | Up | 77.7 | Yes | 30, 66 (ydaS) | ||
| N315-SA0372 | HP (Csb12) | 1.6 | 3.3 | 2.0 | Yes | 25 | — | |
| N315-SA0455 | Translation initiation inhibitor homologue | 3.2 | 6.2 | 2.3 | Yes | 33 | ||
| N315-SA0509 | Conserved HP | 2.0 | 12.1 | 2.0 | ? | — | ||
| N315-SA0528 | HP, similar to hexulose-6-phosphate synthase (Csb4) | 1.8 | 6.8 | 2.0 | Yes | 25 | ||
| N315-SA0529 | Conserved HP (Csb4-1) | 1.9 | 8.7 | 2.0 | Yesd | 25 | ||
| N315-SA0541 | HP, similar to cationic amino acid transporter | 11.3 | 14.4 | 7.7 | Yes | 30 (yhdG) | ||
| N315-SA0572 | HP, similar to esterase/lipase | Up | Up | Up | Yes | |||
| N315-SA0577 | HP, similar to FimE recombinase | Up | Up | Up | ? | |||
| N315-SA0578 | HP, similar to NADH dehydrogenase | Up | Up | Up | Yes | |||
| N315-SA0579 | HP, similar to Na+ H+ antiporter | Up | Up | 4.0 | Yesd | |||
| N315-SA0580 | HP, similar to Na+ H+ antiporter | Up | Up | Up | Yesd | |||
| N315-SA0581 | MnhD homologue, similar to Na+ H+ antiporter subunit | Up | Up | 6.0 | Yesd | |||
| N315-SA0582 | HP, similar to Na+ H+ antiporter | Up | Up | 4.0 | Yesd | |||
| N315-SA0583 | HP, similar to Na+ H+ antiporter | Up | Up | 4.7 | Yesd | |||
| N315-SA0584 | Conserved HP | Up | Up | 5.3 | Yesd | |||
| N315-SA0633 | HP | 2.0 | 8.7 | 2.9 | Yesd | 33 | — | |
| N315-SA0634 | Conserved HP | 1.9 | 6.6 | 2.3 | Yesd | |||
| N315-SA0635 | Conserved HP | 5.1 | 14.8 | 2.8 | Yesd | |||
| N315-SA0636 | Conserved HP | 5.5 | 22.9 | 2.2 | Yesd | |||
| N315-SA0637 | Conserved HP | 5.3 | 24.3 | 3.5 | Yes | — | ||
| N315-SA0658 | HP, similar to plant metabolite dehydrogenases | 3.0 | 10.5 | 2.5 | Yes | — | ||
| N315-SA0659 | HP, similar to CsbB stress response protein | 3.3 | 10.4 | 2.5 | Yesd | 30, 66 (csbB) | ||
| N315-SA0665 | Coenzyme PQQ synthesis homologue | 2.1 | 4.5 | 1.8 | ? | |||
| N315-SA0666 | 6-Pyruvoyl tetrahydrobiopterin synthase homologue | 2.3 | 5.7 | 2.1 | ? | |||
| N315-SA0681 | HP, similar to multidrug resistance protein (Csb29) | 2.4 | Up | Up | Yes | 25 | 30, 66 (bmrU) | |
| N315-SA0721 | Conserved HP | 4.2 | 10.3 | 2.4 | Yes | |||
| N315-SA0722 | Conserved HP | 3.4 | 9.4 | 1.5 | Yesd | |||
| N315-SA0724 | HP, similar to cell division inhibitor | 2.5 | 3.8 | 2.5 | Yes | 30, 66 (yfhF) | ||
| N315-SA0725 | Conserved HP | Up | Up | Up | ? | — | ||
| N315-SA0740 | HP | Up | Up | Up | Yes | — | ||
| N315-SA0741 | Conserved HP | Up | Up | Up | Yesd | — | ||
| N315-SA0748 | HP | 3.0 | Up | 4.8 | Yesd | — | ||
| N315-SA0749 | HP | 2.5 | Up | 6.6 | Yes | — | ||
| N315-SA0751 | HP | 4.3 | 5.7 | 4.1 | ? | — | ||
| N315-SA0752 | HP | Up | Up | Up | Yes | 33 | — | |
| N315-SA0755 | HP, similar to general stress protein 170 | Up | Up | Up | Yes | 30, 66 (ykzA) | ||
| N315-SA0768 | Conserved HP | 2.0 | 5.6 | 4.5 | ? | |||
| N315-SA0772 | Conserved HP | Up | Up | Up | Yes | 33 | 30, 66 (csbD) | |
| N315-SA0774 | HP, similar to ABC transporter ATP-binding protein homologue (Csb10) | 2.1 | 2.0 | 1.4 | Yes | 25 | ||
| N315-SA0780 | HP, similar to hemolysin | 3.3 | Up | 2.2 | Yes | 30, 66 (yqhB) | ||
| N315-SA0781 | HP, similar to 2-nitropropane dioxygenase | 2.2 | Up | 2.0 | Yesd | |||
| N315-SA0933 | HP | 13.1 | 26.9 | 7.1 | Yes | — | ||
| N315-SA1014 | Conserved HP | Up | Up | Up | Yes | |||
| N315-SA1057 | Conserved HP | 2.4 | 3.9 | 3.1 | Yes | |||
| N315-SA1559 | HP, similar to smooth muscle caldesmon | 3.6 | 12.1 | 2.1 | Yesd | — | ||
| N315-SA1560 | HP, similar to general stress protein homolog | 2.8 | 8.2 | 2.2 | Yes | 30, 66 (ytxG) | ||
| N315-SA1573 | HP | 5.9 | 21.0 | 3.0 | Yes | — | ||
| N315-SA1590 | HP | 2.0 | 4.3 | 2.1 | Yes | — | ||
| N315-SA1657 | Conserved HP | 2.0 | 4.5 | 2.4 | Yes | |||
| N315-SA1671 | HP (Csb33) | 3.0 | 9.4 | 2.1 | Yes | 25 | — | |
| N315-SA1692 | Conserved HP (Csb3) | 1.8 | 5.6 | 4.0 | ? | 25 | ||
| N315-SA1697 | HP, similar to protein-tyrosine phosphatase | 2.3 | 5.0 | 3.7 | Yes | 30 (yfkJ) | ||
| N315-SA1698 | HP | 1.3 | 2.9 | 2.0 | Yesd | — | ||
| N315-SA1699 | HP, similar to transporter | 5.0 | 23.1 | 6.1 | Yesd | 30, 66 (yfkH) | ||
| N315-SA1814 | HP, similar to succinyl-diaminopimelate desuccinylase | Up | Up | Up | ? | |||
| N315-SA1903 | Conserved HP | 3.7 | 10.9 | 3.7 | Yesd | |||
| N315-SA1924 | HP, similar to aldehyde dehydrogenase (Csb24) | 3.7 | 26.1 | 3.2 | Yes | 25 | ||
| N315-SA1942 | Conserved HP | 2.3 | 7.9 | 3.6 | ? | |||
| N315-SA1946 | Conserved HP (Csb9) | Up | Up | Up | Yes | 25, 33 | ||
| N315-SA1961 | HP, similar to transcription AT BglG family | 9.7 | 8.2 | Up | Yesd | |||
| N315-SA1980 | Conserved HP | 3.4 | 4.7 | 1.1 | Yesd | — | ||
| N315-SA1981 | Conserved HP | 5.7 | 7.7 | 1.6 | Yes | — | ||
| N315-SA1985 | HP | Up | Up | Up | Yesd | 26 | — | |
| N315-SA1986 | HP | Up | Up | Up | Yes | 26 | — | |
| N315-SA2006 | HP, similar to MHC class II analog | Up | Up | Up | ? | — | ||
| N315-SA2101 | Conserved HP | 2.2 | 3.3 | 1.5 | Yesd | 66 (yrhD) | ||
| N315-SA2102 | Conserved HP | 2.2 | 3.3 | 1.7 | Yes | |||
| N315-SA2104 | HP, similar to suppressor protein SuhB | 2.1 | 2.2 | 1.8 | Yes | |||
| N315-SA2158 | HP, similar to TpgX protein | 2.2 | 3.5 | 2.5 | Yes | |||
| N315-SA2203 | HP, similar to multidrug resistance protein | 2.1 | 3.9 | 2.2 | Yes | |||
| N315-SA2219 | Conserved HP | Up | Up | 3.0 | Yes | 33 | ||
| N315-SA2240 | HP, similar to para-nitrobenzyl esterase chain A | 1.9 | 2.0 | 2.0 | ? | |||
| N315-SA2242 | Conserved HP | Up | Up | Up | ? | |||
| N315-SA2243 | HP, similar to ABC transporter (ATP-binding protein) | Up | Up | Up | ? | |||
| N315-SA2262 | Conserved HP (Csb7) | Up | Up | Up | Yes | 25 | ||
| N315-SA2267 | HP | 3.0 | Up | 3.9 | Yes | — | ||
| N315-SA2298 | Conserved HP | 3.4 | 30.9 | 6.1 | ? | 33 | 30, 66 (ydhT) | |
| N315-SA2309 | Conserved HP | 2.0 | 2.5 | 2.9 | — | |||
| N315-SA2327 | HP, similar to pyruvate oxidase | 51.1 | Up | 17.9 | ? | 30, 66 (ydhP) | ||
| N315-SA2328 | Conserved HP | Up | Up | Up | ? | 66 (yxaC) | ||
| N315-SA2350 | Conserved HP | Up | Up | Up | Yesd | — | ||
| N315-SA2351 | HP, similar to phytoene dehydrogenase | Up | Up | Up | Yesd | — | ||
| N315-SA2352 | HP | Up | Up | Up | Yes | — | ||
| N315-SA2366 | Conserved HP | 7.3 | Up | 4.5 | Yes | — | ||
| N315-SA2367 | Conserved HP | 10.4 | Up | 8.9 | Yes | |||
| N315-SA2374 | Conserved HP | Up | Up | Up | ? | — | ||
| N315-SA2398 | HP | Up | Up | Up | Yes | — | ||
| N315-SA2403 | Conserved HP | 10.3 | Up | 8.7 | Yes | |||
| N315-SA2440 | HP | 2.3 | 5.9 | 1.7 | ? | — | ||
| N315-SA2441 | HP, similar to lipopolysaccharide biosynthesis protein | 2.5 | 6.6 | 2.0 | ? | |||
| N315-SA2442 | Preprotein translocase SecA homologue | 3.5 | 8.5 | 2.0 | ? | |||
| N315-SA2451 | HP | Up | Up | Up | Yes | 33 | — | |
| N315-SA2452 | Conserved HP | Up | Up | 3.5 | ? | |||
| N315-SA2479 | Conserved HP | Up | 4.3 | 4.6 | Yes | — | ||
| N315-SA2485 | HP | Up | Up | Up | Yes | — | ||
| N315-SA2488 | HP | Up | Up | Up | Yes | — | ||
| N315-SA2489 | HP, similar to high-affinity nickel-transport protein | Up | Up | Up | Yesd | — | ||
| N315-SA2491 | Conserved HP | Up | Up | Up | Yes | — | ||
| N315-SAS023 | HP, similar to thioredoxin | 2.1 | 4.6 | 3.2 | ? | — | ||
| N315-SAS049 | HP | Up | Up | Up | Yesd | — | ||
| N315-SAS053 | HP | 4.0 | 12.8 | 2.1 | Yesd | — | ||
| N315-SAS056 | HP | 2.0 | 5.7 | 1.9 | Yes | — | ||
| N315-SAS068 | HP | 5.2 | 5.7 | 3.3 | Yes | — | ||
| N315-SAS082 | HP | Up | Up | Up | ? | — | ||
| N315-SAS083 | HP | Up | Up | Up | ? | — | ||
| N315-SAS089 | HP | 2.6 | 5.7 | 2.3 | ? | — | ||
| COL-SA0866 | HP | Up | Up | Up | ? | — | ||
| COL-SA1046 | HP | 6.6 | 12.0 | 9.0 | Yes | — | ||
| COL-SA2012 | HP, acetyltransferase (GNAT) family | 5.8 | 2.9 | 2.0 | ? | — | ||
| COL-SA2013 | HP | Up | Up | Up | ? | — | ||
| COL-SA2379 | Conserved HP | 2.2 | 17.1 | 3.0 | ? | — | ||
| COL-SA2433 | HP | 2.6 | 3.6 | 2.1 | Yesd | — | ||
| COL-SA2481 | HP | Up | Up | Up | Yesd | — | ||
| COL-SA2595 | HP | 2.3 | 4.1 | 2.1 | ? | — | ||
| COL-SA2631 | Conserved HP | Up | Up | 3.8 | Yes | — | ||
| AAB05395 | HP, ORF 3 of the sarA locus | 11.8 | 46.6 | 6.8 | Yes | 4, 15, 52, 55 | — | |
| CAB60754 | HP | 32.1 | Up | 13.9 | Yes | — | ||
Based on the published sequence of strain N315 (accession no. NC_002745). For genes not present in N315, the gene name and description given are from the COL genome, available from The Institute for Genomic Research Comprehensive Microbial Resource website (http://www.tigr.org), or the respective accession number. ABC, ATP binding cassette; GNAT, GCN5-related N-acetyltransferases; HP, hypothetical protein; MHC, major histocompatibility complex; PTS, phosphotransferase system.
Normalized values in the rsbU+V+W+ sigB+ strain over values in the ΔrsbUVW-sigB mutant. “Up” denotes genes highly downregulated in the ΔrsbUVWsigB mutant, such that the transcripts were below detectable levels and the change could not be accurately calculated.
ORFs preceded by a consensus sequence that resembles the σB consensus sequence for B. subtilis as described by Petersohn et al. (64). Only sequences deviating not more than three nucleotides from the consensus GttTww12-15 gGgwAw (w = a, t) sequence and lying within 500 bp upstream of predicted ORFs were considered σB-dependent promoters. ?, genes or operons are not preceded by a σB consensus promoter that matches the criteria given above.
ORFs likely to form an operon.
References reporting an influence of σB on the respective gene or its gene product in S. aureus or the homologues gene in B. subtilis.
B. subtilis gene names are given in parentheses, if different from those of S. aureus. The absence of a homologous ORF in the B. subtilis genome is indicated by a dash.
TABLE 3.
Genes downregulated by σB
| N315 ORF no.a | N315 genea | N315 descriptiona | Fold changeb in strain:
|
Reference(s) with reported σB dependencec | Regulation by SarAd (reference[s]) | ||
|---|---|---|---|---|---|---|---|
| COL | Newman | GP268 | |||||
| N315-SA2430 | aur | Zinc metalloprotease aureolysin | 7.4 | 6.1 | 9.1 | 36, 84 | Down (16, 37, 84) |
| N315-SA2411 | citM | HP, similar to magnesium citrate secondary transporter | Down | Down | 4.3 | ||
| N315-SA0820 | glpQ | Glycerophosphoryl diester phosphodiesterase | 3.6 | 2.6 | 1.9 | 4 | Down (84) |
| N315-SA1007 | hla | α-Hemolysin precursor | 2.1 | 2.8 | 4.1 | 26, 84 | Up (16) |
| N315-SA2207 | hlgA | γ-Hemolysin component A | 1.7 | 2.0 | 2.1 | ||
| N315-SA2209 | hlgB | γ-Hemolysin component B | 2.2 | 4.2 | Down | Up (16) | |
| N315-SA2208 | hlgC | γ-Hemolysin component C | 2.0 | 4.7 | 4.1 | Up (16) | |
| N315-SA2463 | lip | Triacylglycerol lipase precursor | 2.0 | 6.2 | 2.0 | 44, 84 | Up (84)/Down (16) |
| N315-SA0252 | lrgA | Holin-like protein LrgA | 5.8 | 9.4 | Up (22) | ||
| N315-SA0253 | lrgB | Holin-like protein LrgB | 6.2 | 6.5 | Up (22)/Down (16) | ||
| N315-SA1812 | lukF | HP, similar to synergohymenotropic toxin precursor | 2.7 | 3.9 | Down | ||
| N315-SA1813 | lukM | HP, similar to leukocidin chain lukM precursor | 3.8 | 4.8 | Down | ||
| N315-SA0746 | nuc | Staphylococcal nuclease | 29.7 | 5.1 | Down | 44, 84 | Down (16, 84) |
| N315-SA0091 | plc | l-Phosphatidylinositol phosphodiesterase precurosr | Down | 3.9 | Down | Down (84) | |
| N315-SA0963 | pycA | Pyruvate carboxylase | 2.3 | 1.9 | 2.3 | ||
| N315-SA0259 | rbsD | Ribose permease | 2.9 | 2.8 | 1.5 | ||
| N315-SA0258 | rbsK | Probable ribokinase | 2.8 | 2.3 | 1.3 | ||
| N315-SA1758 | sak | Staphylokinase precursor (protease III) | 2.7 | 7.0 | |||
| N315-SA0128 | sodM | Superoxide dismutase | 4.6 | 2.0 | 2.8 | ||
| N315-SA1631 | splA | Serine protease SplA | Down | 9.9 | Down | 84 | Up (16) |
| N315-SA1630 | splB | Serine protease SplB | Down | 7.9 | Down | Up (16) | |
| N315-SA1629 | splC | Serine protease SplC | Down | Down | Down | 84 | |
| N315-SA1628 | splD | Serine protease SplD | Down | Down | Down | Up (16) | |
| COL-SA1865 | splE | Serine protease SplE | Down | Down | Down | ||
| BAB95617_1 | splF | Serine protease SplF | Down | Down | |||
| N315-SA0901 | sspA | Staphylococcal serine protease (V8 protease) | 3.8 | 2.1 | 3.3 | 36, 84 | Down (36) |
| N315-SA0900 | sspB | Cysteine protease | 3.2 | 2.2 | 4.3 | 36 | Down (16, 36) |
| N315-SA0899 | sspC | Cysteine protease | 3.0 | 1.9 | 3.0 | Down (16) | |
| N315-SA2302 | stpC | HP, similar to ABC transporter | 6.3 | 2.3 | 4.0 | ||
| N315-SA0022 | HP, similar to 5′ nucleotidase | 2.6 | 1.8 | 3.3 | |||
| N315-SA0089 | HP, similar to DNA helicase | 2.4 | Down | 2.1 | |||
| N315-SA0260 | HP, similar to ribose transporter RbsU | 3.0 | 2.6 | 2.3 | |||
| N315-SA0270 | HP, similar to secretory antigen precursor SsaA | 4.6 | Down | Down | |||
| N315-SA0272 | HP, similar to transmembrane protein Tmp7 | 4.4 | Down | Down | |||
| N315-SA0276 | Conserved HP, similar to diarrheal toxin-like protein | 3.7 | Up | ||||
| N315-SA0285 | HP | 2.6 | Down | 3.4 | |||
| N315-SA0291 | HP | 3.1 | 3.3 | ||||
| N315-SA0295 | HP, similar to outer membrane protein precursor | 4.9 | 3.6 | 10.4 | |||
| N315-SA0368 | HP, similar to proton/sodium glutamate symport protein | 2.7 | 3.1 | 1.4 | |||
| N315-SA0841 | HP, similar to cell surface protein Map-w | 5.7 | 3.4 | 2.2 | |||
| N315-SA0977 | 29-kDa cell surface protein | 2.5 | 2.1 | 1.8 | |||
| N315-SA1725 | Staphopain, cysteine protease | 5.9 | 4.2 | 10.6 | 36, 84 | Down (36, 84) | |
| N315-SA1726 | HP | 3.8 | 3.4 | 6.5 | |||
| N315-SA1815 | HP, similar to Na+-transporting ATP synthase | Down | Down | Down | |||
| N315-SA1853 | HP, similar to DNA mismatch repair protein MutS | 2.1 | Down | 4.0 | |||
| N315-SA2132 | HP, similar to ABC transporter (ATP-binding protein) | 2.7 | Down | 2.3 | |||
| N315-SA2133 | Conserved HP | 3.1 | Down | 3.2 | |||
| N315-SA2303 | HP, similar to membrane-spanning protein | Down | 1.8 | Down | |||
| N315-SAS020 | HP, similar to phosphoglycerate mutase | 2.1 | 2.4 | 2.2 | |||
| COL-SA0450 | HP | 2.2 | 2.2 | 3.1 | |||
| COL-SA1884 | HP | 3.3 | Down | Down | |||
| COL-SA2693 | HP | 2.2 | 7.1 | 2.2 | |||
Based on the published sequence of strain N315 (accession no. NC_002745). For genes not present in N315, the gene name and description given are from the COL genome, available from The Institute for Genomic Research Comprehensive Microbial Resource website (http://www.tigr.org) or the respective accession number. HP, hypothetical protein.
Normalized values in the ΔrsbUVW-sigB mutant over values in the rsbU+V+W+ sigB+ strain. “Down” denotes genes highly downregulated in the rsbU+V+W+ sigB strain, such that the transcripts were below detectable levels and the change could not be accurately calculated.
References reporting an influence of σB on the respective gene or its gene product.
References reporting an influence of SarA on the respective gene or its gene product.
Transcriptional start point (tsp) determinations of σB-driven transcripts (15, 33), coupled with σB-dependent in vitro transcription analyses of the asp23 P1 and coa promoters (55), suggest that the promoter region of S. aureus σB-regulated genes contains a consensus sequence that is highly similar to that of B. subtilis σB-regulated genes (GttTww12-15gGgwAw) (64). The similarity of the σB promoter consensus sequences of both species is further corroborated by the findings of Gertz et al. (24, 25), who demonstrated that the S. aureus asp23 P1 promoter is recognized by E-σB in B. subtilis and that all proteins that were identified to be influenced by σB in S. aureus by a proteomic approach are encoded by genes harboring a nucleotide sequence resembling the B. subtilis σB promoter consensus. Most of the genes identified as upregulated by σB in this study were also preceded by nucleotide sequences resembling the σB promoter consensus of B. subtilis, either directly or as part of a putative operon. None of the genes identified as downregulated in a σB-specific manner contained this sequence within their promoter regions.
Genes influenced by σB during early growth stages.
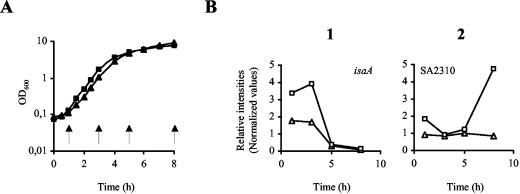
The approach used in experiment 1 proved useful for the identification of a large number of σB-regulated genes (Tables 2 and 3). However, this strategy was likely to miss σB-dependent genes that were expressed only during the early growth stages. In a second approach, the transcriptional profiles of strain Newman and its ΔrsbUVW-sigB mutant, IK184, were analyzed during several growth stages, e.g., 1, 3, 5, and 8 h after inoculation (Fig. 1A). Monitoring of the transcriptional profiles during different growth stages confirmed almost all genes identified by experiment 1 as σB dependent. The experiment also enabled us to identify 23 additional ORFs as positively regulated by σB (Table 4). The majority of these ORFs, represented by transcriptional profile type 1 (Fig. 1B), were expressed primarily during the early growth stages (1 and 3 h after inoculation) while no transcripts were detectable during later growth (5 and 8 h after inoculation). Members of this group include several putative virulence factors such as coa, encoding staphylococcal coagulase, and fnb, encoding fibronectin binding protein A, which have previously been demonstrated to be influenced by σB and confirmed in this study (55, 58, 60). In addition, ORFs N315-SA0620, N315-SA2093, and N315-SA2332, which are all homologues of ssaA of Staphylococcus epidermidis, encoding the highly antigenic staphylococcal secretory antigen A (48), were found to be influenced by σB. Most of the ORFs listed in Table 4 lacked a significant σB consensus promoter in their upstream regions, suggesting that σB indirectly regulates their transcript titers.
FIG. 1.
Expression pattern variation of ORFs influenced positively by σB. (A) Growth curves of S. aureus Newman (▪) and its mutant IK184 (▴). Time points of sampling are indicated by arrows. (B) Examples of expression pattern types of ORFs found to be influenced positively by σB in experiment 2. Transcript levels for Newman (□) and IK184 (▵) cells sampled at different time points of growth (x axis) are shown. Data points were plotted as relative intensity values (y axis).
TABLE 4.
Genes upregulated by σB in strain Newman during early growth phase
| N315 ORF no.a | N315 genea | N315 descriptiona | Fold changeb | σB consensusc,d | Expression profile | References with reported σB dependencee for:
|
|
|---|---|---|---|---|---|---|---|
| S. aureus | B. subtilisf | ||||||
| N315-SA0222 | coa | Staphylocoagulase precursor | 2.4 | Yes | 1 | 55, 60 | — |
| N315-SA2291 | fnb | Fibronectin binding protein A | 2.5 | ? | 1 | 58 | — |
| N315-SA2356 | isuA | Immunodominant antigen A | 2.4 | ? | 1 | — | |
| N315-SA0265 | lytM | Peptidoglycan hydrolase | 3.4 | Yes | 1 | — | |
| N315-SA2093 | ssaA | Secretory antigen precursor SsaA homolog | 2.4 | ? | 1 | — | |
| COL-SA0857 | vwb | Secreted von Willebrand factor-binding protein | 2.6 | ? | 1 | — | |
| N315-SA0336 | HP | 2.1 | ? | 1 | — | ||
| N315-SA0612 | Conserved HP | 3.1 | ? | 2 | — | ||
| N315-SA0620 | Secretory antigen SsaA homologue | 2.7 | ? | 1 | |||
| N315-SA0903 | Conserved HP | 2.5 | ? | 1 | |||
| N315-SA0937 | Cytochrome d ubiquinol oxidase subunit I homolog | 2.2 | ? | 1 | |||
| N315-SA0938 | Cytochrome d ubiquinol oxidase subunit II homolog | 2.0 | ? | 1 | |||
| N315-SA1275 | Conserved HP | 2.6 | ? | 1 | — | ||
| N315-SA1898 | HP, similar to SceD precursor | Up | Yes | 1 | — | ||
| N315-SA2301 | HP, similar to alkaline phosphatase | 2.2 | ? | 2 | |||
| N315-SA2310 | Conserved HP | 2.0 | ? | 2 | |||
| N315-SA2321 | HP | 2.3 | Yes | 2 | — | ||
| N315-SA2332 | HP, similar to secretory antigen precursor SsaA | 2.8 | ? | 1 | — | ||
| N315-SA2355 | Conserved HP | 2.3 | Yes | 1 | — | ||
| N315-SA2378 | Conserved HP | 2.5 | ? | 1 | — | ||
| N315-SA2447 | HP, similar to streptococcal hemagglutinin protein | Up | Yes | 2 | — | ||
| N315-SAS051 | HP | 2.1 | ? | 2 | — | ||
| COL-SA0210 | HP | Up | ? | 1 | — | ||
Based on the published sequence of strain N315 (accession no. NC_002745). For genes not present in N315, the gene name and description given are from the COL genome, available from The Institute for Genomic Research Comprehensive Microbial Resource website (http://www.tigr.org) or the respective accession number. ABC, ATP binding cassette; GNAT, GCN5-related N-acetyltransferases; HP, hypothetical protein; MHC, major histocompatibility complex; PTS, phosphotransferase system.
Normalized values in the rsbU+V+W+ sigB+ strain over values in the ΔrsbUVW-sigB mutant. “Up” denotes genes highly downregulated in the ΔrsbUVWsigB mutant, such that the transcripts were below detectable levels and the change could not be accurately calculated.
ORF preceded by a consensus sequence that resembles the σB consensus sequence for B. subtilis as described by Petersohn et al. (64). Only sequences deviating not more than three nucleotides from the consensus GttTww12-15 gGgwAw (w = a, t) and lying within 500 bp upstream of predicted ORFs were considered σB-dependent promoters. ?, genes or operons are not preceded by a σB consensus promoter that matches the criteria given above.
ORF likely to form an operon.
References reporting an influence of σB on the respective gene or its gene product in S. aureus or the homologues gene in B. subtilis.
B. subtilis gene names are given in parentheses if different from that of S. aureus. The absence of a homologous ORF in the B. subtilis genome is indicated by a dash.
The transcript titers of a number of ORFs were not only increased in the wild-type strain during early growth (1 h after inoculation) but were found to be further enhanced during late growth (8 h after inoculation), as represented by transcription profile type 2 (Fig. 1B). It is conceivable that the expression of these ORFs is again influenced indirectly by σB, most likely via regulator(s) which are mainly active during the late growth phase. The increase in expression observed for these ORFs during the early growth phase may be due to a carryover of the regulators that were produced during late growth in the preculture and may be still active even 1 h after inoculation.
Functional classification of ORFs influenced by σB.
The ORFs influenced by σB represent all functional categories that have been proposed by Kunst et al. (45), e.g., (i) cell envelope and cellular processes, including cell wall production, transport, signal transduction, membrane bioenergetics, and protein secretion; (ii) intermediary metabolism, including carbohydrate metabolism, glycolytic pathways, tricarboxylic acid cycle, and amino acid and lipid metabolism; (iii) information pathways, including DNA modification and repair, RNA synthesis, and regulation; (iv) other functions, such as adaptation to atypical conditions or detoxification; and (v) ORFs similar to proteins with unknown function. The latter group alone comprises 100 of the 251 ORFs regulated by σB, representing a large reservoir of potential factors that may be responsible for phenotypic properties of S. aureus associated with σB activity, such as the development of resistance to methicillin, glycopeptides, and hydrogen peroxide (3, 26, 44, 82) that have not been associated with specific genes.
Chromosomal distribution of σB-regulated genes.
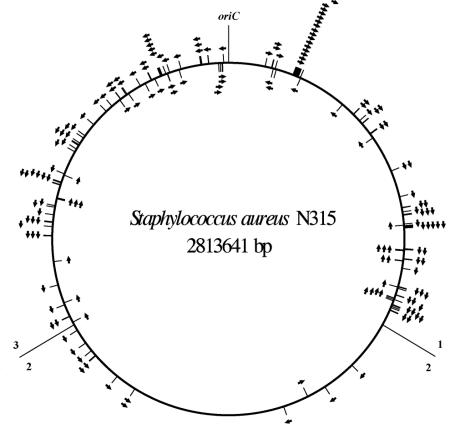
The ORFs that are positively controlled by σB are not evenly distributed over the S. aureus chromosome (Fig. 2) but rather are overabundant in the genomic regions that are close to the origin of replication (oriC). While 77 of 828 ORFs (9.3%) or 69 of 861 ORFs (8%) encoded by the genome fragments 1 and 3, corresponding to positions 1 to 937880 and 1875761 to 2813641, respectively, are influenced by σB, only 12 of 816 (1.5%) of the ORFs encoded by genomic region 2 (positions 937880 to 1875760), which is most distal to oriC, are controlled by σB. The majority of genes and/or operons in these segments are oriented with respect to oriC in a manner that minimizes collisions between the transcribing RNA polymerase and the replication apparatus. Thus, 71.5% of all genes and 77% of the σB-regulated ORFs located on genome fragment 1 are encoded by the clockwise replicating strand, and 72.8% of all genes and 72.5% of the σB-regulated ORFs located on genome fragment 3 are encoded by the counterclockwise strand. It has been suggested by Neidhardt and colleagues (59) that the location of a gene relative to oriC can affect its level of expression. Genes located near the point of origin of replication are present in higher numbers in a rapidly growing cell than those near the terminus, which may be of importance, especially for those genes that are controlled by promoters operating near the maximum possible frequency.
FIG. 2.
Chromosomal distribution and orientation of ORFs upregulated by σB. ORFs and their respective orientations are represented by arrows. The origin of replication (oriC) and the borders of genome fragments 1 to 3 are indicated.
Putative regulators acting downstream of σB.
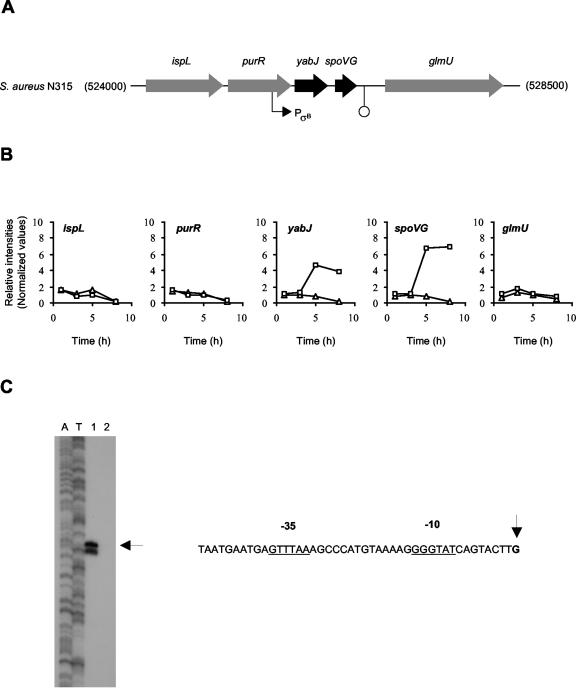
A significant number of ORFs (50 of 176 from experiment 1 and 17 of 23 from experiment 2) found to be upregulated by σB were not preceded by nucleotide sequences resembling the σB promoter consensus. Some of these genes were expressed only in sigB+ strains. It is possible that these ORFs were transcribed by the direct action of E-σB, despite the lack of an obvious σB promoter consensus. Alternatively, it is possible that σB controls the expression of a regulator(s), which would subsequently promote the expression of these genes. Promising candidates for such a scenario are the putative regulator homologues YabJ and SpoVG (N315-SA0455/6), which are likely to be cotranscribed, and were found to be controlled by σB (Fig. 3). The use of a recently described two-plasmid system (33) allowed us to confirm that yabJ expression is driven by σB (Fig. 3C). tsp determination by S1 mapping identified yabJ-specific RNA-protected fragments only in the presence of σB but not in the absence of the alternate transcription factor. A perfectly conserved σB consensus promoter sequence is present upstream of the yabJ tsp, indicating a direct influence of σB on the expression of this gene or operon. YabJ belongs to the highly conserved family of YigF proteins, which have been suggested to influence a variety of biological processes (69). YabJ of B. subtilis was found to have a role in the repression of purA by adenine (69). spoVG, encoding the stage V sporulation protein G, was the first developmentally regulated gene that was cloned from B. subtilis (74), and its regulation has been investigated intensively. However, little is known about the function of this protein. A mutation in spoVG was shown to impair the sporulation of B. subtilis, apparently as a result of disintegration of an immature spore cortex (72). More recent results suggest that SpoVG interferes with or is a negative regulator of the pathway leading to asymmetric septation (53). In addition to S. aureus, spoVG homologues have been found in the genomes of several bacteria, such as Archaeoglobus fulgidus, Borrelia burgdorferi, Listeria monocytogenes, and S. epidermidis, none of which produce spores. Thus, the SpoVG homologues of these organisms are likely to mediate functions other than sporulation. Inactivation of spoVG in a methicillin-resistant S. epidermidis drastically decreased methicillin resistance and the formation of a biofilm (D. Mack, personal communication). Interestingly, both attributes have also been linked positively to σB activity in S. aureus (67, 82). Attempts to inactivate the S. aureus yabJ and spoVG homologues are currently ongoing in our laboratory to elucidate their roles in this organism.
FIG. 3.
The yabJ-spoVG locus of S. aureus. (A) Schematic representation of the yabJ-spoVG operon of S. aureus N315 (GenBank accession no. AP003130). Proposed ORFs and promoter and terminator sequences are indicated. (B) Transcript levels for Newman (□) and IK184 (▵) cells sampled at different time points of growth (x axes). Data points were plotted as relative intensity values (y axes). (C) High-resolution S1 nuclease mapping of the transcriptional start point for yabJ in the E. coli two-plasmid system. The 5′ end-labeled DNA fragment was hybridized with 40 μg of RNA and treated with 100 U of S1 nuclease (as described in Materials and Methods). RNA was isolated from exponentially grown E. coli containing pSA3C and pAC7-sigB (lane1) and pSA3C and pAC7 (lane 2). The RNA-protected DNA fragments were analyzed on DNA sequencing gels together with G+A (lane A) and T+C (lane T) sequencing ladders derived from the end-labeled fragments. The horizontal arrow indicates the position of the RNA-protected fragment, and the vertical arrow indicates the nucleotide corresponding to tsp. Before assigning the tsp, 1.5 nucleotides were subtracted from the length of the protected fragment to account for the difference in the 3′ ends resulting from S1 nuclease digestion and the chemical sequencing reactions. The predicted −35 and −10 boxes are indicated.
Another potential regulator acting downstream of σB is the gene product of ORF N315-SA1961, a homologue of the BglG/SacY family of transcriptional antiterminators (ATs). ATs are regulatory protein factors that bind to specific sites in the nascent mRNA to prevent premature termination of gene transcription and to stimulate elongation by RNA polymerase (83). Expression of N315-SA1961 was found to be highly upregulated in strains harboring an intact sigB operon (Table 2), and the ORF is preceded by a nucleotide sequence (GTTATT-14-GGGTAT) that matches the proposed σB promoter consensus, indicating that the BglG/SacY homologue is controlled directly by σB.
Influence of σB on known regulatory elements.
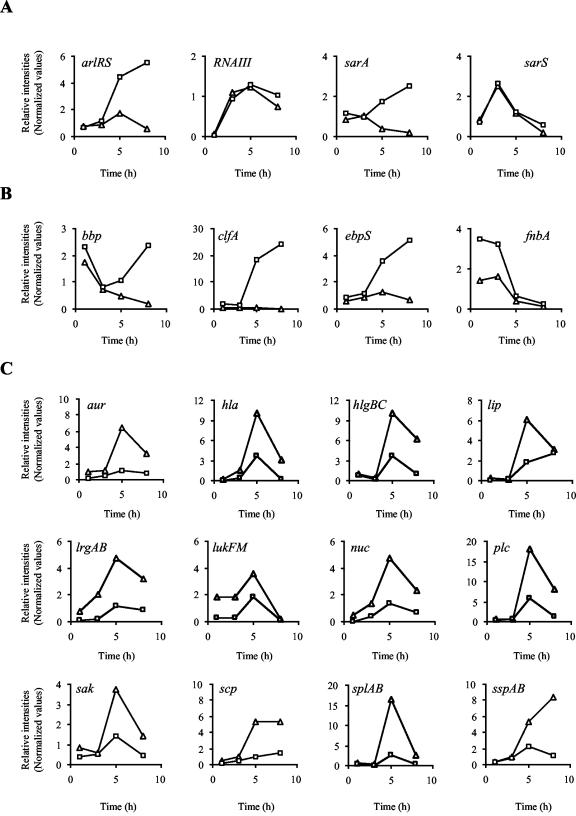
S. aureus possesses an array of virulence factor regulatory elements, such as two-component signal transduction systems and winged-helix transcription regulatory proteins. Presumably, these elements interact to influence different networks of virulence factors on an as-needed basis, thereby providing cells with the necessary arsenal of virulence determinates to respond to environmental changes or stimuli (reviewed in reference 10). The data presented here indicate that three of these virulence regulators, sarA, sarS, and arlRS, are upregulated by σB (Fig. 4A). Transcription of other well-studied virulence regulators, such as Sae and Rot, were not significantly influenced by σB in these studies.
FIG. 4.
Transcription profiles of ORFs influenced by σB. Transcript levels for Newman (□) and IK184 (▵) cells sampled at different time points of growth (x axes). Data points were plotted as relative intensity values (y axes). (A) Influence of σB on known regulatory elements. arlR, autolysis-related locus regulator protein (response regulator); arlS, autolysis-related locus sensor protein (histidine kinase); RNAIII, effector molecule of the agr locus; sarA, staphylococcal accessory regulator A; sarS, staphylococcal accessory regulator S. (B) Adhesions factors upregulated by σB. bbp, bone sialoprotein-binding protein; clfA, clumping factor A; ebpS, elastin binding protein S; fnbA, fibronectin binding protein A. (C) Exoproteins and toxins downregulated by σB. aur, zinc metalloprotease aureolysin; hla, α-hemolysin; hlgBC, γ-hemolysin components B and C; lip, lipase; lrgAB, holin-like proteins LrgA and LrgB; lukF, synergohymenotropic toxin precursor; lukM, leukocidin chain precursor; nuc, staphylococcal nuclease; plc, 1-phosphatidylinositol phosphodiesterase precursor; sak, staphylokinase precursor (protease III); scp, staphopain; splAB, serine proteases SplA and SplB; sspA, staphylococcal serine protease (V8 protease); sspB, cysteine protease SspB.
The staphylococcal accessory regulator A, SarA, a member of the winged-helix transcription proteins is encoded by the sar locus. Although it is well established that expression of the sar locus is in part controlled by the action of σB (4, 15, 52), it is still a matter of debate whether σB has a positive or negative effect on the overall level of SarA production. Much of what is published regarding the influence of σB on SarA expression is difficult to interpret because most of these studies were done in strains, such as RN6390 and 8325-4, that harbor mutations in rsbU, the positive activator of σB, rendering them sigB deficient (26). The discrepancies between the positive influence of σB on SarA production observed by Gertz et al. (25) in a proteomic approach and by Bischoff et al. (4) via reporter gene fusion experiments versus the observed downregulatory effect of σB on SarA production reported by Manna et al. (52) and Cheung et al. (9) may be explained by the fact that, in the latter studies, an rsbU mutant was used as parental strain to compare it with its respective sigB mutant. However, this explanation cannot account for the findings of Horsburgh et al. (34), who did not observe any influence of σB on SarA production either at the transcriptional or at the protein level. The transcriptional profiling data presented here strongly suggest that σB increases the expression of the sar locus (Table 2; Fig. 4A), especially during the later growth stages (5 and 8 h after inoculation). Moreover, a direct correlation between the increase in SarA transcript levels and an increase in SarA protein is indirectly suggested by the findings that the expression of four major extracellular proteases of S. aureus (staphylococcal serine protease V8 [SspA], cysteine protease [SspB], metalloprotease aureolysin [Aur], and staphopain [Scp]) is significantly decreased in sigB+ strains (Table 3; Fig. 4). It was recently demonstrated by Karlsson and Arvidson (36) that transcription of these protease genes was suppressed due to increased σB-dependent expression of SarA. This is further supported by the findings that several of the ORFs found to be downregulated by σB, such as glpQ, encoding glycerophosphoryl diester phosphodiesterase, nuc, encoding staphylococcal thermonuclease, and plc, encoding a 1-phosphatidylinositol phospodiesterase precursor, have previously been demonstrated to be downregulated by SarA (16, 84). It is likely that the increase in expression of these genes found in the ΔrsbUVW-sigB mutants is due to decreased production of SarA. Although appealing, this assumption remains speculative, as both Dunman et al. (16) and Ziebandt et al. (84) used the rsbU-defective RN6390 lineage as the genetic background for their analyses, leaving it open to question what might happen with respect to the sarA regulon in strains carrying an intact sigB operon. The genetic background chosen may also explain the observed discrepancy that several of the genes listed in Table 3 were found to be downregulated by σB but upregulated by SarA. Support for such a process is conferred by the observations that RNAIII expression of the agr locus is known to be promoted by SarA (11) but decreased by σB (4, 34) in an unidentified way that is, however, supposed to be independent from SarA (34).
The expression of a second winged-helix transcription protein, SarS (also known as SarH1), belonging to the family of SarA homologues was previously shown to be influenced by σB (77). This was confirmed in two of the three backgrounds analyzed in this study (Table 2). Interestingly, no difference in sarS expression was observed when comparing strain Newman and its ΔrsbUVW-sigB mutant either in the microarray experiments (Table 2; Fig. 4A) or by Northern blot analysis (data not shown), further demonstrating that strain-to-strain differences influence regulon constituents. Sequencing of the σB promoter regions of sarS of strains Newman and GP268 did not reveal any differences between the respective regions (which were identical with the N315 region corresponding to nucleotides 125868 to 126073 of GenBank accession no. AP003129), leaving the question open as to why expression of sarS in strain Newman is not affected by σB.
The third known virulence regulatory element observed to be influenced by σB was arlRS, encoding a two-component signal transduction system that influences adhesion, autolysis, and extracellular proteolytic activity of S. aureus (19). More recently, it was also demonstrated to decrease expression of the agr locus while increasing the expression of SarA (20). The data obtained from experiment 2 suggest that arlRS of strain Newman is upregulated by σB (Fig. 4A). However, arlRS did not show up in experiment 1 as influenced by σB either in strain COL or strain GP268 and is not preceded by a σB consensus promoter.
Recent results suggest that expression of RNAIII, the effector molecule of the agr locus, is negatively influenced by σB (4, 34). However, results of the two experiments presented here did not effectively corroborate these observations, as although slight differences in RNAIII transcription were detectable between wild-type strains and their respective ΔrsbUVW-sigB mutants (Fig. 4A), changes in expression were not determined to be significant. RNAIII is by far the most prominent RNA molecule produced by S. aureus during the later growth stages. As a result, the RNAIII transcript levels of the wild-type strains already reached amounts that saturated the RNAIII-specific target oligonucleotides represented on the GeneChip, thus impeding the detection of differences in RNAIII transcript levels that might be present between the strain pairs analyzed.
Influence of σB on expression of virulence determinants.
Previous studies demonstrated that σB influences the expression of various factors associated with virulence and pathogenicity of S. aureus (4, 15, 25, 34, 44, 58, 60, 84), which led to the assumption that σB may be important for virulence of this organism (4, 44). However, in vivo studies have failed to demonstrate an effect of σB on the virulence of S. aureus (34, 60), implying that such an assumption is no longer tenable. Alternatively, σB may play a role in pathogenesis; however, the effects of σB-mediated virulence mechanisms do not play a role in the models chosen in those experiments.
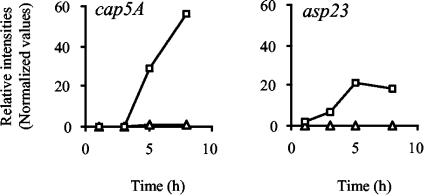
Analysis of the GeneChip data suggests that σB influences the expression of a large number of virulence genes in S. aureus (reviewed in references 10 and 47). Many of these are reported here for the first time as genes that are altered transcriptionally by σB. By comparing the expression profiles of these virulence genes, a pattern has emerged; most of the exoenzymes and toxins produced by S. aureus were negatively influenced by σB (Fig. 4C) while the expression of several adhesins was found to be clearly increased by σB (Fig. 4B). The function of σB in virulence factor production is therefore exactly the opposite of that of RNAIII, which is known to act as a negative regulator of cell wall proteins and a positive regulator of exoenzymes and toxins in a growth phase-dependent manner (Table 5) (10, 62). The decreased amounts of exoprotein and toxin transcripts observed in wild-type strains compared to their respective mutants may in part be a consequence of lower RNAIII transcript levels that are present in strains harboring an intact sigB operon (4, 34). Expression of the cap gene cluster is influenced by a variety of environmental stimuli and affected by several global regulators, such as RNAIII, SarA, and MgrA, (reviewed in reference 63). The microarray data presented here add a further regulator, σB, to this list and suggest that the alternate transcription factor influences cap expression in a growth phase-dependent manner (Fig. 5). While virtually no cap transcripts were detectable during the early growth stages (1 and 3 h), expression of the cap genes increased with ongoing growth (5 and 8 h), being highest at the latest time point analyzed. After 8 h of growth, a >50-fold increase in cap-specific transcripts was observable in strain Newman that was totally missing in its ΔrsbUVW-sigB mutant (Fig. 5). However, the effect of σB on cap expression is likely to be indirect, as the promoter region of the cap operon lacks an obvious σB consensus promoter sequence.
TABLE 5.
Influence of σB on virulence determinants regulated by the agr locus
| Virulence determinant | Genea | Result forb:
|
|
|---|---|---|---|
| agr | σB | ||
| Aureolysin | aur | + | − |
| Capsular polysaccharide synthesis enzyme 5J | cap5J | + | + |
| Clumping factor B | clfB | + | ⊘ |
| Coagulase | coa | − | + |
| Cystein protease | sspC | + | − |
| Enterotoxin A | sea | + | Unknown |
| Enterotoxin B | seb | + | −c |
| Exotoxin 2 | set8 | + | Unknown |
| Factor effecting methicillin resistance B | femB | + | ⊘ |
| Fibronectin-binding protein A | fnbA | − | + |
| Fibronectin-binding protein B | fnbB | − | ⊘ |
| Glycerol ester hydrolyase | geh | + | − |
| α-Hemolysin | hla | + | − |
| β-Hemolysin | hlb | + | −c |
| γ-Hemolysin | hlgBC | + | − |
| δ-Hemolysin | hld | + | ⊘ |
| Hyaluronate lyase | hysA | + | ⊘ |
| Lipase | lip | + | − |
| LrgAB (holin-like proteins) | lrgAB | + | − |
| Myosin-cross-reactive antigen | N315-SA0102 | − | + |
| Phosphatidylinositol-specific phospolipase C | plc | + | − |
| Protein A | spa | − | ⊘ |
| Secretory antigen A | ssaA | − | + |
| Serine protease A, B, D, and F | splA,B,D,F | + | − |
| Staphylokinase | spc | + | − |
| Toxic shock syndrome toxin 1 | tst | + | Unknown |
| V8 protease | sspA | + | − |
Genes that are regulated conversely by agr and σB are shown in boldface type.
Influence of agr and σB on transcription of the respective gene. ⊘, not influenced; +, increased; −, decreased.
Based on transcript levels detected in strains COL and IK183.
FIG. 5.
Transcription profiles of capA and asp23. Transcript levels for Newman (□) and IK184 (▵) cells sampled at different time points of growth (x axes). Data points were plotted as relative intensity values (y axes). The influence of σB on the expression of capA, encoding capsular polysaccharide synthesis enzyme A, and asp23, encoding alkaline shock protein 23, is shown.
The finding that expression of so many virulence genes is significantly altered by σB warrants further investigation to elucidate its role in infectivity of S. aureus in additional models of infection. To date, nothing is known about the expression or activity of σB during the course of infection. S. aureus is known for its ability to cause a variety of unrelated infections (reviewed in reference 50). It is feasible that the σB-dependent downregulation of toxins and exoenzymes, combined with the simultaneous upregulation of adhesins, may enable S. aureus to cause very specific host-pathogen interactions that have not been investigated to date. Recent results indicate that σB is involved in processes that are important for biofilm formation (1, 67); therefore, a comparison of the transcription profile of biofilm cells to the results we have obtained may identify genes that are essential for biofilm formation. Additionally, based on the virulence factor pattern caused by σB, it is tempting to speculate that this alternative transcription factor may also be an important player during nasal colonization, thereby promoting adherence to the host cell matrix without evoking an inflammatory response. Investigations in our laboratories are ongoing to address these questions. It is also quite possible that in vivo conditions leading to S. aureus stress, including those of high temperature at the site of infection, may induce the stress responsive σB factor. Under such conditions, when the host is trying to mount an immune response at the site of infection, it may be more beneficial for the bacterium to produce cell surface components that are involved in camouflaging the organism from the host's defense than to produce exoproteins.
The present study was designed to extensively characterize the genes that are regulated by the alternative sigma factor σB during standard laboratory growth conditions. Under these conditions, a >20-fold increase in the σB-regulated gene asp23 was observed (Fig. 5). In addition, very stringent criteria were used for the identification of σB-regulated genes: (i) transcripts demonstrated the same σB-dependent phenotype in at least two of the three genetic backgrounds tested and (ii) transcripts passed strict statistical cutoff values. Based on these criteria, there was an extremely high correlation between the genes that we identified to be regulated in a σB-dependent manner and previously recorded results. As a result, it is likely that the GeneChip method used accurately identified the genes belonging to the σB regulon of the strains analyzed. While defining the σB regulon, we observed a distinguishable pattern among virulence factors. Subsequent studies that have focused on two S. aureus adhesions (clfA and fnbA) have confirmed that each gene is indeed regulated in a σB-dependent manner and further validated the method used (unpublished data).
The finding that σB downregulates the transcription of secreted virulence factors but upregulates cell surface virulence factors is in direct contrast to the observations of Kupferwasser et al. (46). In that study it was found that salicylic acid mildly induces asp23 (1.9-fold) and corresponds to both the downregulation of certain cell surface adhesions and the upregulation of secreted proteases. Based on the low induction rate of asp23, it is difficult to reconcile whether the virulence factor effects seen in that study are directly mediated by σB versus another salicylic acid-responsive process or a combination of the two. It also raises the question of whether low to moderate levels of σB produce a much different physiological phenotype than the levels tested here. It is also possible that salicylic acid and other stresses that have previously been shown to modulate σB activity direct the expression of portions of the σB regulon. More completely characterizing the σB regulon will allow subsequent experiments to fully address these questions and further understand the role, if any, that the σB regulon plays in pathogenesis.
Acknowledgments
Research in the laboratory of B.B.-B. and M.B. is supported by Swiss National Science Foundation grants 4049.063201 and 3100A0-100234 and by the Forschungskredit der Universität Zürich grant 560030. J.K. is supported by grant 2/3010/23 from the Slovak Academy of Sciences.
We are also grateful to the Wyeth antimicrobial research department for providing us with the necessary materials for the GeneChip experiments.
REFERENCES
- 1.Bateman, B. T., N. P. Donegan, T. M. Jarry, M. Palma, and A. L. Cheung. 2001. Evaluation of a tetracycline-inducible promoter in Staphylococcus aureus in vitro and in vivo and its application in demonstrating the role of sigB in microcolony formation. Infect. Immun. 69:7851-7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger-Bächi, B. 1983. Increase in transduction efficiency of Tn551 mediated by the methicillin resistance marker. J. Bacteriol. 154:533-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bischoff, M., and B. Berger-Bächi. 2001. Teicoplanin stress-selected mutations increasing σB activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 45:1714-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bischoff, M., J. M. Entenza, and P. Giachino. 2001. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 183:5171-5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borukhov, S., and K. Severinov. 2002. Role of the RNA polymerase sigma subunit in transcription initiation. Res. Microbiol. 153:557-562. [DOI] [PubMed] [Google Scholar]
- 6.Burgess, R. R., and L. Anthony. 2001. How sigma docks to RNA polymerase and what sigma does. Curr. Opin. Microbiol. 4:126-131. [DOI] [PubMed] [Google Scholar]
- 7.Chan, P. F., S. J. Foster, E. Ingham, and M. O. Clements. 1998. The Staphylococcus aureus alternative sigma factor σB controls the environmental stress response but not starvation survival or pathogenicity in a mouse abscess model. J. Bacteriol. 180:6082-6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, P., R. E. Ruiz, Q. Li, R. F. Silver, and W. R. Bishai. 2000. Construction and characterization of a Mycobacterium tuberculosis mutant lacking the alternate sigma factor gene, sigF. Infect. Immun. 68:5575-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung, A. L., Y. T. Chien, and A. S. Bayer. 1999. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect. Immun. 67:1331-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung, A. L., S. J. Projan, and H. Gresham. 2002. The genomic aspect of virulence, sepsis, and resistance to killing mechanisms in Staphylococcus aureus. Curr. Infect. Dis. Rep. 4:400-410. [DOI] [PubMed] [Google Scholar]
- 11.Chien, Y., A. C. Manna, and A. L. Cheung. 1998. SarA level is a determinant of agr activation in Staphylococcus aureus. Mol. Microbiol. 30:991-1001. [DOI] [PubMed] [Google Scholar]
- 12.Cole, S. T., R. Brosch, J. Parkhill, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 13.Coynault, C., V. Robbe-Saule, and F. Norel. 1996. Virulence and vaccine potential of Salmonella typhimurium mutants deficient in the expression of the RpoS (sigma S) regulon. Mol. Microbiol. 22:149-160. [DOI] [PubMed] [Google Scholar]
- 14.DeMaio, J., Y. Zhang, C. Ko, D. B. Young, and W. R. Bishai. 1996. A stationary-phase stress-response sigma factor from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 93:2790-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deora, R., T. Tseng, and T. K. Misra. 1997. Alternative transcription factor σSB of Staphylococcus aureus: characterization and role in transcription of the global regulatory locus sar. J. Bacteriol. 179:6355-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunman, P. M., E. Murphy, S. Haney, D. Palacios, G. Tucker-Kellogg, S. Wu, E. L. Brown, R. J. Zagursky, D. Shlaes, and S. J. Projan. 2001. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J. Bacteriol. 183:7341-7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duthie, E. S., and L. L. Lorenz. 1952. Staphylococcal coagulase: mode of action and antigenicity. J. Gen. Microbiol. 6:95-107. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald, J. R., D. E. Sturdevant, S. M. Mackie, S. R. Gill, and J. M. Musser. 2001. Evolutionary genomics of Staphylococcus aureus: insights into the origin of methicillin-resistant strains and the toxic shock syndrome epidemic. Proc. Natl. Acad. Sci. USA 98:8821-8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fournier, B., and D. C. Hooper. 2000. A new two-component regulatory system involved in adhesion, autolysis, and extracellular proteolytic activity of Staphylococcus aureus. J. Bacteriol. 182:3955-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fournier, B., A. Klier, and G. Rapoport. 2001. The two-component system ArlS-ArlR is a regulator of virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 41:247-261. [DOI] [PubMed] [Google Scholar]
- 21.Fraser, C. M., S. J. Norris, G. M. Weinstock, O. White, et al. 1998. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science 281:375-388. [DOI] [PubMed] [Google Scholar]
- 22.Fujimoto, D. F., E. W. Brunskill, and K. W. Bayles. 2000. Analysis of genetic elements controlling Staphylococcus aureus lrgAB expression: potential role of DNA topology in SarA regulation. J. Bacteriol. 182:4822-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaidenko, T. A., and C. W. Price. 1998. General stress transcription factor σB and sporulation transcription factor σH each contribute to survival of Bacillus subtilis under extreme growth conditions. J. Bacteriol. 180:3730-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gertz, S., S. Engelmann, R. Schmid, K. Ohlsen, J. Hacker, and M. Hecker. 2000. Regulation of sigmaB-dependent transcription of sigB and asp23 in two different Staphylococcus aureus strains. Mol. Gen. Genet. 261:558-566. [DOI] [PubMed] [Google Scholar]
- 25.Gertz, S., S. Engelmann, R. Schmid, A.-K. Ziebandt, K. Tischer, C. Scharf, J. Hacker, and M. Hecker. 2000. Characterization of the σB regulon in Staphylococcus aureus. J. Bacteriol. 182:6983-6991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giachino, P., S. Engelmann, and M. Bischoff. 2001. σB activity depends on RsbU in Staphylococcus aureus. J. Bacteriol. 183:1843-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hecker, M., and S. Engelmann. 2000. Proteomics, DNA arrays and the analysis of still unknown regulons and unknown proteins of Bacillus subtilis and pathogenic gram-positive bacteria. Int. J. Med. Microbiol. 290:123-134. [DOI] [PubMed] [Google Scholar]
- 28.Hecker, M., and U. Volker. 2001. General stress response of Bacillus subtilis and other bacteria. Adv. Microb. Physiol. 44:35-91. [DOI] [PubMed] [Google Scholar]
- 29.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, et al. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Helmann, J. D., M. F. Wu, P. A. Kobel, F. J. Gamo, M. Wilson, M. M. Morshedi, M. Navre, and C. Paddon. 2001. Global transcriptional response of Bacillus subtilis to heat shock. J. Bacteriol. 183:7318-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hengge-Aronis, R. 1993. Survival of hunger and stress: the role of rpoS in early stationary phase gene regulation in E. coli. Cell 72:165-168. [DOI] [PubMed] [Google Scholar]
- 32.Heuner, K., C. Dietrich, C. Skriwan, M. Steinert, and J. Hacker. 2002. Influence of the alternative σ28 factor on virulence and flagellum expression of Legionella pneumophila. Infect. Immun. 70:1604-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Homerova, D., M. Bischoff, A. Dumoulin, and J. Kormanec. 2004. Optimization of a two-plasmid system for the identification of promoters recognized by RNA polymerase containing Staphylococcus aureus alternative sigma factor σB. FEMS Microbiol. Lett. 232:173-179. [DOI] [PubMed] [Google Scholar]
- 34.Horsburgh, M. J., J. L. Aish, I. L. White, L. Shaw, J. K. Lithgow, and S. J. Foster. 2002. σB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunt, T. A., W. T. Peng, I. Loubens, and D. G. Storey. 2002. The Pseudomonas aeruginosa alternative sigma factor PvdS controls exotoxin A expression and is expressed in lung infections associated with cystic fibrosis. Microbiology 148:3183-3193. [DOI] [PubMed] [Google Scholar]
- 36.Karlsson, A., and S. Arvidson. 2002. Variation in extracellular protease production among clinical isolates of Staphylococcus aureus due to different levels of expression of the protease repressor sarA. Infect. Immun. 70:4239-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaushal, D., B. G. Schroeder, S. Tyagi, T. Yoshimatsu, C. Scott, C. Ko, L. Carpenter, J. Mehrotra, Y. C. Manabe, R. D. Fleischmann, and W. R. Bishai. 2002. Reduced immunopathology and mortality despite tissue persistence in a Mycobacterium tuberculosis mutant lacking alternative sigma factor, SigH. Proc. Natl. Acad. Sci. USA 99:8330-8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenyon, W. J., D. G. Sayers, S. Humphreys, M. Roberts, and M. P. Spector. 2002. The starvation-stress response of Salmonella enterica serovar Typhimurium requires sigma(E)-, but not CpxR-regulated extracytoplasmic functions. Microbiology 148:113-122. [DOI] [PubMed] [Google Scholar]
- 39.Kormanec, J., and B. Sevcikova. 2000. Identification and transcriptional analysis of a cold shock-inducible gene, cspA, in Streptomyces coelicolor A3(2). Mol. Gen. Genet. 264:251-256. [DOI] [PubMed] [Google Scholar]
- 40.Kormanec, J. 2001. Analyzing the developmental expression of sigma factors with S1-nuclease mapping. Methods Mol. Biol. 160:481-494. [DOI] [PubMed] [Google Scholar]
- 41.Kornblum, J., B. J. Hartmann, R. P. Novick, and A. Tomasz. 1986. Conversion of a homogeneously methicillin-resistant strain of Staphylococcus aureus to heterogeneous resistance by Tn551-mediated insertional inactivation. Eur. J. Clin. Microbiol. 5:714-718. [DOI] [PubMed] [Google Scholar]
- 42.Kovacikova, G., and K. Skorupski. 2002. The alternative sigma factor σE plays an important role in intestinal survival and virulence in Vibrio cholerae. Infect. Immun. 70:5355-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kullik, I., and P. Giachino. 1997. The alternative sigma factor sigB in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch. Microbiol. 167:151-159. [DOI] [PubMed] [Google Scholar]
- 44.Kullik, I., P. Giachino, and T. Fuchs. 1998. Deletion of the alternative sigma factor σB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 180:4814-4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunst, F., N. Ogasawara, I. Moszer, A. M. Albertini, et al. 1997. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390:249-256. [DOI] [PubMed] [Google Scholar]
- 46.Kupferwasser, L. I., M. R. Yeaman, C. C. Nast, D. Kupferwasser, Y. Q. Xiong, M. Palma, A. L. Cheung, and A. S. Bayer. 2003. Salicylic acid attenuates virulence in endovascular infections by targeting global regulatory pathways in Staphylococcus aureus. J. Clin. Investig. 112:222-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuroda, M., T. Ohta, I. Uchiyama, T. Baba, et al. 2001. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357:1225-1240. [DOI] [PubMed] [Google Scholar]
- 48.Lang, S., M. A. Livesley, P. A. Lambert, W. A. Littler, and T. S. Elliott. 2000. Identification of a novel antigen from Staphylococcus epidermidis. FEMS Immunol. Med. Microbiol. 29:213-220. [DOI] [PubMed] [Google Scholar]
- 49.Loewen, P. C., and R. Hengge-Aronis. 1994. The role of the sigma factor sigma S (KatF) in bacterial global regulation. Annu. Rev. Microbiol. 48:53-80. [DOI] [PubMed] [Google Scholar]
- 50.Lowy, F. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520-532. [DOI] [PubMed] [Google Scholar]
- 51.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, E. Dubnau, M. Gomez, and I. Smith. 2002. Role of the extracytoplasmic-function sigma factor sigma(H) in Mycobacterium tuberculosis global gene expression. Mol. Microbiol. 45:365-374. [DOI] [PubMed] [Google Scholar]
- 52.Manna, A. C., M. G. Bayer, and A. L. Cheung. 1998. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J. Bacteriol. 180:3828-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuno, K., and A. L. Sonenshein. 1999. Role of SpoVG in asymmetric septation in Bacillus subtilis. J. Bacteriol. 18:3392-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maxam, A. M., and W. Gilbert. 1980. Sequencing end-labeled DNA with base specific chemical cleavages. Methods Enzymol. 65:449-560. [DOI] [PubMed] [Google Scholar]
- 55.Miyazaki, E., J. M. Chen, C. Ko, and W. R. Bishai. 1999. The Staphylococcus aureus rsbW (orf159) gene encodes an anti-sigma factor of SigB. J. Bacteriol. 181:2846-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morikawa, K., Y. Inose, H. Okamura, A. Maruyama, H. Hayashi, K. Takeyasu, and T. Ohta. 2003. A new staphylococcal sigma factor in the conserved gene cassette: functional significance and implication for the evolutionary processes. Genes Cells 8:699-712. [DOI] [PubMed] [Google Scholar]
- 57.Nadon, C. A., B. M. Bowen, M. Wiedmann, and K. J. Boor. 2002. Sigma B contributes to PrfA-mediated virulence in Listeria monocytogenes. Infect. Immun. 70:3948-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nair, S. P., M. Bischoff, M. M. Senn, and B. Berger-Bächi. 2003. The σB regulon influences internalization of Staphylococcus aureus by osteoblasts. Infect. Immun. 71:4167-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neidhardt, F. C., J. L. Ingraham, and M. Schaechter. 1990. Genetic adaptation: the genome and its plasticity, p. 247-268. In F. C. Neidhardt, J. L. Ingraham, and M. Schaechter (ed.), Physiology of the bacterial cell: a molecular approach. Sinauer Associates, Inc., Sunderland, Mass.
- 60.Nicholas, R. O., T. Li, D. McDevitt, A. Marra, S. Sucoloski, P. L. Demarsh, and D. R. Gentry. 1999. Isolation and characterization of a sigB deletion mutant of Staphylococcus aureus. Infect. Immun. 67:3667-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nickerson, C. A., and R. Curtiss III. 1997. Role of sigma factor RpoS in initial stages of Salmonella typhimurium infection. Infect. Immun. 65:1814-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Novick, R. P. 2000. Pathogenicity factors and their regulation, p. 392-407. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. ASM Press, Washington, D.C.
- 63.O'Riordan, K., and J. C. Lee. 2004. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17:218-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petersohn, A., J. Bernhardt, U. Gerth, D. Hoper, T. Koburger, U. Volker, and M. Hecker. 1999. Identification of σB-dependent genes in Bacillus subtilis using a promoter consensus-directed search and oligonucleotide hybridization. J. Bacteriol. 181:5718-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Price, C. W. 2000. Protective function and regulation of the general stress response in Bacillus subtilis and related gram-positive bacteria, p. 179-197. In G. Storz and R. Hengge-Aronis (ed.), Bacterial stress responses. American Society for Microbiology, Washington, D.C.
- 66.Price, C. W., P. Fawcett, H. Ceremonie, N. Su, C. K. Murphy, and P. Youngman. 2001. Genome-wide analysis of the general stress response in Bacillus subtilis. Mol. Microbiol. 41:757-774. [DOI] [PubMed] [Google Scholar]
- 67.Rachid, S., K. Ohlsen, U. Wallner, J. Hacker, M. Hecker, and W. Ziebuhr. 2000. Alternative transcription factor σB is involved in regulation of biofilm expression in a Staphylococcus aureus mucosal isolate. J. Bacteriol. 182:6824-6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raman, S., T. Song, X. Puyang, S. Bardarov, W. R. Jacobs, Jr., and R. N. Husson. 2001. The alternative sigma factor SigH regulates major components of oxidative and heat stress responses in Mycobacterium tuberculosis. J. Bacteriol. 183:6119-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rappu, P., B. S. Shin, H. Zalkin, and P. Mantsala. 1999. A role for a highly conserved protein of unknown function in regulation of Bacillus subtilis purA by the purine repressor. J. Bacteriol. 181:3810-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rezuchova, B., and J. Kormanec. 2001. A two-plasmid system for identification of promoters recognized by RNA polymerase containing extracytoplasmic stress response sigma(E) in Escherichia coli. J. Microbiol. Methods 45:103-111. [DOI] [PubMed] [Google Scholar]
- 71.Robbe-Saule, V., C. Coynault, and F. Norel. 1995. The live oral typhoid vaccine Ty21a is a rpoS mutant and is susceptible to various environmental stresses. FEMS Microbiol. Lett. 126:171-176. [DOI] [PubMed] [Google Scholar]
- 72.Rosenbluh, A., C. D. Banner, R. Losick, P. C. Fitz-James. 1981. Identification of a new developmental locus in Bacillus subtilis by construction of a deletion mutation in a cloned gene under sporulation control. J. Bacteriol. 148:341-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmid, A. K., and M. E. Lidstrom. 2002. Involvement of two putative alternative sigma factors in stress response of the radioresistant bacterium Deinococcus radiodurans. J. Bacteriol. 184:6182-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Segall, J., and R. Losick. 1977. Cloned Bacillus subtilis DNA containing a gene that is activated early during sporulation. Cell 11:751-761. [DOI] [PubMed] [Google Scholar]
- 75.Silo-Suh, L., S. J. Suh, P. A. Sokol, and D. E. Ohman. 2002. A simple alfalfa seedling infection model for Pseudomonas aeruginosa strains associated with cystic fibrosis shows AlgT (sigma-22) and RhlR contribute to pathogenesis. Proc. Natl. Acad. Sci. USA 99:15699-15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stover, C. K., X. Q. Pham, A. L. Erwin, S. D. Mizoguchi, P. Warrener, M. J. Hickey, F. S. Brinkman, W. O. Hufnagle, D. J. Kowalik, M. Lagrou, R. L. Garber, L. Goltry, E. Tolentino, S. Westbrock-Wadman, Y. Yuan, L. L. Brody, S. N. Coulter, K. R. Folger, A. Kas, K. Larbig, R. Lim, K. Smith, D. Spencer, G. K. Wong, Z. Wu, I. T. Paulsen, J. Reizer, M. H. Saier, R. E. Hancock, S. Lory, and M. V. Olson. 2000. Complete genome sequence of Pseudomonas aeruginosa PA01, an opportunistic pathogen. Nature 406:959-964. [DOI] [PubMed] [Google Scholar]
- 77.Tegmark, K., A. Karlsson, and S. Arvidson. 2000. Identification and characterization of SarH1, a new global regulator of virulence gene expression in Staphylococcus aureus. Mol. Microbiol. 37:398-409. [DOI] [PubMed] [Google Scholar]
- 78.Testerman, T. L., A. Vazquez-Torres, Y. Xu, J. Jones-Carson, S. J. Libby, and F. C. Fang. 2002. The alternative sigma factor sigmaE controls antioxidant defences required for Salmonella virulence and stationary-phase survival. Mol. Microbiol. 43:771-782. [DOI] [PubMed] [Google Scholar]
- 79.Volker, U., B. Maul, and M. Hecker. 1999. Expression of the σB-dependent general stress regulon confers multiple stress resistance in Bacillus subtilis. J. Bacteriol. 181:3942-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wiedmann, M., T. J. Arvik, R. J. Hurley, and K. J. Boor. 1998. General stress transcription factor σB and its role in acid tolerance and virulence of Listeria monocytogenes. J. Bacteriol. 180:3650-3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson, J. A., and P. A. Gulig. 1998. Regulation of the spvR gene of the Salmonella typhimurium virulence plasmid during exponential-phase growth in intracellular salts medium and at stationary phase in L broth. Microbiology 144:1823-1833. [DOI] [PubMed] [Google Scholar]
- 82.Wu, S., H. de Lencastre, and A. Tomasz. 1996. Sigma-B, a putative operon encoding alternate sigma factor of Staphylococcus aureus RNA polymerase: molecular cloning and DNA sequencing. J. Bacteriol. 178:6036-6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yarnell, W. S., and J. W. Roberts. 1999. Mechanism of intrinsic transcription termination and antitermination. Science 284:611-615. [DOI] [PubMed] [Google Scholar]
- 84.Ziebandt, A. K., H. Weber, J. Rudolph, R. Schmid, D. Hoper, S. Engelmann, and M. Hecker. 2001. Extracellular proteins of Staphylococcus aureus and the role of SarA and sigma B. Proteomics 1:480-493. [DOI] [PubMed] [Google Scholar]