Abstract
Objective
To describe three DBS cases which presented with new side effects or loss of benefit from stimulation after long-term follow-up and to discuss the potential contributing factors.
Methods
A University of Florida (UF) database (INFORM) search was performed, identifying three patients, two Parkinson's disease (PD) and one Essential Tremor (ET), with an unexpected change in long-term programming thresholds as compared to initial evaluation. Clinical follow-up, programming, imaging studies, and lead measurements were reviewed. The UF Institutional Review Board (IRB) approved this study.
Results
A substantial increase in the 3rd ventricular width (120%), Evans index (6%), ventricular index (5%), and cella media index (17%) was uncovered. A change in thresholds across lead contacts with a decrease in current densities as well as a relative lateral change of lead location was also observed. Hardware-related complications, lead migration, and impedance variability were not identified.
Conclusions
Potential factors contributing to long-term side effects should be examined during a DBS troubleshooting assessment. Clinicians should be aware that in DBS therapy there is delivery of electricity to a changing brain, and atrophy may possibly affect DBS programming settings as part of long-term follow-up.
Introduction
Deep Brain Stimulation (DBS) has become an effective treatment in select Parkinson's disease (PD), essential tremor (ET), and dystonia cohorts [1], [2], with motor benefits reported at 10 years following implantation [3], [4]. Several groups have suggested recommendations on how to manage and troubleshoot factors that may be responsible for a worse DBS outcome [5], [6] or factors leading to dissatisfaction during long-term management [7]. These issues, which have been previously described, include surgery-related complications (intracerebral hemorrhage, deep cerebral venous hemorrhage/infection, seizure, sterile seroma, pulmonary embolism, pneumonia, perioperative confusion, suboptimal lead placement), hardware-related, (infection, skin erosion, electrode or wire fracture, lead migration, neurostimulator malfunction, neurostimulator migration, pain in region of neurostimulator) and stimulation-related issues. Other possible factors include disease progression, poor selection of DBS candidates, unrealistic patient expectations, improper programming and medication adjustment, tolerance to DBS stimulation, and neuropsychiatric complications [8].
Because of the rapidly growing number of patients with DBS, it is critically important to recognize which factors, and how each of these factors will affect long-term response. We present three patients with previously unrecognized long-term DBS related management issues. These cases led to the investigation of potential mechanism(s) and factors for the stimulation-induced adverse events and loss of benefit.
Methods
The University of Florida (UF) Institutional Review Board (IRB) approved the use of our IRB approved UF INFORM database. Three patients were identified in our database, two PD and one ET, with an unexpected change in long-term thresholds compared to initial evaluation performed 1 month after DBS surgery. The term thresholds, as used in this paper, will define persistent stimulation-induced adverse effects for each electrode in the monopolar mode. Patient records/information was anonymized and de-identified prior to analysis. Analysis included age, gender, diagnosis, disease duration, target, follow-up duration, DBS settings (voltage, pulse width, frequency, impedance, and current density), and Unified Parkinson's Disease Rating Scale (UPDRS) motor or Tolosa-Fahn-Mardsen Tremor Rating Scale (TRS) [9] scores at baseline, immediate postoperative, and long-term follow-up. Current density, which is a measurement of electrical flow, was calculated at 6 months post DBS surgery (once settings were optimized) and at 1 month after the most recent thresholds testing (using the settings tolerated by patients). The formula used was: (V x PW/impedance)/0.06 cm2 [10]. Initial programming thresholds were compared to a follow-up and imaging study was also obtained as part of troubleshooting evaluation.
Lead location and atrophy indexes
Lead localization was measured using CT and MRI fused images. A possible lead migration was assessed applying Cartesian coordinates between two time points, measuring the electrode' tip in the post-operative scan, and comparing it to the most recent lead location (at the time of the side effect) using surgical planning software. Coordinates were assessed relative to the midcommissural point. Standard error measurement of deviation was set at 1.4 mm, as previously reported [11]. In order to assess ventricle size with respect to brain tissue and cerebral atrophy, different indexes (Evans index, ventricular index, cella media index, and maximum width of third ventricle) [12], [13] were obtained from pre-surgical imaging and compared to the most recent brain scan. The Evans index is defined as the maximal frontal horn ventricular width divided by the transverse inner diameter of the skull. The ventricular index is defined as the minimum width of the lateral ventricles divided by the maximum width of the anterior horns of the lateral ventricles. The cella media index is the ratio of biparietal diameter of skull to the maximum external diameter of lateral ventricles at cella media. The maximum width of the third ventricle was measured drawing a line through the long axis of the third ventricle, parallel to the interhemispheric fissure where the third ventricle was most visible. The width (in millimeters) was measured by drawing a second line perpendicular to the first line at its midpoint.
Case Reports
Case 1
A 50 year-old male with PD status post right subthalamic nucleus (STN) DBS implantation 75 months prior, complained of a subacute onset of left facial pulling that fully resolved with DBS deactivation. Surgery was initially indicated for “on state” disabling dyskinesias and tremors unresponsive to levodopa. Baseline pre-operative UPDRS motor score off/on meds was 62/16 with post-operative scores off med/on stim of 37 and on med/on stim of 31 at 6 months. During initial thresholds testing, the voltages required to produce left face/arm paresthesias were 2.9 for contact 0, 3.2 for contact 1, 3.0 for contact 2, and 3.8 for contact 3. Currently, the voltages required to produce muscle pulling, paresthesias, and cloudiness of thinking, were 0.5 for contact 0, 1.2 or contact 1, 1.2 for contact 2, and 0.9 for contact 3. His brain scan revealed an increase in the maximum width of the third ventricle compared to the baseline scan performed six years prior. Ten months after rechecking thresholds, the patient was doing well with lower settings and has complained of only one potential episode of facial pulling which was deemed by the clinical team likely unrelated to stimulation.
Case 2
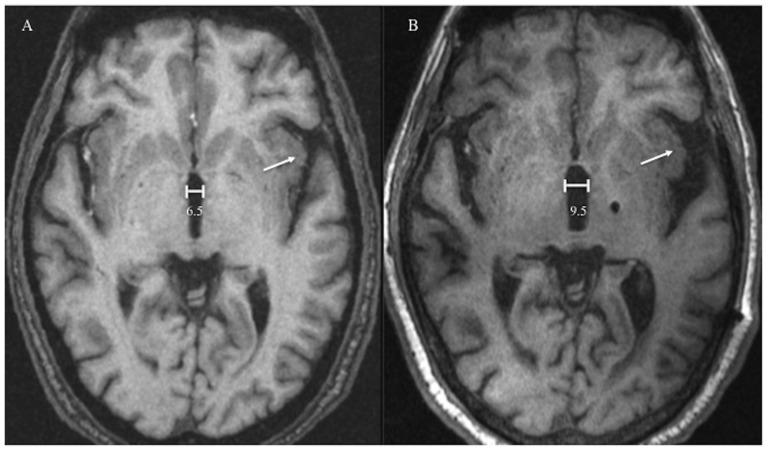
A 63 year-old male with PD status post left STN DBS implantation performed 99 months prior, presented with increased “off” time and re-emergence of troublesome dyskinesias over the last several weeks. The main indication for surgery was his “on state” dyskinesias and severe “off state” periods. His preoperative motor score off/on was 50/12 with post-operative scores off med/on stim of 35 and on med/on stim of 22 at 6 months. The levodopa equivalent daily dosage (LEDD) before surgery was of 1275 mg. During initial thresholds testing, the voltages required to produce right-sided paresthesias and right arm pulling were 2.0 for contact 0, 2.5 for contact 1, 4.0 for contact 2, and 6.0 for contact 3. Currently, the voltages required to produce right arm paresthesias were 0.5 for contact 0, 0.8 or contact 1, 1.5 for contact 2, and 0.8 for contact 3. LEDD at the time of the troubleshooting visit was of 762.5 mg. His MRI revealed an increase in size of the ventricles as compared to his baseline scan, performed 10 years before (Figure 1). Two years after re-programming with lower settings, the patient's symptoms have been controlled, but psychiatric issues have emerged.
Figure 1. Comparison of two MRI scans over 10-year period.
The post-operative scan 10 years after DBS surgery shows an increase in size of the ventricles (millimeters) and widening of the Sylvian fissure (arrow). A: Preoperative scan for DBS targeting; B: Most recent scan.
Case 3
A 65 year-old woman with ET status post left ventralis intermedius nucleus (Vim) nucleus DBS implantation 47 months prior presented with a subacute lack of benefit from DBS. Her indication for DBS included a long history of medically refractory tremor. The baseline TRS motor score was 46. During the initial thresholds testing, the voltages required to produce tingling of the upper extremity were 2.6 for contact 0, and 4.1 for contact 1; contacts 2 and 3 did not produce side effects at 6 or more volts. Currently, the voltages required to produce right arm paresthesias were 1.0 for contact 0, 1.0 or contact 1, 0.3 for contact 2, and 3.3 for contact 3. A recent scan revealed a marked increase in ventricular size as compared to the initial scan following DBS surgery. Twenty months after reprogramming, the patient reported feeling well, and no adjustment in settings have been required. Table 1 describes patient's characteristics with subsequent motor scores. Table 2 compares the initial with the most recent threshold testing, revealing decrements in the amount of voltage tolerated in cases, as well as decrements in current densities in two patients.
Table 1. Demographic and clinical characteristics of patients.
| Patient 1 | Patient 2 | Patient 3 | |
| Gender | Male | Male | Female |
| Age, y | 50 | 63 | 65 |
| Diagnosis | PD | PD | ET |
| Disease duration, y | 17 | 24 | >20 |
| Target | R STN | L STN | L Vim |
| Follow-up, mo. | 75 | 99 | 47 |
| UPDRS-III | N/A | ||
| Pre-op off/on | 62/16 | 50/12 | |
| Post-op off med/on stim | 27 | 35 | |
| Post-op on med/on stim | 21 | 22 | |
| 5 y follow-up | |||
| off med/on stim | 19 | 33 | |
| on med/on stim | 13 | 22 | |
| 8 y follow-up | N/A | ||
| off med/on stim | 41 | ||
| on med/on stim | 21 | ||
| TRS motor | N/A | N/A | |
| Pre-operative | 46 | ||
| 4 y off/on stim | 26/25 |
PD = Parkinson's disease; ET = Essential Tremor; R = Right; L = Left; STN = subthalamic nucleus; Vim = ventralis intermedius nucleus; UPDRS = Unified Parkinson's Disease Rating Scale; TRS = Tremor Rating Scale.
Table 2. Comparison between initial and current thresholds.
| Contact | Initial (Voltage – Side effect) | Currently (Voltage – Side effect) | Δ Volts | Δ Time (months) | |
| Patient 1 | 0 | 2.9 – tingling left hand | 0.5 – bilateral hand tingling | −2.4 | 75 |
| 1 | 3.2 – pulling face, cheek, jaw | 1.2 – left neck and face pulling | −2.0 | ||
| 2 | 3.0 – tingling left face/arm | 1.2 – cloudiness of thinking | −1.8 | ||
| 3 | 3.8 – tingling left face/arm | 0.9 – pulling left face and tongue | −2.9 | ||
| Impedance | 1291 Ω | Postop - 1285 Ω; Chronic - 747 Ω | |||
| Current density | 3.15 µC/cm2/phase | 1.99 µC/cm2/phase | |||
| Patient 2 | 0 | 2.0 – tingling right face | 0.5 – tingling right hand | −1.5 | 99 |
| 1 | 2.5 – tingling right face | 0.8 – tingling right hand | −1.7 | ||
| 2 | 4.0 – pulling right face | 1.5 – tingling right hand | −2.5 | ||
| 3 | 6.0 – pulling right face | 0.8 – tingling right hand | −5.2 | ||
| Impedance | 848 Ω | Postop – 915 Ω; Chronic - 730 Ω | |||
| Current density | 5.49 µC/cm2/phase | 6.16 µC/cm2/phase | |||
| Patient 3 | 0 | 2.6 – bilateral arm tingling | 1 – tingling hand | −2.5 | 47 |
| 1 | 4.1 – tingling right arm | 1 – tingling hand | −4.0 | ||
| 2 | 6.0 – no side effects | 0.3 – tingling hand | −5.7 | ||
| 3 | 6.0 – no side effects | 3.3 – tingling hand | −2.7 | ||
| Impedance | 1099 Ω | Postop - 1343 Ω; Chronic - 658 Ω | |||
| Current density | 10.55 µC/cm2/phase | 7.13 µC/cm2/phase |
Lead Location and Ventricular Size
Considering the standard error of deviation (set at 1.4 mm) when measuring lead location, long-term postoperative lead positions were slightly different across the cohort. The DBS leads were overall located at a more lateral position in recent scans in cases 2 and 3 (Δ = 2.68 and 1.88, respectively), as compared to the initial measurements. In case 2, the collar angle changed from 13 to 15, while in the two other cases, the arc and collar angles remained the same. When comparing atrophy indexes from the initial and most recent brain scans, there was a substantial change in the 3rd ventricular width (120%), Evans index (6%), ventricular index (5%), and cella media index (17%) across the patients (as shown in Table 3).
Table 3. Two time-point comparison of percentage change in brain atrophy measurements and lead measurements.
| 3rd Ventricle Width | Evans index | Ventricular Index | Cella Media Index | Δ AP | Δ Lateral | Δ Axial | |
| Patient 1 | 300% | 5.7% | 7.5% | 5.5% | 0.32 | 1.2 | 0.16 |
| Patient 2 | 37.3% | 9.4% | 6.6% | 15.1% | 0.1 | 2.68 | 0.5 |
| Patient 3 | 22.5% | 4.1% | 1.1% | 29.1% | 0.52 | 1.88 | 0.26 |
| Mean | 120% | 6% | 5% | 17% | - | - | - |
Change in lead position was measured using a distance formula: √ (Xpre−Xpost)2+(Ypre−Ypost)2+(Zpre−Zpost)2. AP = antero-posterior.
Discussion
We present a series of three cases with long-term DBS related management issues and an unexpected change in long-term programming thresholds as compared to the initial evaluation.
Factors possibly underpinning the findings
Our patients presented with long-term issues after several years of DBS related stimulation-induced benefit. This presentation does not occur in typical surgery-related complications, since most of these issues are acute. Hardware-related complications should be considered as a potential factor for side effects or a loss of benefit, and these types of complications have been reported in 4.8% of cases [6], [14]. However, careful examination of the neurostimulator, impedances, as well associated current drains revealed no issues. In addition, plain x-rays of the DBS system were also normal, excluding a DBS lead/wire break. Another important issue could have been impedance variability [15]. However, the impedances across all cases decreased over time, and the values remained within reasonably normal ranges (Table 2), and these would therefore be unlikely to have resulted in the large changes in programming thresholds.
Lead migration was another possible hardware-related factor. Small shifts in position could have contributed to induction of side effects or to the loss of benefit [16]. Perioperative brain shift could also account for differences in lead location [17]. It is important to consider that by comparing the lead location measurements, a lateral shift was documented in two of our patients. This lateral shift could at least in part be explained by atrophy [18].
Brain and nuclei atrophy hypothesis
Stimulation-induced side effects result from the spread of electrical current into brain regions other than the region intended for stimulation. The most interesting finding in our patients was the unexpected reduced programming thresholds that were uncovered following long-term follow-up. DBS-related adverse effects at relatively low levels of stimulation could suggest a suboptimally placed DBS lead. Besides the slight lateral lead shift found in two of our cases, a substantial change in atrophy indices was also manifested. It is unknown how atrophy may affect lead location, but presumably there would be a relationship. Change in atrophy indices could theoretically affect lead position by simply slightly repositioning the DBS lead closer to surrounding structures.
Brain atrophy is expected with aging and is known to be hastened by neurodegeneration [19]. Annual rates of brain atrophy have been reported to be 0.32%/year in healthy adults [20] with a median volume loss of 10.35 ml/year in PD patients [21]–[23]. There has not been a standardized rate of brain atrophy for DBS patients reported. Regarding deep brain structures, thalamic and Vim total volumes are approximately 13000 mm3 and 218 mm3, respectively [24], and these decline over time have been reported in normal subjects [25], [26], and likely will also decline in affected subjects. Similarly, STN shrinks in volume with time [27]. We observed patients with an increase (worsening) in atrophy measurements (Table 2). The Vim's long narrow shape, measuring ∼8 mm (dorsal–ventral) by ∼3 mm (anterior–posterior) by ∼12 mm (medial–lateral) [28] and the typical trajectory of the DBS lead could be hypothesized to result in spreading current into an unintended and adjacent region (capsule or sensory areas). This effect could similarly occur in STN, considering its small size (150–240 mm3) [21], [29], and considering that STN atrophy and shifts in the lateral direction with increasing age [18], could also potentially result in spread of stimulation outside of the intended region. Atrophy could theoretically contribute to the clinically manifested symptoms and in the reduced programming threshold tolerances and subsequent decrement in current densities in two of our patients. The globus pallidus internus (GPi) is a larger structure (450 mm3), and its shape could possibly be more forgiving, but not immune to disease related atrophy and spread of stimulation [30]. No GPi DBS cases were observed in our clinic which actively follows over 600 patients, however a larger sample size and more careful follow-up will be needed. Figure 2 illustrates the DBS atrophy hypothesis, which would posit that similar amounts of electricity are delivered to a shrinking brain (assuming stability of impedance measurements). The mechanisms resulting in atrophy in this small group of patients is unknown and will need to be clarified in future studies which should include long-term cognition and other relevant measures. Future studies will need to measure nuclei atrophy with more sophisticated imaging, such as volumetry or tractography, and to compare to contralateral non-stimulated nuclei in unilateral cases.
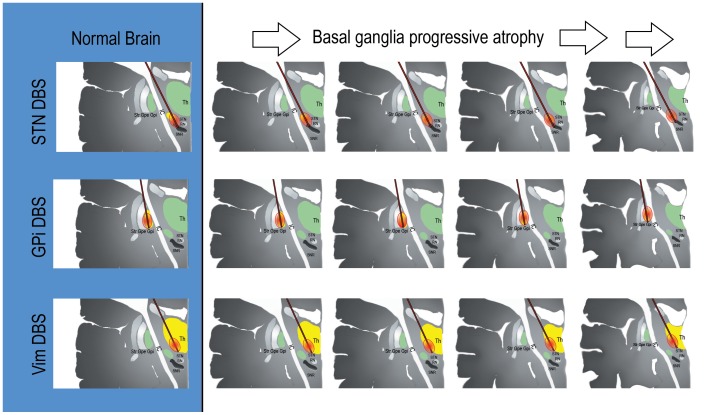
Figure 2. Theoretical figure including atrophy which may play a role in delayed stimulation induced side effects.
Progressive 10%, 20%, 30%, and 40% atrophy with stimulation spread to other structures can be observed following columns to the right. DBS = Deep Brain Stimulation; Str = striatum; GPe = globus pallidus externus; GPi = globus pallidus internus; IC = internal capsule; Th = thalamus; STN = subthalamic nucleus; Vim = ventralis intermedius nucleus; RN = red nucleus; SNR = substantia nigra pars reticulata. This figure is theoretical and shows how atrophy may potentially affect lead location and stimulation fields.
Other potential long-term issues
Disease progression could possibly explain the worsening of symptoms over time, as was observed in cases 2 and 3. However, assessment of the UPDRS and TRS motor scores suggested that the symptoms remained well controlled with chronic stimulation over time. Although worsening of tremor in ET patients with long-term follow-up is expected, and has been previously observed to be related to disease progression [31], the appearance of sensory side effects at lower voltages would suggests a different cause. Motor side effects, as in case 1, could have been a manifestation of dystonia, rather than internal capsule current spread. This notion though possible, was unlikely, since the symptoms continued to worsen with increasing stimulation voltage, and the symptoms abated when turning off the DBS. Additionally, there were programming threshold changes across all four DBS lead contacts.
In conclusion, unexpected emergent stimulation induced side effects or loss of benefit in DBS cases can be potentially important clinical issues that may manifest more frequently in longer-term DBS management. Brain atrophy and disease progression may possibly play a role in at least some of these patients; however, changes in lead position, and long-term pathophysiological changes cannot be completely ruled out. Clinicians should be aware that in DBS therapy there is delivery of electricity to a changing brain and that several factors may affect the response to long-term stimulation. It is therefore very important for DBS patients to have close follow-up and monitoring even after many years post-implant.
Acknowledgments
We thank Russell Moore for assistance with data collection, and the National Parkinson Foundation, Tyler's Hope, and the Bachmann-Strauss Foundation for support of the center. This manuscript was run through the iThenticate system provided by the University of Florida and the 1st author takes all responsibility for ensuring originality.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This work was supported by the University of Florida Foundation, the UF INFORM database, and partly by the Japan Society for Promotion of Science, and St. Luke life Institute in Japan. The sponsors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Okun MS (2012) Deep-brain stimulation for Parkinson's disease. N Engl J Med 367: 1529–1538. [DOI] [PubMed] [Google Scholar]
- 2. Hariz M (2012) Twenty-five years of deep brain stimulation: celebrations and apprehensions. Mov Disord 27: 930–933. [DOI] [PubMed] [Google Scholar]
- 3. Zibetti M, Merola A, Rizzi L, Ricchi V, Angrisano S, et al. (2011) Beyond nine years of continuous subthalamic nucleus deep brain stimulation in Parkinson's disease. Mov Disord 26: 2327–2334. [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez-Oroz MC, Moro E, Krack P (2012) Long-term outcomes of surgical therapies for Parkinson's disease. Mov Disord 27: 1718–1728. [DOI] [PubMed] [Google Scholar]
- 5. Okun MS, Rodriguez RL, Foote KD, Sudhyadhom A, Bova F, et al. (2008) A case-based review of troubleshooting deep brain stimulator issues in movement and neuropsychiatric disorders. Parkinsonism Relat Disord 14: 532–538. [DOI] [PubMed] [Google Scholar]
- 6. Farris S, Vitek J, Giroux ML (2008) Deep brain stimulation hardware complications: the role of electrode impedance and current measurements. Mov Disord 23: 755–760. [DOI] [PubMed] [Google Scholar]
- 7. Farris S, Giroux M (2013) Retrospective review of factors leading to dissatisfaction with subthalamic nucleus deep brain stimulation during long-term management. Surg Neurol Int 4: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks WJ (2011) Deep brain stimulation management. Cambridge New York: Cambridge University Press. 167 p. p. [Google Scholar]
- 9.Jankovic J, Tolosa E (1993) Parkinson's disease and movement disorders. 2nd ed. Baltimore: Williams & Wilkins. pp. 1 online resource (xxii, 618 pages). [Google Scholar]
- 10. Fakhar K, Hastings E, Butson CR, Foote KD, Zeilman P, et al. (2013) Management of deep brain stimulator battery failure: battery estimators, charge density, and importance of clinical symptoms. PLoS One 8: e58665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Uitti RJ, Tsuboi Y, Pooley RA, Putzke JD, Turk MF, et al. (2002) Magnetic resonance imaging and deep brain stimulation. Neurosurgery 51: 1423–1428 discussion 1428–1431. [PubMed] [Google Scholar]
- 12. Morishita T, Foote KD, Okun MS (2010) INPH and Parkinson disease: differentiation by levodopa response. Nat Rev Neurol 6: 52–56. [DOI] [PubMed] [Google Scholar]
- 13. Obuchi T, Katayama Y, Kobayashi K, Oshima H, Fukaya C, et al. (2008) Direction and predictive factors for the shift of brain structure during deep brain stimulation electrode implantation for advanced Parkinson's disease. Neuromodulation 11: 302–310. [DOI] [PubMed] [Google Scholar]
- 14. Shih LC, LaFaver K, Lim C, Papavassiliou E, Tarsy D (2013) Loss of benefit in VIM thalamic deep brain stimulation (DBS) for essential tremor (ET): how prevalent is it? Parkinsonism Relat Disord 19: 676–679. [DOI] [PubMed] [Google Scholar]
- 15. Cheung T, Nuno M, Hoffman M, Katz M, Kilbane C, et al. (2013) Longitudinal Impedance Variability in Patients with Chronically Implanted DBS Devices. Brain Stimul 6: 746–751. [DOI] [PubMed] [Google Scholar]
- 16. Blomstedt P, Hariz MI (2005) Hardware-related complications of deep brain stimulation: a ten year experience. Acta Neurochir (Wien) 147: 1061–1064 discussion 1064. [DOI] [PubMed] [Google Scholar]
- 17. Hunsche S, Sauner D, Maarouf M, Poggenborg J, Lackner K, et al. (2009) Intraoperative X-ray detection and MRI-based quantification of brain shift effects subsequent to implantation of the first electrode in bilateral implantation of deep brain stimulation electrodes. Stereotact Funct Neurosurg 87: 322–329. [DOI] [PubMed] [Google Scholar]
- 18. Keuken MC, Bazin PL, Schafer A, Neumann J, Turner R, et al. (2013) Ultra-high 7T MRI of structural age-related changes of the subthalamic nucleus. J Neurosci 33: 4896–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Munivenkatappa A, Bagepally BS, Saini J, Pal PK (2013) In vivo Age-related Changes in Cortical, Subcortical Nuclei, and Subventricular Zone: A Diffusion Tensor Imaging Study. Aging Dis 4: 65–75. [PMC free article] [PubMed] [Google Scholar]
- 20. Scahill RI, Frost C, Jenkins R, Whitwell JL, Rossor MN, et al. (2003) A longitudinal study of brain volume changes in normal aging using serial registered magnetic resonance imaging. Arch Neurol 60: 989–994. [DOI] [PubMed] [Google Scholar]
- 21. Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, et al. (2005) Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging 26: 1261–1270 discussion 1275–1268. [DOI] [PubMed] [Google Scholar]
- 22. Goodro M, Sameti M, Patenaude B, Fein G (2012) Age effect on subcortical structures in healthy adults. Psychiatry Res 203: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hu MT, White SJ, Chaudhuri KR, Morris RG, Bydder GM, et al. (2001) Correlating rates of cerebral atrophy in Parkinson's disease with measures of cognitive decline. J Neural Transm 108: 571–580. [DOI] [PubMed] [Google Scholar]
- 24. Henderson JM, Carpenter K, Cartwright H, Halliday GM (2000) Loss of thalamic intralaminar nuclei in progressive supranuclear palsy and Parkinson's disease: clinical and therapeutic implications. Brain 123 Pt 7: 1410–1421. [DOI] [PubMed] [Google Scholar]
- 25. Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A (2004) Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiol Aging 25: 185–192. [DOI] [PubMed] [Google Scholar]
- 26. Keller SS, Gerdes JS, Mohammadi S, Kellinghaus C, Kugel H, et al. (2012) Volume estimation of the thalamus using freesurfer and stereology: consistency between methods. Neuroinformatics 10: 341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fjell AM, Walhovd KB (2010) Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci 21: 187–221. [DOI] [PubMed] [Google Scholar]
- 28. Butson CR, McIntyre CC (2006) Role of electrode design on the volume of tissue activated during deep brain stimulation. J Neural Eng 3: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, Lozano AM (2004) The subthalamic nucleus in the context of movement disorders. Brain 127: 4–20. [DOI] [PubMed] [Google Scholar]
- 30. Raz N, Rodrigue KM, Kennedy KM, Head D, Gunning-Dixon F, et al. (2003) Differential aging of the human striatum: longitudinal evidence. AJNR Am J Neuroradiol 24: 1849–1856. [PMC free article] [PubMed] [Google Scholar]
- 31. Favilla CG, Ullman D, Wagle Shukla A, Foote KD, Jacobson CEt, et al. (2012) Worsening essential tremor following deep brain stimulation: disease progression versus tolerance. Brain 135: 1455–1462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.