Abstract
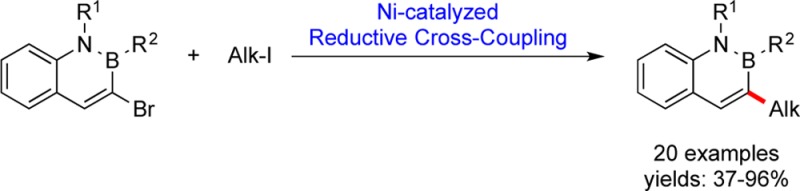
Conditions have been developed for the reductive cross-coupling of 3-bromo-2,1-borazaronaphthalenes with primary and secondary alkyl iodides. This method allows direct alkylation of azaborine cores, providing efficient access to functionalized isosteres of naphthalene derivatives.
Azaborines represent a class of molecules that bear a B–N bond in place of a C=C unit within an aromatic ring. These molecules possess aromaticity and stability similar but not identical to their corresponding all-carbon analogues. 2,1-Borazaronaphthalenes have received a significant amount of attention because of their potentially impactful properties in the fields of pharmaceuticals and materials science.1
Having developed a general and convenient route for the construction of the 2,1-borazaronaphthalene core from 2-aminostyrene derivatives,2 we sought methods for the selective installation of various substituents about the assembled ring. For placement of substituents at the 3-position, the utilization of substituted alkenes of the styrene starting material failed to provide useful quantities of the desired target structures (eq 1).
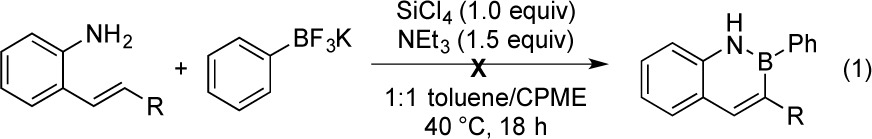 |
1 |
Consequently, another approach, selective halogenation at the 3-position followed by substitution, was developed. The halogenation of 2,1-borazaronaphthalenes was first reported by Dewar in 1961 and was shown to be selective for reaction at the C-3 position, although yields were modest and the scope of the process was not extensively explored.3 More recent progress on the functionalization of 2,1-borazaronaphthalenes was achieved through modified conditions for the bromination, combined with subsequent Pd-catalyzed cross-coupling of 2,1-borazaronaphthalenes (Scheme 1).2
Scheme 1. Bromination and Suzuki–Miyaura Cross-Coupling of 2-Phenyl-2,1-borazaronaphthalenes.
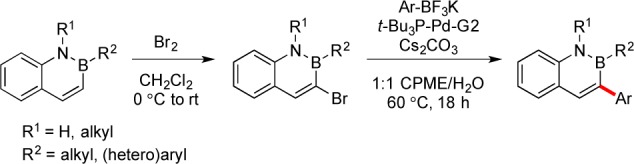
This method allowed aryl and heteroaryl substrates to be successfully introduced to the ring system, but the path forward to install a variety of alkyl substituents at this position was unclear. Thus, although alkyl cross-coupling methods held some promise as a means to extend elaboration of the borazines at the 3-position, a primary goal was to demonstrate the incorporation of diverse alkyl substructures, and limitations on both the availability of the requisite organometallic starting materials and concerns about conversions in such cross-coupling reactions appeared daunting. Specifically, we sought the installation of saturated, heterocyclic ring systems, which are highly valued as moieties that possess nonplanar character, while allowing incorporation of hydrogen-bonding heteroatoms.4 Methods to insert these nonaromatic heterocycles onto aryl systems through typical sp2–sp3 cross-coupling methods have proven quite ineffective, yielding little to none of the desired products.5 Consequently, we pursued a more direct pathway for the efficient introduction of alkyl substituents onto the azaborine core.
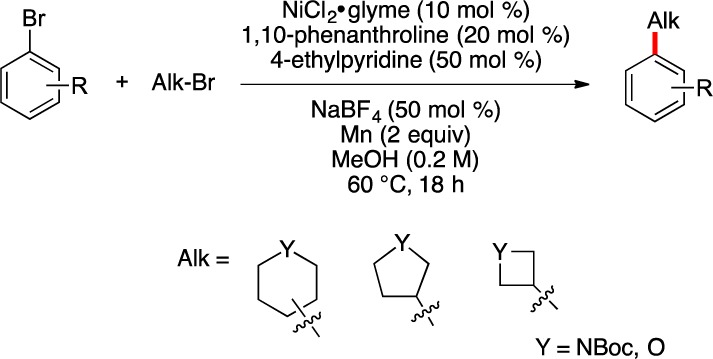
Based on the stability of 2,1-borazaronaphthalenes to palladium catalysis, a metal-promoted reductive cross-coupling pathway6 for the introduction of alkyl halides onto 2,1-borazaronaphthalenes was envisioned. Weix et al.7 and Gong et al.8 have carried out extensive work in the area of reductive cross-couplings in the past decade, illustrating the ability to introduce alkyl halides to aromatic halides under Ni-catalyzed conditions. Recently, application of modified conditions based on these investigations that allow the reductive cross-coupling of nonaromatic heterocyclic bromides with aryl and heteroaryl bromides was reported (Scheme 2).9 We sought to extend a parallel coupling to 3-bromo-2,1-borazaronaphthalene systems, which we anticipated would react in a similar fashion with alkyl bromides.
Scheme 2. Reductive Cross-Coupling of Aryl Bromides with Non-aromatic Heterocyclic Bromides.
Investigation into the reactivity of 3-bromo-2,1-borazaronaphthalenes under reductive coupling conditions was begun using 3-bromo-2-phenyl-2,1-borazaronaphthalene in the presence of N-Boc-4-bromopiperidine. Under the conditions recently developed for the reductive coupling of nonaromatic heterocyclic bromides with aryl bromides,9 a 44% yield of the desired product was obtained (Scheme 3). The major side product observed was protodehalogenation of the azaborine.
Scheme 3. Reductive Coupling of N-Boc-4-bromopiperidine with 3-Bromo-2-phenyl-2,1-borazaronaphthalene.
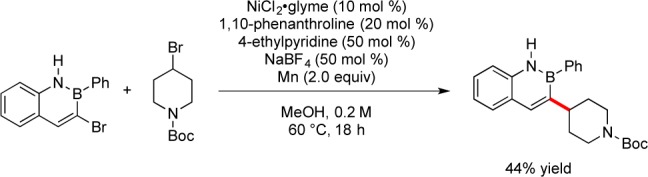
Optimization on this system was begun by testing several bipyridine ligands, and the use of 4,4′-dimethyl-2,2′-bipyridine led to increased conversion to the desired product. Employing 1 equiv of 4-ethylpyridine (from 0.5 equiv) decreased the amount of observed protodehalogenation of the azaborine. Several inorganic salts were tested as additives (e.g., MgCl2, CsI, LiCl, ZnBr2, KBF4, NaI), but NaBF4 led to markedly cleaner reactions than the other salts explored. The use of N-Boc-4-iodopiperidine in place of the corresponding bromide allowed the reaction to be carried out at 40 °C, and the formation of side products was significantly diminished. Various polar solvents were tested under the reaction conditions, and it was determined that both MeOH and DMA served as good reaction solvents in combination with nonpolar cosolvents. The best solvent combinations were mixtures of either DMA or MeOH with cyclohexane, and variation of the ratios of these solvent combinations showed that a 2:1 cyclohexane/DMA mixture was optimal (Table 1). Reduction of the amount of alkyl iodide to 1 equiv (from 1.2 equiv) was detrimental to the reaction, as was lowering the temperature below 40 °C. The use of anhydrous, degassed solvents was determined to be unnecessary, allowing the coupling to be conducted in solvents that did not require degassing or drying prior to use. Lowering the catalyst loading from 10 to 5 mol % Ni did not decrease the conversion to any significant extent.
Table 1. Optimization of Cross-Coupling Conditions.
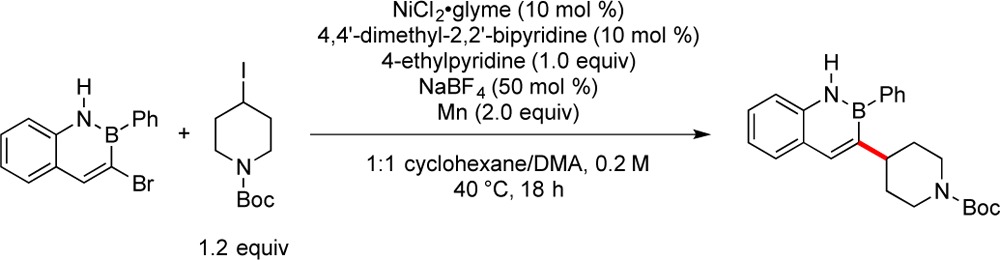
| entry | modification | P/ISa |
|---|---|---|
| 1 | none | 2.78 |
| 2 | temp = rt | 2.58 |
| 3 | 1:1 cyclohexane/MeOH | 2.07 |
| 4 | 2:1 cyclohexane/DMA | 3.10 |
| 5 | 2:1 DMA/cyclohexane | 2.51 |
| 6 | anhydrous, degassed solvent | 2.65 |
| 7 | 1.0 equiv of Alk-I | 2.61 |
| 8 | 5 mol % of Ni, 5 mol % of L | 2.75 |
Ratio of product to internal standard.
With optimized conditions in hand, the reductive cross-coupling of N-Boc-4-iodopiperidine was carried out with a variety of 3-bromo-2,1-borazaronaphthalenes (Table 2). The reaction conditions were amenable to the incorporation of various aromatic moieties on boron, including fluoro-, methoxy-, and carbomethoxy-substituted phenyl rings (entries 2–5). Sterically hindered B-heterocyclic 2,1-borazaronaphthalenes were successfully engaged in the reaction, such that the dibenzofuran and dibenzothiophene systems afforded the cross-coupled products in high yield (entries 6 and 7). Alkyl substituents on boron were also tolerated under the reaction conditions (entries 8–10). We were delighted to see that N-substituted systems reacted under the developed conditions (entries 11–13). An allyl substitution on nitrogen is not cleaved during the coupling, demonstrating the inherent aromaticity and stability of 2,1-borazaronaphthalenes.
Table 2. Reductive Cross-Coupling of N-Boc-4-iodopiperidine with Various Azaborinesa.
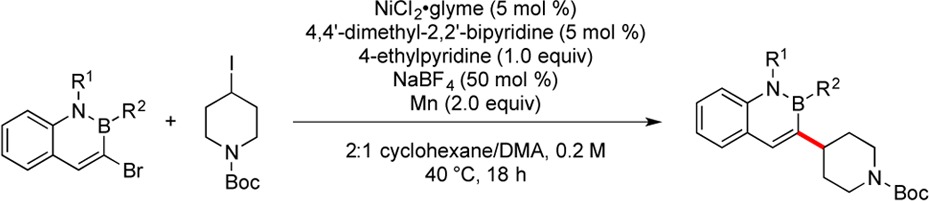
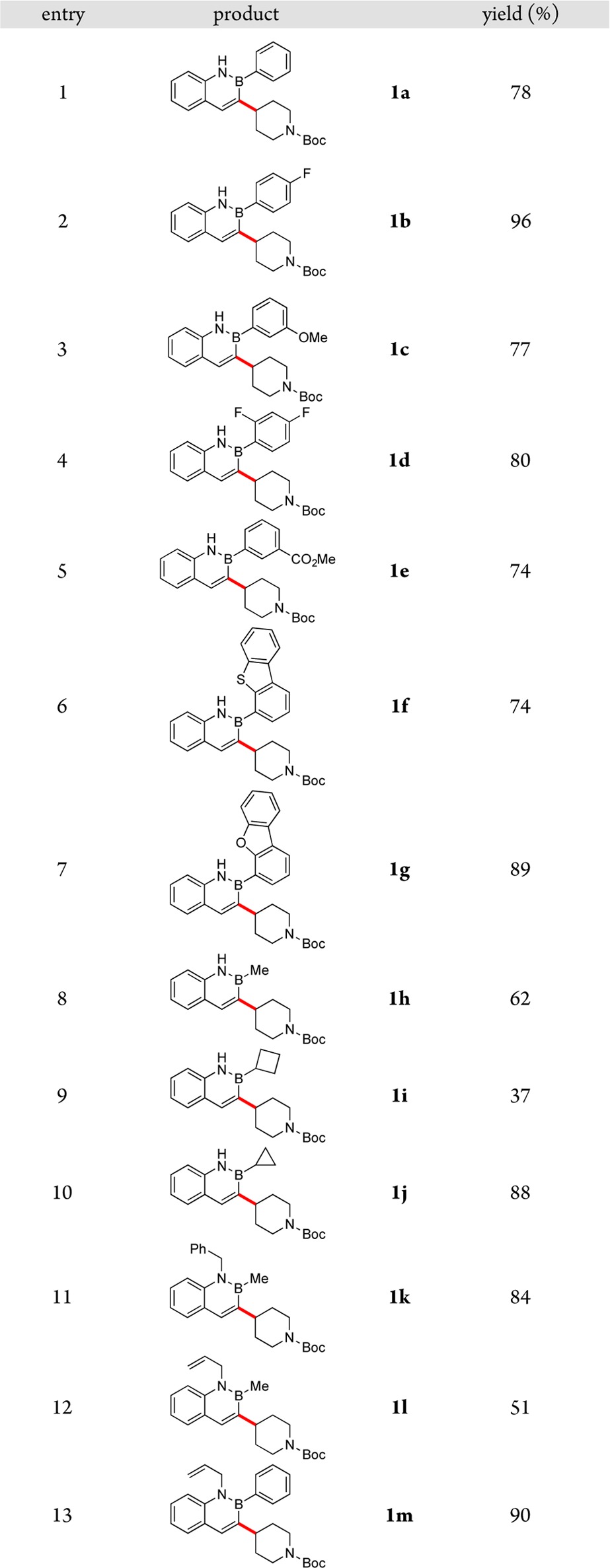
Reactions performed on 0.5 mmol scale unless otherwise noted.
The developed method was next extended to the cross-coupling of 3-bromo-2-phenyl-2,1-borazaronaphthalene with a variety of alkyl iodides (Table 3). Other nonaromatic heterocyclic iodides were successfully cross-coupled, including azetidine, tetrahydropyran, tetrahydrofuran, and oxetane systems (entries 1–5). The scalable nature of the couplings was demonstrated by carrying out the reaction of 3-bromo-2-phenyl-2,1-borazaronaphthalene with N-Boc-3-iodoazetidine on a 3.0 mmol scale (2.5 mol % of Ni, decreased from 5 mol % of Ni on a 0.5 mmol scale) without a decrease in yield (entry 2). The method was further extended to show application to non-heteroatom-containing alkyl iodides, and both secondary and primary alkyl iodides were readily cross-coupled (entries 6–8). Attempts to employ tertiary alkyl iodides or sterically hindered secondary alkyl iodides (e.g., 2-iodoadamantane) resulted in low conversion to product (less than 20%).
Table 3. Reductive Cross-Coupling of 3-Bromo-2-phenyl-2,1-borazaronaphthalene with Various Alkyl Iodides*.
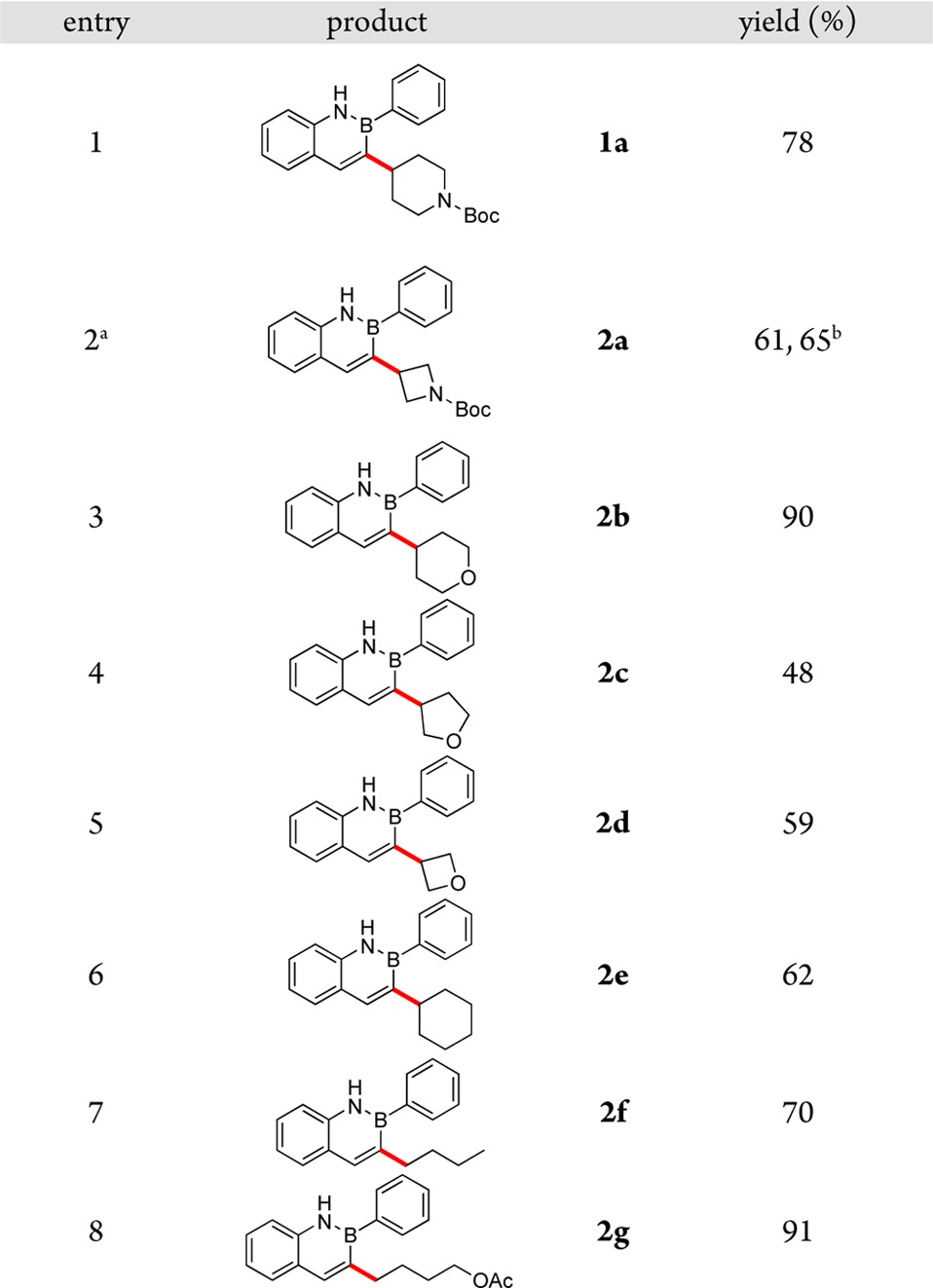
Reactions performed on 0.5 mmol scale unless otherwise noted.
Reaction carried out at 50 °C.
3.0 mmol scale; modification to reaction conditions: 2.5 mol % of NiCl2·glyme, 2.5 mol % of 4,4′-dimethyl-2,2′-bipyridine.
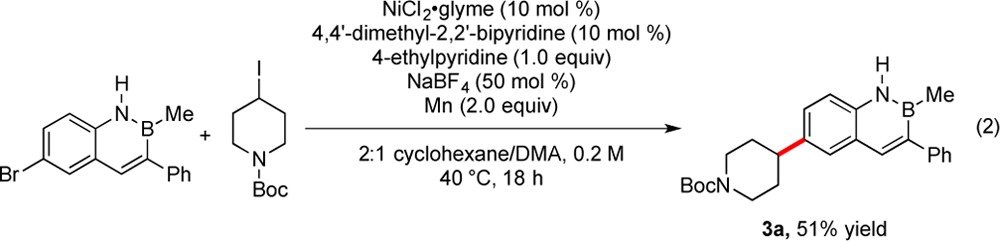
To demonstrate the applicability of this method toward alkylation of azaborine cores at another position, N-Boc-4-iodopiperidine was cross-coupled with 6-bromo-2-methyl-3-phenyl-2,1-borazaronaphthalene (eq 2). The reaction proceeded in 51% yield without any change in the developed conditions, illustrating the potential for this method to be extended beyond 3-bromo-2,1-borazaronaphthalene systems.
 |
2 |
In conclusion, a method has been developed for the reductive cross-coupling of 3-bromo-2,1-borazaronaphthalenes with alkyl iodides. The method allows the coupling of azaborine cores bearing aryl or alkyl substituents on boron and also tolerates alkyl substitution on nitrogen. Primary and secondary alkyl iodides, including an important class of nonaromatic heterocycles, are capable of reacting under the developed conditions. This method provides a valuable, direct route to alkylated, functionalized azaborine systems.
Acknowledgments
This research was supported by the NIGMS (R01 GM-081376) and Eli Lilly. Frontier Scientific is acknowledged for their generous donation of potassium organotrifluoroborates. Dr. Rakesh Kohli (University of Pennsylvania) is acknowledged for acquisition of HRMS spectra.
Supporting Information Available
Complete experimental procedures and characterization data (1H, 13C, 11B NMR, IR, mp, HRMS). This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
† These authors contributed equally.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Liu Z.; Marder T. B. Angew. Chem., Int. Ed. 2008, 47, 242. [DOI] [PubMed] [Google Scholar]; b Bosdet M. J. D.; Piers W. E. Can. J. Chem. 2009, 87, 8. [Google Scholar]; c Campbell P. G.; Marwitz A. J. V.; Liu S.-Y. Angew. Chem., Int. Ed. 2012, 51, 6074. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Marwitz A. J. V.; Matus M. H.; Zakharov L. N.; Dixon D. A.; Liu S.-Y. Angew. Chem., Int. Ed. 2009, 48, 973. [DOI] [PubMed] [Google Scholar]; e Campbell P. G.; Abbey E. R.; Neined D.; Grant D. J.; Dixon D. A.; Liu S.-Y. J. Am. Chem. Soc. 2010, 132, 18048. [DOI] [PubMed] [Google Scholar]; f Abbey E. R.; Zakharov L. N.; Liu S.-Y. J. Am. Chem. Soc. 2008, 130, 7520. [DOI] [PubMed] [Google Scholar]
- Molander G. A.; Wisniewski S. R. Manuscript submitted.
- Dewar M. J. S.; Dietz R. J. Org. Chem. 1961, 26, 3253. [Google Scholar]
- Loverling F.; Bikker J.; Humblet C. J. Med. Chem. 2009, 52, 6752. [DOI] [PubMed] [Google Scholar]
- Allwood D. M.; Blakemore D. C.; Brown A. D.; Ley S. V. J. Org. Chem. 2014, 79, 328. [DOI] [PubMed] [Google Scholar]
- For examples of reductive cross-coupling reactions, see:; a Durandetti M.; Nédélec J.-Y.; Périchon J. J. Org. Chem. 1996, 61, 1748. [DOI] [PubMed] [Google Scholar]; b Durandetti M.; Gosmini C.; Périchon J. Tetrahedron 2007, 63, 1146. [Google Scholar]; c Gosmini C.; Bassene-Ernst C.; Durandetti M. Tetrahedron 2009, 65, 6141. [Google Scholar]; d Yan C.-S.; Peng Y.; Xu X.-B.; Wang Y.-W. Chem.—Eur. J. 2012, 18, 6039. [DOI] [PubMed] [Google Scholar]; e Gomes P.; Gosmini C.; Perichon J. Org. Lett. 2003, 5, 1043. [DOI] [PubMed] [Google Scholar]; f Cherney A. H.; Kadunce N. T.; Reisman S. E. J. Am. Chem. Soc. 2013, 135, 7442. [DOI] [PubMed] [Google Scholar]; g Leon T.; Correa A.; Martin R. J. Am. Chem. Soc. 2013, 135, 1221. [DOI] [PubMed] [Google Scholar]; h Knappke C. E. I.; Grupe S.; Gartner D.; Corpet M.; Gosmini C.; Jacobi von Wangelin A. Chem.—Eur. J. 2014, 20, 6828. [DOI] [PubMed] [Google Scholar]
- a Everson D. A.; Shrestha R.; Weix D. J. J. Am. Chem. Soc. 2010, 132, 920. [DOI] [PubMed] [Google Scholar]; b Everson D. A.; Jones B. A.; Weix D. J. J. Am. Chem. Soc. 2012, 134, 6146. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Biswas S.; Weix D. J. J. Am. Chem. Soc. 2013, 135, 16192. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Everson D. A.; Buonomo J. A.; Weix D. J. Synlett 2014, 14, 3352. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Everson D. A.; Weix D. J. J. Org. Chem. 2014, 79, 4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Yu X.; Yang T.; Wang S.; Xu H.; Gong H. Org. Lett. 2011, 13, 2138. [DOI] [PubMed] [Google Scholar]; b Xu H.; Zhao C.; Qian Q.; Deng W.; Gong H. Chem. Sci. 2013, 4, 4022. [Google Scholar]; c Wang S.; Qian Q.; Gong H. Org. Lett. 2012, 14, 3352. [DOI] [PubMed] [Google Scholar]
- Molander G. A.; Traister K. M.; O’Neill B. J. Org. Chem. 2014, 79, 5771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


