Abstract
Numerous gram-negative bacteria communicate and regulate gene expression through a cell density-responsive mechanism termed quorum sensing (QS), which involves the synthesis and perception of diffusible N-acyl-homoserine lactones (AHL). In this study we genetically and physiologically characterized the Burkholderia thailandensis DW503 QS network. In silico analysis of the B. thailandensis genome revealed the presence of at least three AHL synthases (AHS) and five transcriptional regulators belonging to the LuxIR family of proteins. Mass spectrometry demonstrated that wild-type B. thailandensis synthesizes N-hexanoyl-homoserine lactone (C6-HSL), N-octanoyl-homoserine lactone (C8-HSL), and N-decanoyl-homoserine lactone (C10-HSL). Mutation of the btaI1 (luxI) AHS gene prevented accumulation of C8-HSL in culture supernatants, enhanced beta-hemolysis of sheep erythrocytes, increased lipase production, and altered colony morphology on swarming and twitching motility plates. Disruption of the btaI3 (luxI) AHS prevented biosynthesis of C6-HSL and increased lipase production and beta-hemolysis, whereas mutagenesis of the btaI2 (luxI) allele eliminated C10-HSL accumulation and reduced lipase production. Complementation of the btaI1 and btaI3 mutants fully restored the synthesis of C8-HSL and C6-HSL to parental levels. In contrast, mutagenesis of the btaR1, btaR3, btaR4, and btaR5 (luxR) transcriptional regulators had no effect on AHL accumulation, enhanced lipase production, and resulted in extensive beta-hemolysis on sheep blood agar plates. Furthermore, interruption of the btaI1, btaR1, and btaR3 genes altered colony morphology on twitching and swarming motility plates and induced pigmentation. Additionally, phenotypic microarray analysis indicated that QS in B. thailandensis both positively and negatively affects the metabolism of numerous substrates, including citric acid, formic acid, glucose 6-phosphate, capric acid, γ-hydroxybutyric acid, and d-arabinose. These results demonstrate that mutagenesis of the B. thailandensis QS system affects various cellular processes, including lipase production, swarming and twitching motility, beta-hemolysis of sheep erythrocytes, and carbon metabolism and/or transport.
Burkholderia thailandensis is a gram-negative motile rod that is commonly found in stagnant waters, soils, and rice paddies in the central and northeastern areas of Thailand (5). B. thailandensis is genetically and physiologically similar to Burkholderia pseudomallei, the etiologic agent of melioidosis (5, 22, 45). Biochemically, these two organisms have similar abilities to metabolize various carbon sources, with the exception of l-arabinose, which only B. thailandensis assimilates (45). Most striking are the virulence differences between B. thailandensis and B. pseudomallei. Brett et al. reported that the 50% lethal dose of B. pseudomallei in a hamster model was <10 CFU, whereas the 50% lethal dose of B. thailandensis was >106 CFU (5).
Quorum sensing (QS) is an N-acyl-homoserine lactone (AHL) microbial communication system that is employed by gram-negative bacteria to regulate gene expression and has been shown to both positively and negatively control various cellular processes (2, 4, 6, 8, 9, 11, 14, 15, 27, 28, 30, 33, 34, 37, 44). Recent studies have demonstrated that members of the genus Burkholderia encode functional QS systems that regulate AHL biosynthesis and secretion of extracellular virulence factors and contribute to the in vivo pathogenicity of Burkholderia cepacia (3, 10, 23, 29, 30, 42).
In this study, we both genetically and biochemically characterized the B. thailandensis QS network and determined that at least three functional AHL synthases (AHS) (LuxI) and five transcriptional regulators (LuxR), two of which are orphaned (not flanked by a luxI homologue) for a putative luxI gene (btaR4 and btaR5), are present. The products of the B. thailandensis AHS genes were identified as N-hexanoyl-homoserine lactone (C6-HSL), N-octanoyl-homoserine lactone (C8-HSL), and N-decanoyl-homoserine lactone (C10-HSL). By creating merodiploids in each of the B. thailandensis QS alleles, we demonstrate that QS affects lipase production, colony morphology on swarming and twitching motility plates, beta-hemolysis of sheep erythrocytes, and the metabolism and/or transport of numerous carbon sources.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and cloning vectors used in this study are described in Table 1. B. thailandensis, Agrobacterium tumefaciens A136, and Escherichia coli were cultured in Luria-Bertani (LB) broth or on LB agar at either 30 or 37°C. For screening of recombinant clones, E. coli was grown on LB agar plates containing 25 μg of kanamycin (Sigma, St. Louis, Mo.) per ml and 50 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal) (Sigma) per ml by using standard procedures (36).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Descriptiona | Reference or source |
|---|---|---|
| Escherichia coli strains | ||
| SM10 | Mobilizing strain; RP4 tra genes; Kmr | 41 |
| TOP10 | Used for cloning and blue-white screening | Invitrogen |
| Bioreporter strain A136 | Agrobacterium tumefaciens strain lacking the Ti plasmid | 19 |
| Burkholderia thailandensis strains | ||
| DW503 | Derived from E264; Δ(amrR-oprA) (Kms Gms Sms); rpsL (Smr) | 7 |
| BTRJ1 | DW503 derivative; I1::pGSV3; Gmr | This study |
| BTRJ2 | DW503 derivative; I2::pGSV3; Gmr | This study |
| BTRJ3 | DW503 derivative; I3::pGSV3; Gmr | This study |
| BTRJ4 | DW503 derivative; R1::pGSV3; Gmr | This study |
| BTRJ5 | DW503 derivative; R2::pGSV3; Gmr | This study |
| BTRJ6 | DW503 derivative; R3::pGSV3; Gmr | This study |
| BTRJ7 | DW503 derivative; R4::pGSV3; Gmr | This study |
| BTRJ8 | DW503 derivative; R5::pGSV3; Gmr | This study |
| BTRJ9 | BTRJ1 containing plasmid pBHR1-I1 | This study |
| BTRJ10 | BTRJ3 containing plasmid pBHR1-I3 | This study |
| BTRJ11 | BTRJ6 containing plasmid pBHR1-R3 | This study |
| BTRJ12 | BTRJ8 containing plasmid pBHR1-R5 | This study |
| Plasmids | ||
| pGSV3 | Mobilizable suicide vector; Gmr | 13 |
| pCR2.1-TOPO | TA cloning vector; Kmr Apr | Invitrogen |
| pBHR1 | Mobilizable broad-host-range expression vector; Kmr Cmr | MoBiTec |
| pRUI1 | Contains a 369-bp PCR product from the 1026b I1 synthase gene | This study |
| pRUI2 | Contains a 360-bp PCR product from the 1026b I2 synthase gene | This study |
| pRUI3 | Contains a 398-bp PCR product from the 1026b I3 synthase gene | This study |
| pRUR1 | Contains a 397-bp PCR product from the 1026b R1 transcriptional regulator | This study |
| pRUR2 | Contains a 424-bp PCR product from the 1026b R2 transcriptional regulator | This study |
| pRUR3 | Contains a 402-bp PCR product from the 1026b R3 transcriptional regulator | This study |
| pRUR4 | Contains a 391-bp PCR product from the 1026b R4 transcriptional regulator | This study |
| pRUR5 | Contains a 401-bp PCR product from the 1026b R5 transcriptional regulator | This study |
| pBHR1-btaI1 | pBHR1 carrying the btaI1 AHS synthase gene from DW503; Kmr | This study |
| pBHR1-btaI3 | pBHR1 carrying the btaI3 AHS synthase gene from DW503; Kmr | This study |
| pBHR1-btaR5 | pBHR1 carrying the btaR5 gene from DW503; Kmr | This study |
Kmr, kanamycin resistance; Kms, kanamycin sensitivity; Gmr, gentamicin resistance; Gms, gentamicin sensitivity; Smr, streptomycin resistance; Sms, streptomycin sensitivity; Apr, ampicillin resistance.
Cloning of the B. thailandensis internal gene amplicons and mutant construction.
Primer design for each B. thailandensis QS allele was performed using the B. pseudomallei K96243 luxIR homologues recovered by blastx analysis using the B. cepacia cepIR and Pseudomonas aeruginosa lasIR and rhlIR genes as query sequences (http://www.sanger.ac.uk/). Genomic DNA for PCR amplification was purified with a MasterPure DNA purification kit (Epicentre Technologies, Madison, Wis.). The PCR cycling parameters used for internal gene amplification, the methods used for mutating each B. thailandensis QS gene, and site-specific integration were confirmed by using the methods of Ulrich and DeShazer (43).
AHL reporter assays and mass spectrometry (MS).
The accumulation of AHLs by wild-type B. thailandensis and each QS mutant was analyzed by using the bioreporter strain A. tumefaciens A136 (19), which responds to exogenously secreted AHLs that are of various sizes and chemical compositions. Thin-layer chromatography (TLC) plates were overlaid with A. tumefaciens A136 (inoculated into LB soft agar containing 50 μg of X-Gal per ml) and incubated for 36 to 48 h at 30°C or until adequate color development was achieved.
AHLs were purified from culture supernatants and separated on TLC plates as described by Shaw et al. (40). Preparative TLC spots visually identified as containing AHLs were scraped and extracted three times with 1 ml of methylene chloride (B&J HPLC grade; VWR Scientific, Bridgeport, N.J.). Residual debris was pelleted by centrifugation at 4,000 rpm (Eppendorf 5415C) for 10 min. Supernatants were pooled and evaporated at 50°C under a gentle stream of nitrogen. Dried samples were reconstituted in 100 μl of 50% acetonitrile (B&J HPLC grade; VWR Scientific) in 0.1% formic acid (Sigma), and aliquots (20 μl) were injected onto a PepMap C18 column (150 mm by 1 mm; 5 μm; 100 Å; LC Packings, San Francisco, Calif.). An ABI 140B syringe pump (Applied Biosystems, Foster City, Calif.) provided a flow rate of 50 μl/min, and a 20-min gradient of 0 to 100% solvent B (0.1% formic acid in 95% acetonitrile) was used to elute the compounds of interest. Solvent A was 0.1% formic acid. The column effluent was directed into an LCQ DECA ion trap mass spectrometer equipped with an API II electrospray interface (Finnigan, San Jose, Calif.). The transfer capillary temperature was 350°C. Full-scan, positive-ion mass spectra were acquired by scanning from m/z 100 to m/z 335 in 1.5 s. For identification, extracts were fragmented by collision-induced dissociation of the corresponding [M+H]+ ion by using a relative collision energy setting of 19. The spectra were acquired by scanning from m/z 50 to m/z 335 in 1.5 s. MS-MS spectra of unknown compounds were compared to those of standards acquired under the same conditions in order to confirm the identities of the unknown.
Exoproduct secretion and motility analysis.
Siderophore activity was measured on CAS agar plates by using methods described previously (38). Briefly, wild-type B. thailandensis and each QS mutant were replicated onto CAS agar plates and incubated for 24 to 48 h at 37°C. Iron removal, indicating siderophore secretion, was assayed by measuring the blue-orange halo surrounding each inoculation site. Protease, lipase, and phospholipase C (PLC) production (zone sizes) was determined by using previously described methods (12). Briefly, protease secretion was measured on 3% skim milk agar plates, lipase production was measured on tributyrin plates, and PLC synthesis was measured on egg yolk agar plates. Proteolytic activity (peptide degradation), lipase production (with polyoxyethylene sorbitan Tween 40 as the substrate), and PLC production (lecithin cleavage) were confirmed by measuring zone sizes 24 to 48 h postinoculation. Beta-hemolysis was analyzed on 5% sheep blood agar plates. Positive reactions, as indicated by clearing or lysis of erythrocytes surrounding the bacterial colonies, were determined 48 to 72 h following inoculation. Colony morphologies on twitching and swarming motility plates were examined by using methods described by Köhler et al. and Reimmann et al. (26, 35).
Analysis of substrate utilization by B. thailandensis and each QS mutant.
Carbon utilization was monitored in duplicate by using BiOLOG phenotypic microarrays (BiOLOG, Hayward, Calif.). B. thailandensis and each mutant derivative were cultured on R2A agar plates (BiOLOG) for 24 h at 37°C. Cell densities (as determined from plate scrapings) were adjusted by using IF-0 inoculating fluid (BiOLOG), inoculating (100 μl per well) into PM1 and PM2 assay plates (BiOLOG), and incubating at 37°C for 24 to 48 h or until adequate color development was achieved.
RESULTS
B. thailandensis has multiple luxIR genes.
By using the cepIR, lasIR, and rhlIR genes as query sequences, several luxIR genes were identified in the B. pseudomallei K96243 genome. Incorporating the B. pseudomallei K96243 QS genes for primer design (Table 2), the B. thailandensis QS alleles were PCR amplified (small internal gene amplicons for mutant construction), cloned, sequenced, and used in silico to search the B. thailandensis genome (The Institute for Genomic Research) for putative luxIR homologues. As with B. pseudomallei, in silico analysis of the B. thailandensis genome revealed the presence of at least three luxI and five luxR transcriptional regulators (Table 3). Database (blastx) search results further established that B. thailandensis encodes at least three AHSs and five putative transcriptional regulators, two of which are orphaned (no flanking luxI gene was identified) for cognate AHS alleles (btaR4 and btaR5) belonging to the LuxIR family of proteins (Table 3). The btaIR1 and btaIR2 gene pairs are disrupted by intergenic regions, while the btaIR3 genes are transcribed in the same direction (data not shown). The structural organization, including the surrounding alleles, is identical to that reported for Burkholderia mallei (R. L. Ulrich, D. DeShazer, H. Hines, and J. A. Jeddeloh, submitted for publication). The B. thailandensis genome was also screened in silico for potential LuxS and QscR homologues, none of which were identified, suggesting that B. thailandensis encodes only an AHL-based communication system.
TABLE 2.
Primers used for PCR amplification of internal gene amplicons
| Genea | Directionb | Primer sequence | Amplicon size (bp) |
|---|---|---|---|
| btaI1 | F | 5′-CCGCGACGACGACGGGGAAATC-3′ | 369 |
| R | 5′-TCGATCCAGCACGCGACGACCAT-3′ | ||
| btaI2 | F | 5′-ATAAGCGCCGCGCAACTGGATTCC-3′ | 360 |
| R | 5′-CAGGATCGCCGTATTGCGGTGAGC-3′ | ||
| btaI3 | F | 5′-TCGCGGGCCGATTGAACGAACTGC-3′ | 398 |
| R | 5′-GAGCGACGCGGCCACCGTGAGCAC-3′ | ||
| btaR1 | F | 5′-CGGCTTCGAATATTGCTGCTATGG-3′ | 397 |
| R | 5′-GAGAAAACGGCTCATCAGCGAGTG-3′ | ||
| btaR2 | F | 5′-AGCGACCGGCCCGTGACCTGGAG-3′ | 424 |
| R | 5′-CGGCCTGTATCTTGTTCGTGGAG-3′ | ||
| btaR3 | F | 5′-AGACGTCGTCTCGCTGCACTATCC-3′ | 402 |
| R | 5′-ACCCACGTGAGGCACATCTGTTCG-3′ | ||
| btaR4 | F | 5′-GGCGTTCGACAGATGAAACACGAC-3′ | 391 |
| R | 5′-GCTCATCTGGCACGACGACCTCTA-3′ | ||
| btaR5 | F | 5′-CGCGTGCCGTGGCCGCTGTCCA-3′ | 401 |
| R | 5′-CCGCGCTCCGGGTCCGCCATCAG-3′ |
btaI1 to btaI3 represent AHSs, while btaR1 to btaR5 encode transcriptional regulators.
F, forward; R, reverse.
TABLE 3.
Protein homology searches for the B. thailandensis QS genes
| Gene | % Identity | % Similarity | Homologya | Protein accession no.b |
|---|---|---|---|---|
| btaI1 | 96 | 97 | B. pseudomallei AHS | AF501236_1 |
| 96 | 97 | B. mallei AHS | AF525414_1 | |
| 80 | 88 | B. cepacia AHS CepI | AF330012_1 | |
| btaI2 | 48 | 65 | B. cepacia AHS BviI | AAD12727_1 |
| 48 | 65 | Burkholderia ambifaria AHS BafI | AAK27305_1 | |
| 48 | 65 | B. cepacia AHS CepI | AF330012_1 | |
| btaI3 | 40 | 54 | B. mallei AHS | AF525414_1 |
| 40 | 54 | B. pseudomallei AHS | AF501236_1 | |
| 38 | 51 | Ralstonia solanacearum AHS SolI | CAD17074_1 | |
| btaR1 | 80 | 88 | B. vietnamiensis AHL receptor | AF333007_1 |
| 80 | 89 | Burkholderia stabilis AHL receptor | AF330021_1 | |
| 80 | 89 | B. cepacia AHL receptor | AF330020_1 | |
| btaR2 | 43 | 64 | R. solanacearum transcriptional activator protein | CAD17929_1 |
| 40 | 58 | R. solanacearum transcriptional activator protein SolR | AF330023_1 | |
| 42 | 59 | B. cepacia AHL receptor CepR | AF330023_1 | |
| btaR3 | 45 | 62 | Burkholderia fungorum DNA-binding protein | ZP_00030469.1 |
| 36 | 57 | Bradyrhizobium japonicum transcriptional regulatory protein | NP_768520.1 | |
| 35 | 56 | Mesorhizobium loti unknown protein | BAB52313.1 | |
| btaR4 | 35 | 50 | Pseudomonas aeruginosa PAO1 transcriptional regulator | AE004616_1 |
| 33 | 49 | Listonella anguillarum transcriptional regulator VanR | AAC45213.1 | |
| 35 | 50 | R. solanacearum transcriptional activator protein SolR | CAD17075.1 | |
| btaR5 | 51 | 66 | R. solanacearum transcriptional activator protein SolR | CAD17929.1 |
| 41 | 56 | B. cepacia transcriptional activator BviR | AF296284_2 | |
| 39 | 57 | R. solanacearum transcriptional activator protein SolR | CAD17075.1 |
LuxI or LuxR proteins similar to that of B. thailandensis.
GenBank protein accession number.
Characterization and detection of the AHLs produced by B. thailandensis.
In order to characterize the AHLs synthesized by B. thailandensis, the A. tumefaciens A136 bioreporter strain was used, which allowed detection of multiple AHLs with variable side chain lengths and chemical modifications (C4-HSL) (40). To confirm that strains BTRJ1 (btaI1), BTRJ2 (btaI2), and BTRJ3 (btaI3) were deficient in AHL synthesis, TLC overlays with A. tumefaciens A136 were examined. As shown in Fig. 1, lanes 2 to 4, BTRJ1 (btaI1), BTRJ2 (btaI2), and BTRJ3 (btaI3) failed to produce detectable levels of C8-HSL, C10-HSL, and C6-HSL, respectively. However, it should be noted that AHL detection in this study was dependent on the sensitivity of the bioreporter strain, and it is possible that B. thailandensis produces additional AHLs at concentrations below the limit of detection with A. tumefaciens A136. To determine if any of the five LuxR homologues encoded by B. thailandensis affected AHL biosynthesis, TLC overlays were performed with AHL extracts from each transcriptional regulator mutant. Although the A. tumefaciens A136 bioassay is only semiquantitative, it appeared that the remaining B. thailandensis LuxR QS mutants (BTRJ4 to BTRJ8) accumulated similar levels of C6-HSL, C8-HSL, or C10-HSL, suggesting that these LuxR proteins have no effect on AHL production (data not shown). To determine if AHL synthesis could be complemented, wild-type copies of btaI1 and btaI3 were heterologously expressed in the corresponding luxI mutants. Both BTRJ1 (btaI1) and BTRJ3 (btaI3) were fully complemented, and AHL production was restored to parental levels (Fig. 1, lanes 5 and 6). Attempts to clone and maintain the btaI2 gene in E. coli were unsuccessful.
FIG. 1.
Synthesis of the AHL molecules produced by B. thailandensis and each luxI mutant. AHL extracts were spotted (10 μl) onto C18 reversed-phase TLC plates and visualized by using an A. tumefaciens A136 overlay. Lane 1, wild-type B. thailandensis; lane 2, BTRJ1 (btaI1); lane 3, BTRJ2 (btaI2); lane 4, BTRJ3 (btaI3); lane 5, BTRJ9 (pBHR1-btaI1); and lane 6, BTRJ10 (pBHR1-btaI3).
To determine the chemical structure of each AHL produced by B. thailandensis, MS of the TLC-purified molecules was performed. Figures 2D to F show that B. thailandensis synthesized C8-HSL, C6-HSL, and C10-HSL. The mass spectra for the C6-HSL extract and the standard compound had ions at m/z 200.0 and m/z 200.2, respectively, in addition to a product ion at m/z 102.0, which is characteristic of the lactone ring (Fig. 2A and D). The spectra for the C8-HSL extract and the standard had molecular ions at m/z 228.3 and m/z 227.9, respectively, and also contained the AHL signature lactone ion at m/z 102.0 (Fig. 2B and E). Like that of C6-HSL and C8-HSL, fragmentation of the C10-HSL standard and extract produced ion products at m/z 102 (lactone ring) and m/z 155 representing the acyl side chain of C10-HSL (Fig. 2C and F).
FIG. 2.
Structural analysis of the AHLs biosynthesized by B. thailandensis. (A) Synthetic C6-HSL; (B) synthetic C8-HSL; (C) synthetic C10-HSL; (D to F) AHLs (C6-HSL, C8-HSL, and C10-HSL, respectively) extracted from an overnight culture of B. thailandensis.
Mutagenesis of the B. thailandensis QS system results in altered lipase and beta-hemolysis phenotypes.
Semiquantitative plate assays for detection of beta-hemolysis and protease, siderophore, lipase, and PLC production were performed with each B. thailandensis QS mutant. Disruption of the B. thailandensis QS system had no effect on protease secretion (as measured on 3% skim milk agar plates) or PLC activity (as assayed on egg yolk agar plates) and had a marginal effect on siderophore production (measured on CAS agar plates) (data not shown).
Unlike the effects on protease, PLC, and siderophore synthesis, mutagenesis of the B. thailandensis QS genes both positively and negatively affected lipase production. Strains BTRJ2 (btaI2) and BTRJ5 (btaR2) exhibited reductions in the zone radii on lipase plates, while BTRJ1 (btaI1), BTRJ3 (btaI3), BTRJ4 (btaR1), BTRJ6 (btaR3), BTRJ7 (btaR4), and BTRJ8 (btaR5) exhibited zone sizes that were larger than those for wild-type B. thailandensis (data not shown). Table 4 summarizes the phenotypes characterized in this investigation. Addition of exogenous AHL (C8-HSL for BTRJ1, C10-HSL for BTRJ2, and C6-HSL for BTRJ3) at a concentration of 200 nM to lipase plates marginally complemented BTRJ2 (btaI2) and BTRJ3 (btaI3), but not BTRJ1 (btaI1) (data not shown). However, trans complementation with the parental btaI1 gene in BTRJ1 (btaI1) restored the defective lipase phenotype (data not shown). Cloning the B. thailandensis luxR homologues in the broad-host-range expression vector pBHR1 was problematic, and only the btaR3 and btaR5 genes were successfully cloned and maintained in E. coli. As a result, complementation of the remaining mutants (BTRJ4 [btaR1], BTRJ5 [btaR2], and BTRJ7 [btaR4]) was not pursued. The elevated lipolytic phenotypes observed for BTRJ6 (btaR3) and BTRJ8 (btaR5) were reduced to parental levels by expression of the functional allele in trans (data not shown).
TABLE 4.
Phenotype characterization of the B. thailandensis DW503 QS mutants
| Mutant | Protease | PLC | Lipase | Swarming motility | Twitching motility | Beta-hemolysis | C6-HSL | C8-HSL | C10-HSL | Carbon sourcesb
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Formic acid | Glucose 6-phosphate | Capric acid | γ-Hydroxybutyric acid | d-Arabinose | Citric acid | ||||||||||
| BTRJ1 (btaI1) | NAa | NA | − | v | v | − | NA | + | NA | NA | NA | − | − | NA | NA |
| BTRJ2 (btaI2) | NA | NA | + | NA | NA | NA | NA | NA | + | NA | NA | − | − | NA | NA |
| BTRJ3 (btaI3) | NA | NA | − | NA | NA | − | + | NA | NA | NA | NA | − | − | − | NA |
| BTRJ4 (btaR1) | NA | NA | − | NA | v | − | NA | NA | NA | NA | NA | − | − | − | − |
| BTRJ5 (btaR2) | NA | NA | + | NA | NA | − | NA | NA | NA | NA | NA | − | − | − | NA |
| BTRJ6 (btaR3) | NA | NA | − | NA | v | − | NA | NA | NA | + | NA | − | − | NA | − |
| BTRJ7 (btaR4) | NA | NA | − | NA | NA | NA | NA | NA | NA | NA | NA | − | − | NA | NA |
| BTRJ8 (btaR5) | NA | NA | − | NA | NA | − | NA | NA | NA | NA | + | − | − | − | NA |
NA, not affected; −, negatively regulated; +, positively regulated; v, different from wild-type B. thailandensis.
For carbon sources, negatively regulated indicates a gain of function and positively regulated indicates a loss of function.
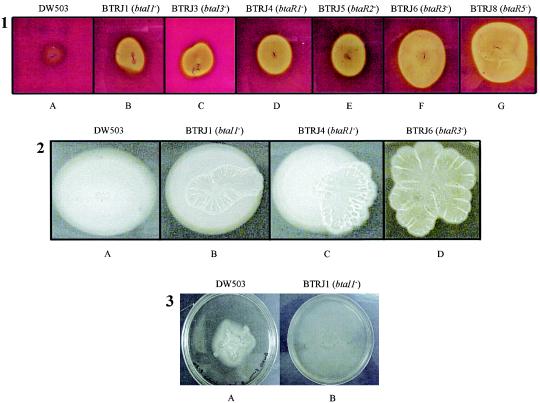
A recent study revealed that B. pseudomallei produces a heat-stable extracellular toxin, determined to be a rhamnolipid, that is hemolytic and cytotoxic to both phagocytic (HL60) and nonphagocytic (HeLa) cell lines (24). Lipid extracts from B. thailandensis and each QS mutant indicated that these strains synthesize a rhamnolipid that is structurally analogous to the B. pseudomallei hemolytic rhamnolipid (data not shown). To determine if QS in B. thailandensis was involved in the beta-hemolysis of sheep erythrocytes, each QS mutant was screened on blood agar plates. BTRJ1 (btaI1), BTRJ3 (btaI3), BTRJ4 (btaR1), BTRJ5 (btaR2), BTRJ6 (btaR3), and BTRJ8 (btaR5) displayed hyper-beta-hemolytic phenotypes (Fig. 3, panel 1). Particularly striking were the levels of beta-hemolysis observed for strains BTRJ6 (btaR3) and BTRJ8 (btaR5−) on sheep blood agar plates (Fig. 3, panel 1). In contrast, BTRJ2 (btaI2) and BTRJ7 (btaR4) produced zones of beta-hemolysis equivalent to those of wild-type B. thailandensis (data not shown). Interestingly, trans complementation of BTRJ1 (btaI1), BTRJ6 (btaR3), and BTRJ8 (btaR5) did not reduce beta-hemolysis to parental levels. Also, exogenous addition of C8-HSL or C6-HSL (200 nM) to blood agar plates inoculated with BTRJ1 (btaI1) and BTRJ3 (btaI3), respectively, failed to reduce bacterial hemolysis to parental levels.
FIG. 3.
Mutations in the B. thailandensis QS system result in hyper-beta-hemolysis of sheep erythrocytes and altered colony morphologies on twitching and swarming motility plates. Experiments were performed in triplicate, as described in Materials and Methods. Panel 1 shows beta-hemolytic activity, while panels 2 and 3 show the results of twitching and swarming motility assays, respectively. DW503 represents wild-type B. thailandensis, btaI corresponded to luxI mutants, and btaR was disrupted luxR homologues.
Disruption of the B. thailandensis QS system results in altered colony morphologies on swarming and twitching motility plates.
Several studies have demonstrated that QS is involved in bacterial motility and plays an integral role in biofilm maturation (1, 21, 35). To determine if QS influenced motility in wild-type B. thailandensis and each QS mutant, bacterial strains were analyzed on a medium that promotes swarming and twitching motility. Compared to wild-type B. thailandensis, the QS mutants BTRJ1 (btaI1), BTRJ4 (btaR1), and BTRJ6 (btaR3) displayed altered growth phenotypes on twitching mofility plates (Fig. 3, panel 2). Wild-type B. thailandensis colonies had a mucoidal, symmetrical morphology without visible pigmentation (Fig. 3, panel 2). BTRJ1 (btaI1) and BTRJ4 (btaR1) exhibited a wrinkled phenotype in which cells proliferated from the center of the inoculation site and grew on the surface of the underlying colony (Fig. 3, panel 2). Interestingly, BTRJ6 (btaR3) produced a faint orange pigment and displayed extensive wrinkling without the glistening appearance observed for wild-type B. thailandensis (Fig. 3, panel 2). Furthermore, BTRJ6 (btaR3) did not exhibit the symmetrical concentric colony morphology of the parental strain. Heterologous expression of the wild-type genes (btaI1 and btaR3) in BTRJ1 (btaI1) and BTRJ6 (btaR3) and addition of C8-HSL (200 nM) to the twitching motility plates failed to restore the defective phenotype (data not shown). Likewise, swarming motility was affected by mutagenesis of the B. thailandensis QS system. On swarming motility plates, wild-type B. thailandensis grew in an irregular and spreading fashion at 24 h, and the colony margins were undulating or wavy and had a glistening appearance (Fig. 3, panel 3). Interestingly, most of the QS mutants tested in this study on swarming motility plates resembled (in terms of size and shape of growth) wild-type B. thailandensis, except for BTRJ1 (btaI1), which exhibited a hyperswarming phenotype (Fig. 3, panel 3). Complementation of this luxI mutant by heterologous expression of the btaI1 gene in BTRJ1 or exogenous addition of C8-HSL (200 nM) to the swarming motility plate was negligible.
Mutagenesis of the B. thailandensis QS system affects carbohydrate metabolism.
Recent studies have linked QS to numerous physiological processes, including transcription, translation, amino acid biosynthesis, chemotaxis, and, interestingly, carbohydrate utilization (37, 44). To determine if carbon metabolism is linked to QS, wild-type B. thailandensis and each QS mutant were assayed by using the BiOLOG phenotypic microarray kit. Mutation of the btaR1 (BTRJ4) and btaR3 (BTRJ6) genes negatively affected (i.e., gain of function) citric acid catabolism (Table 4). The substrates that were positively affected (with a loss of function) included formic acid (BTRJ6 [btaR3]) and glucose 6-phosphate (BTRJ8 [btaR5]) (Table 4). Individual mutagenesis of each of the B. thailandensis luxIR alleles positively influenced capric acid and γ-hydroxybutyric acid metabolism (Table 4). Furthermore, mutations in the btaI3 (BTRJ3), btaR1 (BTRJ4), btaR2 (BTRJ5), and btaR5 (BTRJ8) QS alleles resulted in a gain-of-function phenotype (negatively regulated in B. thailandensis) for d-arabinose utilization (Table 4). It should be noted that disruption of the B. thailandensis QS system imposed no apparent bacterial growth phenotype (growth curve for optical density at 600 nm) when organisms were cultured in complex medium (LB medium).
DISCUSSION
QS is a complex mode of intra- and interspecies communication utilized by both gram-positive and gram-negative bacteria. Signaling mechanisms of this type, which function in response to population density, have been shown to regulate the transcription of various genes associated with human and plant infections (20). In turn, this provides microorganisms with an ecological niche that allows subversion of host defense mechanisms.
Recent studies have demonstrated that Burkholderia species encode functional QS networks that direct the biosynthesis of numerous AHL molecules, regulate virulence factor production, and contribute to animal pathogenicity (3, 10, 23, 25, 29-31, 42). In silico analysis of the B. thailandensis genome revealed the presence of at least three luxI and five luxR homologues. Using MS analysis after TLC separation, this study demonstrated that B. thailandensis produces the signaling molecules C8-HSL (BtaI1), C6-HSL (BtaI3), and C10-HSL (BtaI2). Similar findings have been reported for B. cepacia and Burkholderia vietnamiensis (10, 29). Although genetically related, especially with regard to the similarity of encoded (at the DNA level) luxIR homologues, B. pseudomallei synthesizes a wide range of additional AHLs, including N-(3-hydroxyoctanoyl)-homoserine lactone, N-(3-hydroxydecanoyl)-homoserine lactone, and N-(3-oxotetradecanoyl)-homoserine lactone, which are not detected in B. thailandensis. Furthermore, disruption of the B. pseudomallei luxI genes (three total) has a negligible effect on AHL accumulation (Ulrich et al., submitted for publication). Mutagenesis of the btaI1, btaI2, and btaI3 alleles prevented the accumulation of C8-HSL, C10-HSL, and C6-HSL in culture supernatants, respectively (Fig. 1, lanes 2, 3, and 4). However, it is possible that additional signaling molecules not recognized by A. tumefaciens A136 are synthesized. In numerous QS networks, luxI expression has been shown to be regulated by LuxR proteins (16-18, 29, 39). Although the results are preliminary, disruption of the B. thailandensis luxR genes appeared not to have an effect on AHL accumulation (data not shown).
In B. cepacia the secretion of exoproducts (lipases, proteases, and siderophores), which enhance microbial virulence, is regulated at the level of transcription via QS (29, 30). DeShazer et al. demonstrated that B. pseudomallei produces several potential extracellular virulence factors (12). Because of the genetic and biochemical relatedness between B. thailandensis and B. pseudomallei, each QS mutant and wild-type B. thailandensis were tested for protease, PLC, lipase, and siderophore production. Using semiquantitative plate assays, it was found that QS in B. thailandensis both positively and negatively regulates lipase production (Table 4). Similar findings have been reported for B. cepacia K52-2 (29, 30). Disruption of the cepR allele increased ornibactin secretion, reduced lipase activity, and resulted in a protease-defective phenotype (29). Deletion of the cepI gene also resulted in hyperproduction of ornibactin and eliminated protease activity (29). Interestingly, with the multiple B. thailandensis QS mutants tested, only mutagenesis of the btaIR2 genes resulted in a reduction in lipase production (Table 4). These results suggest that the btaIR2 gene products function as positive regulators of lipase synthesis. Unlike the results obtained with mutations in the B. cepacia cepIR genes, which eliminated protease activity (30), analysis of protease and PLC biosynthesis in each B. thailandensis QS mutant suggested that these exoproducts are not regulated in a cell density-dependent manner via QS.
Both swarming and twitching motility are modes of bacterial translocation that allow microorganisms to avoid inhibitory compounds, promote nutrient uptake, and establish cell-to-cell contact with plant and animal tissues. Several investigations have shown that QS is involved in the regulation of bacterial motility (25, 26, 35). In B. cepacia, swarming motility is affected by QS, and mutagenesis of the cepIR genes results in a pronounced reduction in swarming motility (25). Disruption of the B. thailandensis QS system revealed that both twitching and swarming motility were affected (Fig. 3, panels 2 and 3). On twitching motility plates, BTRJ1 (btaI1), BTRJ4 (btaR1), and BTRJ6 (btaR3) produced a wrinkled phenotype that was not apparent in wild-type B. thailandensis (Fig. 3, panel 2). In addition, the appearance of the colony in the wrinkled domain was rough instead of the characteristic smooth and glistening appearance observed for B. thailandensis (Fig. 3, panel 2). Interestingly, BTRJ6 (btaR3) also produced an uncharacterized pale orange pigment not found in wild-type B. thailandensis. It should be noted that extensive incubation (5 to 8 days) of B. thailandensis at 30 or 37°C resulted in altered colony morphologies similar to the wrinkled appearance observed for BTRJ6 (btaR3). However, prolonged incubation of B. thailandensis (in our laboratory) has never induced pigment biosynthesis. Subtle variations (colony size and shape) in swarming motility were also observed for each B. thailandensis QS mutant tested (data not shown). The most prominent difference on motility plates was observed for BTRJ1 (btaI1), which exhibited a hyperswarming phenotype. The mechanisms for these defects in twitching and swarming motility following mutagenesis of the B. thailandensis QS system are unclear and remain to be determined. Analysis of pilus (twitching) and flagellum biosynthesis (by electron microscopy) in BTRJ1 (btaI1), BTRJ4 (btaR1), and BTRJ6 (btaR3) revealed no differences compared to wild-type B. thailandensis (data not shown). These findings suggest that disruption of the B. thailandensis QS system has no effect on pilus and flagellum biosynthesis, indicating that an additional factor(s) associated with QS contributes to the observed twitching and swarming motility defects.
A recent study characterized a cytotoxic and hemolytic exolipid synthesized by B. pseudomallei (24). Elevated levels of cytotoxicity and hemolysis were detected in supernatant extracts of B. pseudomallei that had reached stationary phase (24). Considering the relatedness of B. thailandensis and B. pseudomallei strains, we tested wild-type B. thailandensis and each QS mutant for hemolytic activity on sheep blood agar plates. Disruption of the B. thailandensis QS system, particularly the luxR homologues, resulted in hyper-beta-hemolysis of erythrocytes, suggesting that QS regulates a factor(s) that contributes to beta-hemolysis (Fig. 3, panel 1). The mechanism (protein or lipid) for this hyper-beta-hemolysis is not fully understood. However, using TLC and MS, we purified and characterized a monounsaturated rhamnolipid from B. thailandensis that is chemically analogous to the B. pseudomallei hemolytic rhamnolipid (data not shown). In addition, using semiquantitative plate assays (egg yolk agar), we found that QS has no effect on PLC production which could also contribute to hemolysis (Table 4). It is conceivable the hyper-beta-hemolytic phenotypes observed in this study following disruption of the B. thailandensis QS system were the result of increased rhamnolipid production. Attempts to quantify the rhamnolipid in each B. thailandensis QS mutant were unsuccessful, and additional studies will be necessary to determine the mechanisms of this beta-hemolysis.
Preliminary analysis using a semiquantitative approach (BiOLOG phenotypic array plates) suggested that QS either directly or indirectly plays a role in the central metabolism of B. thailandensis (Table 4). Interestingly, mutagenesis of the btaR5 gene (BTRJ8) resulted in a positive phenotype, that is, a substrate that was positively regulated by QS in B. thailandensis for the metabolism of glucose 6-phosphate (Table 4). A similar finding, at the transcriptional level, was recently reported by Schuster et al., who found that the P. aeruginosa QS system regulates the NADPH-dependent glucose-6-phosphate dehydrogenase, an essential enzyme needed for glucose metabolism (37). Another interesting phenotype affected by QS in B. thailandensis is d-arabinose utilization (Table 4). Although l-arabinose is structurally different, l-arabinose utilization is one of the assays used to distinguish B. thailandensis, which assimilates l-arabinose, from B. pseudomallei, which cannot use l-arabinose as a primary carbon source (45). Disruption of the btaI3 (BTRJ3), btaR1 (BTRJ4), btaR2 (BTRJ5), and btaR5 (BTRJ8) genes resulted in a positive phenotype for d-arabinose utilization; that is, disruption conferred the ability to use this substrate as a sole carbon source (Table 4). However, neither BTRJ4 (btaR1), BTRJ5 (btaR2), nor BTRJ8 (btaR5) grew in M9 minimal broth (36) with d-arabinose as the primary substrate (data not shown). These results were likely concentration (d-arabinose) dependent and will require further analysis. Also, considering the results of Schuster et al. (37), it is possible that QS regulates the biosynthesis of an additional factor(s) needed to support the growth of B. thailandensis in d-arabinose minimal medium. However, these findings suggest that QS affects carbon metabolism in B. thailandensis. The mechanisms for these differences in carbon utilization remain to be determined, but it is plausible that B. thailandensis employs QS in the environment to prevent excess energy expenditure for carbon acquisition and metabolism when adequate substrates are present in situ.
Complementation of the numerous defective phenotypes observed in this study was unsuccessful. Similar results have been reported for B. cepacia, in which lipase activity was not restored to parental levels by heterologous expression of the cepR and cepIR genes in trans (29). The disruption cassettes used in this investigation were not designed to create in-frame mutations within the target gene. It is possible that the complementation difficulties are the result of polar effects on downstream alleles (i.e., transcriptional regulators) that are required for normal swarming and twitching motility in addition to lipase production. Furthermore, the relative copy number of the gene being transcribed and translated could result in complementation problems, especially when the hypothesis described by Schuster et al. (37), which stated that LuxR levels are essential for activation of QS-controlled genes, is applied to B. thailandensis. Another possibility is that the B. thailandensis QS system forms a hierarchy; that is, there is cross- regulation of the luxI and luxR genes, and overexpression of these genes disrupts this regulatory cascade. However, full restoration of C8-HSL and C6-HSL accumulation in BTRJ1 (btaI1) and BTRJ3 (btaI3) was achieved by trans expression of the btaI1 and btaI3 genes, respectively. The latter finding suggests that the relative concentration of the signaling molecule is essential for effective complementation of defects in swarming motility (BTRJ1 [btaI1]) and lipase production.
Extensive genetic analysis will be required to determine the functional relationship between QS and the environmental survival of B. thailandensis. As in B. cepacia, mutations in the B. thailandensis QS system resulted in both positive and negative regulation of numerous phenotypes (Table 4). With regard to the multiple B. thailandensis luxR mutants assayed for phenotypic variations in this investigation and the findings of Medina et al. (32), who demonstrated that the rhlR transcriptional regulator is both an activator when it is associated with the signaling molecule C4-HSL and an antiactivator when it is not bound to C4-HSL, we hypothesize that the B. thailandensis LuxR transcriptional regulators have dual functions for modulation of twitching and swarming motility, lipase production, and substrate utilization (Table 4). Similar to data obtained for P. aeruginosa, B. cepacia, and B. thailandensis, disruption of the solIR QS system in Ralstonia solanacearum resulted in a 1.7-fold increase in polygalacturonase synthesis (18). Negative gene regulatory mechanisms of this type may provide bacteria a way to reduce energy expenditure for protein synthesis in environments that contain adequate substrates and cofactors. With the multiple phenotypes altered by mutagenesis of the B. thailandensis QS system, it is apparent that extensive molecular analysis, likely by expression profiling studies with whole-genome DNA microarrays, will be required to unravel the complex regulatory circuits encoded by the QS network.
Acknowledgments
Special thanks are extended to Ernst Brueggemann for performing the extensive MS analysis and to William Nierman at The Institute for Genomic Research for providing access to the B. thailandensis genome project. We also thank Melanie Ulrich, Chris Cote, Erica Wargo, and Katheryn Kenyon for critically reviewing the manuscript. We also thank David DeShazer for his excellent technical assistance.
The opinions, interpretations, conclusions, and recommendations in this paper are those of the authors and are not necessarily endorsed by the U.S. Army.
REFERENCES
- 1.Atkinson, S., J. P. Throup, G. S. Stewart, and P. Williams. 1999. A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol. Microbiol. 33:1267-1277. [DOI] [PubMed] [Google Scholar]
- 2.Bainton, N. J., B. W. Bycroft, S. R. Chhabra, P. Stead, L. Gledhill, P. J. Hill, C. E. Rees, M. K. Winson, G. P. Salmond, G. S. Stewart, et al. 1992. A general role for the lux autoinducer in bacterial cell signalling: control of antibiotic biosynthesis in Erwinia. Gene 116:87-91. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin, A., P. A. Sokol, J. Parkhill, and E. Mahenthiralingam. 2004. The Burkholderia cepacia epidemic strain marker is part of a novel genomic island encoding both virulence and metabolism-associated genes in Burkholderia cenocepacia. Infect. Immun. 72:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck von Bodman, S., and S. K. Farrand. 1995. Capsular polysaccharide biosynthesis and pathogenicity in Erwinia stewartii require induction by an N-acylhomoserine lactone autoinducer. J. Bacteriol. 177:5000-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brett, P. J., D. DeShazer, and D. E. Woods. 1998. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int. J. Syst. Bacteriol. 48:317-320. [DOI] [PubMed] [Google Scholar]
- 6.Brint, J. M., and D. E. Ohman. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155-7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burtnick, M., A. Bolton, P. Brett, D. Watanabe, and D. Woods. 2001. Identification of the acid phosphatase (acpA) gene homologues in pathogenic and non-pathogenic Burkholderia spp. facilitates TnphoA mutagenesis. Microbiology 147:111-120. [DOI] [PubMed] [Google Scholar]
- 8.Cao, J. G., and E. A. Meighen. 1989. Purification and structural identification of an autoinducer for the luminescence system of Vibrio harveyi. J. Biol. Chem. 264:21670-21676. [PubMed] [Google Scholar]
- 9.Chapon-Herve, V., M. Akrim, A. Latifi, P. Williams, A. Lazdunski, and M. Bally. 1997. Regulation of the xcp secretion pathway by multiple quorum-sensing modulons in Pseudomonas aeruginosa. Mol. Microbiol. 24:1169-1178. [DOI] [PubMed] [Google Scholar]
- 10.Conway, B. A., and E. P. Greenberg. 2002. Quorum-sensing signals and quorum-sensing genes in Burkholderia vietnamiensis. J. Bacteriol. 184:1187-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies, D. G., M. R. Parsek, J. P. Pearson, B. H. Iglewski, J. W. Costerton, and E. P. Greenberg. 1998. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science 280:295-298. [DOI] [PubMed] [Google Scholar]
- 12.DeShazer, D., P. J. Brett, M. N. Burtnick, and D. E. Woods. 1999. Molecular characterization of genetic loci required for secretion of exoproducts in Burkholderia pseudomallei. J. Bacteriol. 181:4661-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeShazer, D., D. M. Waag, D. L. Fritz, and D. E. Woods. 2001. Identification of a Burkholderia mallei polysaccharide gene cluster by subtractive hybridization and demonstration that the encoded capsule is an essential virulence determinant. Microb. Pathog. 30:253-269. [DOI] [PubMed] [Google Scholar]
- 14.Dunphy, G., C. Miyamoto, and E. Meighen. 1997. A homoserine lactone autoinducer regulates virulence of an insect-pathogenic bacterium, Xenorhabdus nematophilus (Enterobacteriaceae). J. Bacteriol. 179:5288-5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eberl, L., M. K. Winson, C. Sternberg, G. S. Stewart, G. Christiansen, S. R. Chhabra, B. Bycroft, P. Williams, S. Molin, and M. Givskov. 1996. Involvement of N-acyl-l-hormoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol. Microbiol. 20:127-136. [DOI] [PubMed] [Google Scholar]
- 16.Engebrecht, J., K. Nealson, and M. Silverman. 1983. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32:773-781. [DOI] [PubMed] [Google Scholar]
- 17.Engebrecht, J., and M. Silverman. 1984. Identification of genes and gene products necessary for bacterial bioluminescence. Proc. Natl. Acad. Sci. USA 81:4154-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flavier, A. B., L. M. Ganova-Raeva, M. A. Schell, and T. P. Denny. 1997. Hierarchical autoinduction in Ralstonia solanacearum: control of acyl-homoserine lactone production by a novel autoregulatory system responsive to 3-hydroxypalmitic acid methyl ester. J. Bacteriol. 179:7089-7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuqua, C., and S. C. Winans. 1996. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J. Bacteriol. 178:435-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuqua, C., S. C. Winans, and E. P. Greenberg. 1996. Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulators. Annu. Rev. Microbiol. 50:727-751. [DOI] [PubMed] [Google Scholar]
- 21.Glessner, A., R. S. Smith, B. H. Iglewski, and J. B. Robinson. 1999. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of twitching motility. J. Bacteriol. 181:1623-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godoy, D., G. Randle, A. J. Simpson, D. M. Aanensen, T. L. Pitt, R. Kinoshita, and B. G. Spratt. 2003. Multilocus sequence typing and evolutionary relationships among the causative agents of melioidosis and glanders, Burkholderia pseudomallei and Burkholderia mallei. J. Clin. Microbiol. 41:2068-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gotschlich, A., B. Huber, O. Geisenberger, A. Togl, A. Steidle, K. Riedel, P. Hill, B. Tummler, P. Vandamme, B. Middleton, M. Camara, P. Williams, A. Hardman, and L. Eberl. 2001. Synthesis of multiple N-acylhomoserine lactones is wide-spread among the members of the Burkholderia cepacia complex. Syst. Appl. Microbiol. 24:1-14. [DOI] [PubMed] [Google Scholar]
- 24.Haussler, S., M. Nimtz, T. Domke, V. Wray, and I. Steinmetz. 1998. Purification and characterization of a cytotoxic exolipid of Burkholderia pseudomallei. Infect. Immun. 66:1588-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber, B., K. Riedel, M. Hentzer, A. Heydorn, A. Gotschlich, M. Givskov, S. Molin, and L. Eberl. 2001. The cep quorum-sensing system of Burkholderia cepacia H111 controls biofilm formation and swarming motility. Microbiology 147:2517-2528. [DOI] [PubMed] [Google Scholar]
- 26.Köhler, T., L. K. Curty, F. Barja, C. van Delden, and J. C. Pechère. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182:5990-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latifi, A., M. Foglino, K. Tanaka, P. Williams, and A. Lazdunski. 1996. A hierarchical quorum-sensing cascade in Pseudomonas aeruginosa links the transcriptional activators LasR and RhIR (VsmR) to expression of the stationary-phase sigma factor RpoS. Mol. Microbiol. 21:1137-1146. [DOI] [PubMed] [Google Scholar]
- 28.Latifi, A., M. K. Winson, M. Foglino, B. W. Bycroft, G. S. Stewart, A. Lazdunski, and P. Williams. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333-343. [DOI] [PubMed] [Google Scholar]
- 29.Lewenza, S., B. Conway, E. P. Greenberg, and P. A. Sokol. 1999. Quorum sensing in Burkholderia cepacia: identification of the LuxRI homologs CepRI. J. Bacteriol. 181:748-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewenza, S., and P. A. Sokol. 2001. Regulation of ornibactin biosynthesis and N-acyl-l-homoserine lactone production by CepR in Burkholderia cepacia. J. Bacteriol. 183:2212-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lutter, E., S. Lewenza, J. J. Dennis, M. B. Visser, and P. A. Sokol. 2001. Distribution of quorum-sensing genes in the Burkholderia cepacia complex. Infect. Immun. 69:4661-4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medina, G., K. Juarez, B. Valderrama, and G. Soberon-Chavez. 2003. Mechanism of Pseudomonas aeruginosa RhlR transcriptional regulation of the rhlAB promoter. J. Bacteriol. 185:5976-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochsner, U. A., and J. Reiser. 1995. Autoinducer-mediated regulation of rhamnolipid biosurfactant synthesis in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 92:6424-6428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179:5756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reimmann, C., N. Ginet, L. Michel, C. Keel, P. Michaux, V. Krishnapillai, M. Zala, K. Heurlier, K. Triandafillu, H. Harms, G. Defago, and D. Haas. 2002. Genetically programmed autoinducer destruction reduces virulence gene expression and swarming motility in Pseudomonas aeruginosa PAO1. Microbiology 148:923-932. [DOI] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 37.Schuster, M., C. P. Lostroh, T. Ogi, and E. P. Greenberg. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J. Bacteriol. 185:2066-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwyn, B., and J. B. Neilands. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47-56. [DOI] [PubMed] [Google Scholar]
- 39.Seed, P. C., L. Passador, and B. H. Iglewski. 1995. Activation of the Pseudomonas aeruginosa lasI gene by LasR and the Pseudomonas autoinducer PAI: an autoinduction regulatory hierarchy. J. Bacteriol. 177:654-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw, P. D., G. Ping, S. L. Daly, C. Cha, J. E. Cronan, Jr., K. L. Rinehart, and S. K. Farrand. 1997. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc. Natl. Acad. Sci. USA 94:6036-6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology 1:784-791. [Google Scholar]
- 42.Sokol, P. A., U. Sajjan, M. B. Visser, S. Gingues, J. Forstner, and C. Kooi. 2003. The CepIR quorum-sensing system contributes to the virulence of Burkholderia cenocepacia respiratory infections. Microbiology 149:3649-3658. [DOI] [PubMed] [Google Scholar]
- 43.Ulrich, R. L., and D. DeShazer. 2004. Type III secretion: a virulence factor delivery system essential for the pathogenicity of Burkholderia mallei. Infect. Immun. 72:1150-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner, V. E., D. Bushnell, L. Passador, A. I. Brooks, and B. H. Iglewski. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J. Bacteriol. 185:2080-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wuthiekanun, V., M. D. Smith, D. A. Dance, A. L. Walsh, T. L. Pitt, and N. J. White. 1996. Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. J. Med. Microbiol. 45:408-412. [DOI] [PubMed] [Google Scholar]