Abstract
Background
The two isoforms of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), 1 with a long cytoplasmic domain (CEACAM1-L) and 1 with a short (CEACAM1-S), are involved in different signaling pathways. β2-spectrin (β2SP) is an adaptor protein that plays critical roles in the proper control of Smad access to activate receptors involved in regulation of TGF-β signaling. In this study, we examined the association between CEACAM1 isoform balance and hepatocellular carcinoma (HCC) malignant potential and investigated the possibility of a molecular interaction between CEACAM1 and β2SP.
Methods
Immunohistochemical analysis was carried out with CEACAM1-L or CEACAM1-S antibodies on 154 HCC tissues to correlate with the factors of malignancy. Invasion assay was performed for the effect of CEACAM1 expression on HCC cell lines. Moreover, immunohistochemical analysis and immunoprecipitation analysis were performed to investigate the association between CEACAM1 isoform balance and β2SP.
Results
In immunohistochemical analysis, CEACAM1-L expression dominance was a risk factor for HCC recurrence (p = 0.04) and was significantly associated with a shorter survival compared with CEACAM1-S expression dominance. Invasion assay indicated that CEACAM1-4L-transfected HLF and PLC/PRF/5 cells showed significantly increased invasion (p<0.0001) and CEACAM1-4S-transfected HLF cells showed significantly decreased invasion. Immunohistochemical analysis of β2SP suggested that the HCCs with CEACAM1-L-dominant expression were more strongly stained with β2SP than the HCCs with CEACAM1-S-dominant expression (p = 0.013), and coprecipitation assays indicated that CEACAM1-L could bind to β2SP.
Conclusions
CEACAM1-L may enhance the HCC invasiveness through an interaction with β2SP and subsequent effects on TGF-β signaling.
Hepatocellular carcinoma (HCC) is common cancer in Southeast Asia, and it is the third leading cause of death from cancer in the world.1,2 Currently, the curative treatments for HCC consist of surgical resection, percutaneous ablation, and liver transplantation. However, long-term survival following these treatments remains unsatisfactory because of the high rate of recurrence and metastasis.2 A better understanding of the molecular mechanisms responsible for tumor malignancy and recurrence should lead to more effective treatments for HCC.
One possible therapeutic target is carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), which is a type-I transmembrane glycoprotein member of the carcinoembryonic antigen family and a member of the immunoglobulin superfamily.3,4 Expression of CEACAM1 is downregulated in the early-phase cancers of the liver, prostate, bladder, colon, lung, breast, and endometrium.5–11 Therefore, CEACAM1 may play an important role in preventing carcinogenesis. The loss of CEACAM1 expression in HCC indicates high metastatic potential and is associated with poor survival after hepatectomy.12 However, several studies have revealed that CEACAM1 expression is involved in malignant progression of colon cancer, gastric cancer, melanoma, and lung cancer.13–16
CEACAM1 has several isoforms that are produced by alternative mRNA splicing, which results in 1–4 Ig-like extracellular domains and either a long cytoplasmic tail (CEACAM1-L) or a short one (CEACAM1-S). The long cytoplasmic domain contains 2 immunoreceptor tyrosine-based inhibitory motifs that bind SH2-containing protein tyrosine phophatase-1 and phophatase-2.17 In contrast, the short cytoplasmic domain isoform is thought to have no such motifs, but binds to cytoskeletal proteins via Ser phosphorylated residues.18 We previously reported that CEACAM1-L expression in colorectal tumor cells indicates lymph node metastasis, hematogenous metastasis, and a short survival time, and that CEACAM1-L expression in colorectal cancer cell lines promotes invasion and migration.13 These facts suggest that the long and short cytoplasmic domain isoforms play divergent roles in different signaling pathways.
For example, the TGF-β signaling pathway may be affected by the CEACAM1 isoform balance. TGF-β signaling plays an important role in inducing the epithelial-mesenchymal transition, which plays a pivotal role in the dissemination of malignant hepatocytes and is a key process that drives intrahepatic metastasis.19–22 β2-spectrin (β2SP) is an adaptor protein that plays critical roles in the proper control of Smad access to activate receptors involved in regulation of TGF-β signaling.23,24
In this retrospective study, we investigated the association between CEACAM1 cytoplasmic isoform balance and HCC malignant potential, and we evaluated the effect of CEACAM1 isoform balance on invasion of HCC cells. In addition, we examined the possibility that a molecular interaction between CEACAM1 and β2SP affects TGF-β signaling.
MATERIALS AND METHODS
Patients
A total of 154 patients with HCC who underwent hepatectomy at Wakayama Medical University Hospital between April 1995 and March 2007 were enrolled in this study. Written informed consent was obtained from all patients, and the study was conducted in accordance with the protocol approved by the Declaration of Helsinki and the guidelines of the Ethics Review Committee of Wakayama Medical University Hospital.
Immunohistochemistry
The techniques have been described previously.13 The sections were incubated with monoclonal mouse antibodies against CEACAM1 (29H2; Abcam, Cambridge, MA) at a dilution of 1:300, with polyclonal rabbit antibodies against CEACAM1-L (generously provided by John E. Shively, Beckman Research Institute of the City of Hope, CA) at a dilution of 1:500, with polyclonal rabbit antibodies against CEACAM1-S (generously provided by J.E. Shively) at a dilution of 1:500, or with polyclonal rabbit antibodies against β2SP (generously provided by Lopa Mishra, MD Anderson Cancer Center, Houston, TX) at a dilution of 1:500.23–25 Negative controls were treated the same way without the primary antibodies. Adjacent non-neoplastic liver tissue was used as a positive control.
Evaluation of Immunohistochemical Staining
Expression status was evaluated by scoring both the intensity and the range of staining. The intensity of the staining was scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong); the range of the general staining intensity in the tissue was scored as 0 (0–5 %), 1 (6–33 %), 2 (34–66 %), or 3 (67–100 %).26 The ranges of staining and intensity scores were then added to obtain a total score. The sections were reviewed twice by two individuals, including a pathologist (S.Y. and Y.N.), who were blinded to the clinical parameters.
Cell Lines
There were three human HCC cell lines used in this study. HepG2 was obtained from the Cell Resource Center for Biomedical Research of the Institute of Development, Aging, and Cancer of Tohoku University (Miyagi, Japan). PLC/PRF/5 and HLF were obtained from the Japanese Collection of Research Bioresources cell bank (Osaka, Japan).
Cell Transfection, RNA Interference, and Clone Selection
The pHβApr-1-neo expression plasmids encoding either human CEACAM1-4L or CEACAM1-4S were transfected.27,28 The human CEACAM1-specific shRNA plasmid set was purchased from SABiosciences (SureSilencing shRNA plasmid for human CEACAM1; Frederick, MD). The design of shRNA plasmid and techniques have been described previously.13
RNA Isolation and RT-PCR
Total RNA was extracted with the RNeasy Mini Kit (Qiagen, Hilden, Germany) and then cDNA was synthesized using the Reverse Transcription System (Promega Co, Madison, WI). The primers designed to amplify CEACAM1 gene and techniques and primer design have been described previously.
Western Blot Analysis
The techniques have been described previously13. Immunoblotting experiments were performed with monoclonal mouse antibodies against CEACAM1 (T84.1) at a dilution of 1:2000, polyclonal rabbit antibodies against CEACAM1-L at a dilution of 1:2000, monoclonal mouse antibodies against β-actin (AC-15; Sigma-Aldrich, St. Louis, MO) at a dilution of 1:2500, or polyclonal rabbit antibodies against β2SP (ab72239; Abcam) at a dilution of 1:2000.
Growth Proliferation Assay
Proliferation assay was performed using BrdU Labeling and Detection Kit III (Roche, Mannheim, Germany), in accordance with the manufacturer’s instruction. Briefly, cells, HLF or PLC/PRF/5 cells transfected with empty vector, CEACAM1-4L, or CEACAM1-4S, were seeded in 96-well plates at a density of 15,000 cells per well with growth medium. After 72 h, they were incubated with BrdU labeling medium for 3 h at 37 °C in 5% CO2. Cells were ethanol-fixed for 30 min at −20 °C, washed, and incubated with anti-BrdU working solution for 30 min at 37 °C. After incubation with anti-mouse-immunoglobulin-fluorescein working solution for 30 min at 37 °C, cells were washed and examined by microplate reader (SH-9000, Corona Electric, Ibaraki, Japan). Results were expressed as mean ± SD values. Each measurement was performed in quadruplicate.
Cell Death Detection Assay
Cell Death Detection ElisaPlus kit (Roche) was used to measure DNA fragmentation as a marker for apoptosis according to the manufacturer’s instructions. Cells, HLF or PLC/PRF/5 cells transfected with empty vector, CEACAM1-4L, or CEACAM1-4S, were seeded in 96-well plates at a density of 5000 cells per well. After 24 h, the medium was replaced with serum-free medium and incubated for an additional 48 h at 37 °C in 5 % CO2. Cytoplasmic fractions of HCC cells were transferred into streptavidin-coated 96-well plates, incubated with biotinylated mouse antihistone antibody and peroxidase-conjugated mouse anti-DNA antibody at room temperature for 2 h, and examined by microplate reader (SH-9000). Results were expressed as mean ± SD values. Each measurement was performed in quadruplicate.
Invasion Assay
Invasion assay was measured using Biocoat Matrigel invasion chambers (BD Biosciences). The techniques have been described previously.13 Each measurement was performed in quadruplicate. The percentage invasion was calculated as the ratio of the number of cells invading through the Matrigel membrane to the number of cells migrating through the control membrane.
Immunoprecipitation Analysis
Cells were harvested and homogenized in whole-cell lysis buffer (Active Motif, Carlsbad, CA), and the cell lysates were clarified by centrifugation. Equal amounts of cell lysates (250 μg) were incubated for 4 h at 4 °C with monoclonal mouse antibodies against CEACAM1 (1 μg) (GM8G5; Santa Cruz Biotechnology, Inc., CA) or with polyclonal rabbit antibodies against β2SP (1 μg) (ab72239; Abcam). Protein G Magnetic Beads (25 μL, Active Motif) were added, and incubation was continued for 1 hour. After centrifugation, the pellet was rinsed four times with complete Co-IP/wash buffer (Active Motif).
Immunofluorescence Assay
For the immunofluorescence assay, cells were fixed with 4 % formaldehyde solution in PBS for 45 min. After washing with PBS, cells were blocked in PBS containing 3 % bovine serum albumin for 30 min at room temperature and subsequently incubated with a rabbit polyclonal antibody against Smad3 (51–1500; Invitrogen, Carlsbad, CA) at a dilution of 1:100 for 1 h at room temperature. Cells were washed to remove primary antibody, followed by incubation with the secondary goat anti-rabbit antibody conjugated with Alexa Fluor-488 (A-11008; Invitrogen) at a dilution of 1:500 for 1 h at room temperature. After incubation, cells were washed and mounted.
Statistical Analysis
We corrected for continuity by means of Child-Pugh score, UICC-TNM classification, or each median value of age and laboratory data.29,30 To measure background features, we analyzed tumor characteristics and laboratory data using the Fisher exact test and the Mann–Whitney U test. Survival rates were analyzed by means of the Kaplan–Meier method and the log-rank test. Risk factors for recurrence were analyzed using the Cox proportional hazards model. In vitro data for more than 3 groups were analyzed by the Bonferroni test. All analyses were performed with Statview software (ver. 5.0; SAS Institute Inc., Cary, NC). Statistical significance was defined as p < 0.05.
RESULTS
Relationship Between CEACAM1 Cytoplasmic Domain Isoform Balance and Malignant Potential and Poor Survival of HCC Patients After Hepatectomy
Among 154 patients, 131 (85.1 %) exhibited CEACAM1-positive staining of the HCCs. Immunohistochemical analyses with antibodies specific to CEACAM1-L and CEACAM1-S were performed for the 131 CEACAM1-positive samples. CEACAM1, CEACAM1-L, and CEACAM1-S were stained at the bile canaliculi of trabecular and the apical portion of pseudoglandular (Fig. 1a). We evaluated expression status by scoring both the intensity and the range of CEACAM1 staining (Fig. 1b). CEACAM1-L-dominant expression was observed in 67 patients (44 %), and CEACAM1-S-dominant expression was observed in 61 patients (40 %). There were three patients who had the same score for CEACAM1-L and CEACAM1-S. Comparison of clinicopathologic variables between the CEACAM1-L-dominant and CEACAM1-S-dominant expression groups indicated that CEACAM1-L-dominant expression was significantly correlated with multiple HCCs (p = 0.0005), the presence of capsule infiltration (p = 0.01), and the presence of vascular invasion (p = 0.03) (Table 1).
FIG. 1.

Immunohistochemical staining of CEACAM1 in hepatocellular carcinoma tissue and survival analyses in patients with hepatocellular carcinoma after hepatectomy in relation to CEACAM1 cytoplasmic domain isoform balance. a Staining pattern of CEACAM1, CEACAM1-L, or CEACAM1-S at the bile canaliculi of trabecular and the apical portion of pseudoglandular. b Immunohistochemical staining intensity of CEACAM1 (×400 magnification): strong, moderate, weak. c Recurrence-free survival rate of the CEACAM1-L-dominant and CEACAM1-S-dominant expression groups. d Disease-specific survival rate of the CEACAM1-L-dominant and CEACAM1-S-dominant expression groups
TABLE 1.
Clinicopathologic variables for CEACAM1-L-dominant and CEACAM1-S-dominant expression groups
| Variable | CEACAM1-L-dominant expression | CEACAM1-S-dominant expression | p valuea |
|---|---|---|---|
| Gender, n (male/female) | 49/18 | 44/17 | >0.99 |
| Age (mean ± SD, year) | 65.8 ± 10.1 | 66.9 ± 8.96 | 0.68 |
| Hepatitis B surface antigen, n (positive/negative) | 12/55 | 8/53 | 0.48 |
| Hepatitis C virus antibody, n (positive/negative) | 45/22 | 32/29 | 0.11 |
| Alcohol abuse (≥50 g daily), n (presence/absence) | 11/56 | 18/43 | 0.09 |
| Serum albumin (mean ± SD, g/dL) | 3.87 ± 0.525 | 3.95 ± 0.470 | 0.23 |
| Serum bilirubin (mean ± SD, mg/dL) | 0.899 ± 0.394 | 0.844 ± 0.347 | 0.39 |
| Prothrombin activity (mean ± SD, %) | 84.0 ± 13.8 | 84.9 ± 13.0 | 0.93 |
| Serum aspartate aminotransferase (AST) (mean ± SD, IU/L) | 56.6 ± 27.4 | 56.2 ± 37.1 | 0.56 |
| Serum alanine aminotransferase (ALT) (mean ± SD, IU/L) | 55.9 ± 31.2 | 60.2 ± 47.7 | 0.80 |
| Hepaplastin-test (HPT) (mean ± SD, %) | 88.9 ± 17.5 | 91.8 ± 17.6 | 0.42 |
| Platelet count (mean ± SD, ×104/μL) | 15.6 ± 7.32 | 16.6 ± 5.97 | 0.21 |
| Child-Pugh classification, n (class A/class B) | 57/10 | 58/3 | 0.08 |
| Number of tumorsb, n (single/multiple) | 38/29 | 52/9 | 0.0005 |
| Maximum tumor sizeb (mean ± SD, cm) | 4.93 ± 3.73 | 4.57 ± 3.08 | 0.54 |
| Growth patternc, n (expansive/invasive) | 58/9 | 58/7 | 0.79 |
| Fibrous capsule formationc, n (presence/absence) | 50/17 | 52/13 | 0.54 |
| Capsule infiltrationc,d, n (absence/presence) | 16/34 | 30/22 | 0.01 |
| Septum formationc, n (presence/absence) | 49/18 | 43/22 | 0.45 |
| Serosa infiltrationc, n (absence/presence) | 48/19 | 56/9 | 0.06 |
| Vascular invasionc, n (absence/presence) | 37/30 | 48/17 | 0.03 |
| Edmondson–Steiner classification, n (I plus II/III plus IV) | 56/11 | 56/5 | 0.19 |
| Fibrosis staging, n (F0–F3/F4) | 34/33 | 31/30 | >0.99 |
| Hepatitis activity grading, n (A0 plus A1/A2 plus A3) | 14/53 | 19/42 | 0.23 |
n number of patients, SD standard deviation, IU international unit
Fisher exact test or Mann–Whitney U test
Evaluated on image-findings
Evaluated on pathological findings
102 HCCs had capsules
Univariate analysis revealed that multiple HCCs, a maximum tumor size of ≥5 cm, the presence of serosa infiltration, the presence of vascular invasion, and CEACAM1-L-dominant expression were significant predictive factors for recurrence (Table 2). On the basis of multivariate analysis, multiple HCCs (HR, 2.45; 95 % CI 1.59–3.78), maximum tumor size of ≥5 cm (HR 1.70; 95 % CI 1.13–2.55), and CEACAM1-L-dominant expression (HR 1.49; 95 % CI 1.01–2.19) were selected as the independent predictive factors for recurrence.
TABLE 2.
Univariate and multivariate analyses of recurrence-free survival in 154 patients
| Variable | Univariate analysis
|
Multivariate analysis
|
||||
|---|---|---|---|---|---|---|
| HR | 95 % CI | p valuea | HR | 95 % CI | p valuea | |
| Gender male | 0.87 | 0.57–1.31 | 0.50 | |||
| Age ≥66 years | 1.06 | 0.74–1.53 | 0.74 | |||
| Positive for hepatitis B surface antigen | 1.02 | 0.70–1.48 | 0.93 | |||
| Positive for hepatitis C virus antibody | 1.15 | 0.80–1.67 | 0.45 | |||
| Presence of alcohol abuse (≥50 g daily) | 0.71 | 0.46–1.09 | 0.12 | |||
| Child-Pugh classification (class B) | 1.54 | 0.86–2.74 | 0.15 | |||
| Serum aspartate aminotransferase (AST) ≥51 IU/L | 1.16 | 0.81–1.67 | 0.41 | |||
| Serum alanine aminotransferase (ALT) ≥47 IU/L | 0.93 | 0.65–1.34 | 0.70 | |||
| Hepaplastin test (HPT) ≥88.8 % | 0.90 | 0.63–1.30 | 0.59 | |||
| Platelet count <15 × 104/μL | 1.24 | 0.86–1.79 | 0.24 | |||
| Multiple tumorsb | 3.14 | 2.13–4.62 | <0.0001 | 2.45 | 1.59–3.78 | <0.0001 |
| Maximum tumor sizeb ≥5 cm | 1.92 | 1.33–2.78 | 0.0005 | 1.70 | 1.13–2.55 | 0.01 |
| Invasive growth of tumorc | 1.06 | 0.61–1.82 | 0.85 | |||
| Absence of fibrous capsule formationc | 1.00 | 0.67–1.50 | 0.99 | |||
| Presence of capsule infiltrationc,d | 1.30 | 0.84–2.01 | 0.24 | |||
| Absence of septum formationc | 1.27 | 0.85–1.89 | 0.25 | |||
| Presence of serosa infiltrationc | 1.71 | 1.12–2.62 | 0.01 | 1.01 | 0.63–1.62 | 0.97 |
| Presence of vascular invasionc | 1.91 | 1.32–2.75 | 0.0005 | 1.28 | 0.85–1.92 | 0.24 |
| Edmondson-Steiner classification (III or IV) | 1.55 | 0.98–2.45 | 0.060 | |||
| Fibrosis staging (F4) | 1.43 | 0.99–2.06 | 0.057 | |||
| Hepatitis activity grading (A2 or A3) | 0.98 | 0.65–1.47 | 0.91 | |||
| CEACAM1-L-dominant expression | 1.59 | 1.10–2.30 | 0.01 | 1.49 | 1.01–2.19 | 0.04 |
| Loss of CEACAM1 expression | 1.36 | 0.81–2.28 | 0.24 | |||
HR hazard ratio, 95 % CI 95 % confidence interval, IU international unit
Cox proportional hazard test
Evaluated on image-findings
Evaluated on pathological findings
102 HCCs had capsules
The period of recurrence-free survival after hepatectomy was significantly shorter in patients with CEACAM1-L-dominant expression than in those with CEACAM1-S-dominant expression (Fig. 1c). Moreover, the period of disease-specific survival was also significantly shorter in patients with CEACAM1-L-dominant expression (Fig. 1d).
CEACAM1 Cytoplasmic Isoform and Proliferation and Apoptosis of HCC Cells
To determine whether CEACAM1 isoform expression affects proliferation and apoptosis of HCC cells, we performed growth proliferation assay and cell death detection assay for CEACAM1-4L or CEACAM1-4S transfected HLF or PLC/PRF/5 cells. There is no difference among them. CEACAM1 cytoplasmic isoform expression did not have any effect on HCC cells proliferation. On the other hand, overexpression of CEACAM1-4L significantly suppressed apoptosis in HCC cell line HLF (p = 0.029) and PLC/PRF/5 (p = 0.043) (Supplemental Fig. 1).
CEACAM1 Cytoplasmic Isoform and Invasion of HCC Cells
We examined the expression levels of CEACAM1 transcript and protein expression in HCC cell lines. CEACAM1 transcripts of all HCC cell lines were detected by RT-PCR analyses; however, CEACAM1 protein was only detected in HepG2 cells. The protein levels of other two cell lines were probably below thresholds of detections, although transcripts were present. To investigate the effect of CEACAM1-4L or CEACAM1-4S overexpression on HCC cell invasion, we selected loss-of-CEACAM1-expression cell lines HLF and PLC/PRF/5, which exhibit high and low invasiveness, respectively. HLF and PLC/PRF/5 cells stably transfected with CEACAM1-4L or CEACAM1-4S showed elevated CEACAM1-4L and CEACAM1-4S protein expression compared with vector-transfected cells (Fig. 2a, c). CEACAM1-4L overexpression significantly promoted invasion of HLF cells and PLC/PRF/5 cells and enhanced expression of CEACAM1-4S inhibited invasion of HLF cells (Fig. 2b, d, p < 0.0001). To investigate the effect of inhibition of CEACAM1 expression on invasion, we recruited HepG2 cells expressing CEACAM1. Stable CEACAM1 shRNA inhibited CEACAM1 protein expression compared with the vector control (Fig. 2e). Invasion chamber experiments showed that CEACAM1 shRNA significantly suppressed invasion of HepG2 cells (Fig. 2f, p = 0.01).
FIG. 2.

CEACAM1 cytoplasmic isoform balance modulation of hepatocellular carcinoma cell invasion. a RT-PCR analysis and western blot analysis with cell lysates from parental HLF cell (Lane 1) and HLF cells transfected with empty vector (Lane 2), CEACAM1-4L (Lane 3), or CEACAM1-4S (Lane 4). b In vitro invasion assay using Matrigel invasion chambers with parental HLF cells (Lane 1) and HLF cells transfected with empty vector (Lane 2), CEACAM1-4L (Lane 3), or CEACAM1-4S (Lane 4). c RT-PCR analysis and western blot analysis performed with cell lysates from parental PLC/PRF/5 cells (Lane 1) and PLC/PRF/5 cells transfected with empty vector (Lane 2), CEACAM1-4L (Lane 3), or CEACAM1-4S (Lane 4). d In vitro invasion assay using Matrigel invasion chambers with parental PLC/PRF/5 cells (Lane 1) and PLC/PRF/5 cells transfected with empty vector (Lane 2), CEACAM1-4L (Lane 3), or CEACAM1-4S (Lane 4). e RT-PCR analysis and western blot analysis performed with cell lysates from parental HepG2 cells (Lane 1) and HepG2 cells transfected with negative control RNA (Lane 2) or shRNA targeting CEACAM1 (Lane 3). f In vitro invasion assay using Matrigel invasion chambers with parental HepG2 cells (Lane 1) and HepG2 cells transfected with negative control RNA (Lane 2) or shRNA targeting CEACAM1 (Lane 3)
CEACAM1 Cytoplasmic Isoform Expression and β2SP Expression
CEACAM1 binds to actin, as does β2SP, which is involved in the TGF-β signaling pathway.18,31–33 A molecular interaction between CEACAM1 and β2SP may modulate the invasiveness of HCC cells. To determine whether there was any correlation between CEACAM1 and β2SP expression, we performed immunochemical staining of HCC clinical samples from CEACAM1-L-dominant and CEACAM1-S-dominant expression groups with β2SP antibodies. Expression status was evaluated by scoring both the intensity and the range of β2SP staining. The median β2SP staining score was 5 (mean 4.43; range 0–6; SD 1.26), and these 128 patients were divided into 2 groups according to the median score: 70 patients had β2SP staining scores of ≥5, and 58 patients had β2SP staining scores of <5. Statistical analysis indicated that the HCCs with CEACAM1-L-dominant expression were more strongly stained for β2SP than the HCCs with CEACAM1-S-dominant expression (p = 0.013).
CEACAM1-L Cytoplasmic Isoform Binding with β2SP
To determine whether the CEACAM1 isoforms could bind to β2SP, we performed coprecipitation assays. HLF and PLC/PRF/5 cells lysates were initially immunoprecipitated with β2SP antibodies and then subsequently western blotted with CEACAM1 antibodies. Large amounts of β2SP and CEACAM1 complexes were detected in the CEACAM1-4L-overexpressing HLF and PLC/PRF/5 cells (Fig. 3A, B). Conversely, when HLF and PLC/PRF/5 cells lysates were immunoprecipitated with CEACAM1 antibodies and then western blotted with β2SP antibodies, large amounts of CEACAM1 and β2SP complexes were detected in CEACAM1-4L-transfected HLF and PLC/PRF/5 cells (Fig. 3C, D). CEACAM1-4S and β2SP were coprecipitated in HLF cells (Fig. 3A, C); however, it was weaker than coprecipitation of CEACAM1-4L and β2SP.
FIG. 3.
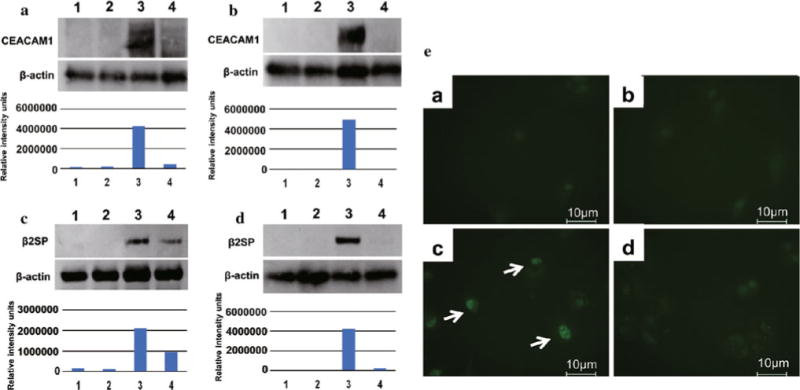
Coimmunoprecipitation analysis of interaction between CEACAM1 and β2SP, and nuclear translocation of Smad3. Immunoprecipitated with anti-β2SP and immunoblotted with anti-CEACAM1. A HLF (Lane 1) and HLF transfected with empty vector (Lane 2), CEACAM1-4L (Lane 3) or CEACAM1-4S (Lane 4). B PLC/PRF/5 (Lane 1) and PLC/PRF/5 transfected with empty vector (Lane 2), CEACAM1-4L (Lane 3) or CEACAM1-4S (Lane 4). Immunoprecipitated with anti-CEACAM1 and immunoblotted with anti-β2SP. C HLF (Lane 1) and HLF transfected with empty vector (Lane 2), CEACAM1-4L (Lane 3) or CEACAM1-4S (Lane 4). D PLC/PRF/5 (Lane 1) and PLC/PRF/5 transfected with empty vector (Lane 2), CEACAM1-4L (Lane 3) or CEACAM1-4S (Lane 4). E Immunofluorescence staining of Smad3 in PLC/PRF/5 cells. PLC/PRF/5 (a) and PLC/PRF/5 transfected with empty vector (b), CEACAM1-4L (c), or CEACAM1-4S (d)
CEACAM1-L Cytoplasmic Isoform and TGF-β/Smad Signaling Pathway
To determine whether there was any correlation between CEACAM1 and TGF-β/Smad3 signaling pathway, we performed immunofluorescent staining with Smad3 antibodies of HCC cell lines transfected with empty vector, CEACAM1-4L, or CEACAM1-4S. In invasion assay, PLC/PRF/5 cell transfected with CEACAM1-4L exhibited more enhanced invasiveness than HLF cells; therefore, we examined immunofluorescent staining assay with PLC/PRF/5 cells. In immunofluorescent staining with Smad3, the nucleus of PLC/PRF/5 cell with CEACAM1-4L (Fig. 3E-c) stained more strongly than parental PLC/PRF/5 cell (Fig. 3E-a) and PLC/PRF/5 cell transfected with empty vector (Fig. 3E-b), or CEACAM1-4S (Fig. 3E-d).
DISCUSSION
CEACAM1 expression is downregulated in HCC, and this protein is thought to function as a tumor suppressor.5,12,34,35 However, in this study, we observed that 131 of 154 patients diagnosed with HCC with tumors of 4.5–5 cm expressed CEACAM1 and that the incidence of HCCs with loss of CEACAM1 expression was low. These results suggest that CEACAM1 does not function as a tumor suppressor in advanced HCC. We analyzed the relationship between the CEACAM1 isoform balance and clinicopathological factors and found that CEACAM1-L-dominant expression was significantly correlated with multiple HCCs, capsule infiltration, and vascular invasion. These results suggest CEACAM1-L is involved in HCC cell invasion and metastasis. Multivariate analysis demonstrated that multiple HCCs, maximum tumor size of ≥5 cm, and CEACAM1-L-dominant expression were independent predictive factor for recurrence, indicating that CEACAM1-L-dominant expression in HCC tissues is indicative of the malignant potential.
Kaplan–Meier analysis of recurrence-free survival showed that CEACAM1-L-dominant expression was associated with HCC recurrence. Postoperative HCC recurrence pattern is thought to occur by two pathways: intrahepatic metastasis and multicentric carcinogenesis resulting from chronic hepatitis. Some reports have shown that early recurrence arises mainly from intrahepatic metastases, whereas late recurrence is more likely to be multicentric, based on a cutoff of 2 years after surgery.36,37 According to our analysis, the recurrence rate in patient with CEACAM1-L-dominant expression was more significant more than 2 years after hepatectomy than within 2 more years hepatectomy (p = 0.0006 and 0.002, respectively). This result suggests CEACAM1-L-dominant expression is associated with intrahepatic metastasis recurrence by means of tumor-related factors such as cancer cell invasion. Therefore, we investigated the effect of CEACAM1 cytoplasmic domain isoform balance on cancer cell invasion. The long cytoplasmic domain contains 2 immunoreceptor tyrosine-based inhibitory motifs that can be phosphorylated by Src kinases and can bind SH2-containing protein tyrosine phosphatase-1 and phosphatase-2.17,38 In contrast, CEACAM1-S lacks these tyrosine residues. The roles of CEACAM1 in tumor invasion were not clear, but it is may be presumed that the isoform balance would have an important role in cancer tumorigenesis and invasion because of the differences between the cytoplasmic domain isoforms. We have previously reported CEACAM1-L-dominant expression in colorectal cancer is correlated with metastasis to lymph nodes and blood vessels, and it involved poor survival rate; furthermore, we showed that CEACAM1 induces hollow spheroid formation, which is correlated with colorectal cancer metastasis beyond the invasion front.13,39 In this study, we found overexpression of CEACAM1-4L suppressed apoptosis and promoted HCC cell invasion, whereas CEACAM1-4S suppressed invasion. These results suggest CEACAM1 isoform balance plays a crucial role in cancer cell survival and invasion.
The mechanism by which CEACAM1 isoform balance modulates invasion remains to be investigated. Here, we focused on the expression of β2SP, which is involved in TGF-β signaling. β2SP is a Smad adaptor protein and plays an important role in the localization and signaling of Smads, leading to the tumor-suppressing functions of the TGF-β pathway.25,40 β2SP haploinsufficiency mice develop HCCs, and 11 % of human HCC cells express a splice site mutation in β2SP.41,42 In addition, β2SP inhibits the epithelial-mesenchymal transition.43 Our data showed that CEACAM1-L-dominant expression in clinical samples of HCCs were more correlated with β2SP expression than CEACAM1-S-dominant expression. In our in vitro data, CEACAM1-4L can bind to β2SP, and CEACAM1-4L expression promotes nuclear translocation of Smad3, suggesting that CEACAM1-4L is able to modulate TGF-β/Smad signaling pathway. It remains to be investigated why overexpression of β2SP in HCCs promotes invasion of HCC cells. Further studies are needed for clarification of the mechanism by which CEACAM1 enhances invasiveness.
In conclusion, CEACAM1-L isoform expression dominance is associated with invasion, metastasis, and recurrence in HCC. One of the mechanisms by which CEACAM1-L enhances the invasiveness of HCC cells may involve interaction with β2SP and subsequent effects on TGF-β signaling.
Supplementary Material
Acknowledgments
Grant-in-Aid No. 20591554 and 23591904 from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and Takeda Science Foundation, Japan.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1245/s10434-013-3460-1) contains supplementary material, which is available to authorized users.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Llovet JM, Beaugrand M. Hepatocellular carcinoma: present status and future prospects. J Hepatol. 2003;38(Suppl 1):S136–49. doi: 10.1016/s0168-8278(02)00432-4. [DOI] [PubMed] [Google Scholar]
- 3.Svenberg T. Carcinoembryonic antigen-like substances of human bile. Isolation and partial characterization. Int J Cancer. 1976;17:588–96. doi: 10.1002/ijc.2910170506. [DOI] [PubMed] [Google Scholar]
- 4.Williams AF, Barclay AN. The immunoglobulin superfamily—domains for cell surface recognition. Annu Rev Immunol. 1988;6:381–405. doi: 10.1146/annurev.iy.06.040188.002121. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka K, Hinoda Y, Takahashi H, Sakamoto H, Nakajima Y, Imai K. Decreased expression of biliary glycoprotein in hepatocellular carcinomas. Int J Cancer. 1997;74:15–9. doi: 10.1002/(sici)1097-0215(19970220)74:1<15::aid-ijc3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Busch C, Hanssen TA, Wagener C, OBrink B. Down-regulation of CEACAM1 in human prostate cancer: correlation with loss of cell polarity, increased proliferation rate, and Gleason grade 3 to 4 transition. Hum Pathol. 2002;33:290–8. doi: 10.1053/hupa.2002.32218. [DOI] [PubMed] [Google Scholar]
- 7.Kleinerman DI, Dinney CP, Zhang WW, Lin SH, Van NT, Hsieh JT. Suppression of human bladder cancer growth by increased expression of C-CAM1 gene in an orthotopic model. Cancer Res. 1996;56:3431–5. [PubMed] [Google Scholar]
- 8.Neumaier M, Paululat S, Chan A, Matthaes P, Wagener C. Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc Natl Acad Sci USA. 1993;90:10744–8. doi: 10.1073/pnas.90.22.10744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohwada A, Takahashi H, Nagaoka I, Kira S. Biliary glycoprotein mRNA expression is increased in primary lung cancer, especially in squamous cell carcinoma. Am J Respir Cell Mol Biol. 1994;11:214–20. doi: 10.1165/ajrcmb.11.2.8049082. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Simpson JF, Glackin C, Riethorf L, Wagener C, Shively JE. Expression of biliary glycoprotein (CD66a) in normal and malignant breast epithelial cells. Anticancer Res. 1998;18:3203–12. [PubMed] [Google Scholar]
- 11.Bamberger AM, Riethdorf L, Nollau P, Naumann M, Erdmann I, Götze J, et al. Dysregulated expression of CD66a (BGP, C-CAM), an adhesion molecule of the CEA family, in endometrial cancer. Am J Pathol. 1998;152:1401–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Cruz PV, Wakai T, Shirai Y, Yokoyama N, Hatakeyama K. Loss of carcinoembryonic antigen-related cell adhesion molecule 1 expression is an adverse prognostic factor in hepatocellular carcinoma. Cancer. 2005;104:354–60. doi: 10.1002/cncr.21159. [DOI] [PubMed] [Google Scholar]
- 13.Ieda J, Yokoyama S, Tamura K, Takifuji K, Hotta T, Matsuda K, et al. Re-expression of CEACAM1 long cytoplasmic domain isoform is associated with invasion and migration of colorectal cancer. Int J Cancer. 2011;129:1351–61. doi: 10.1002/ijc.26072. [DOI] [PubMed] [Google Scholar]
- 14.Kinugasa T, Kuroki M, Takeo H, Matsuo Y, Ohshima K, Ya-mashita Y, et al. Expression of four CEA family antigens (CEA, NCA, BGP and CGM2) in normal and cancerous gastric epithelial cells: up-regulation of BGP and CGM2 in carcinomas. Int J Cancer. 1998;76:148–53. doi: 10.1002/(sici)1097-0215(19980330)76:1<148::aid-ijc23>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Brummer J, Ebrahimnejad A, Flayeh R, Schumacher U, Löning T, Bamberger AM, et al. cis Interaction of the cell adhesion molecule CEACAM1 with integrin beta(3) Am J Pathol. 2001;159:537–46. doi: 10.1016/s0002-9440(10)61725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sienel W, Dango S, Woelfle U, Morresi-Hauf A, Wagener C, Brummer J, et al. Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 promotes progression of non-small cell lung cancer. Clin Cancer Res. 2003;9:2260–6. [PubMed] [Google Scholar]
- 17.Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, et al. The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J Biol Chem. 1999;274:335–44. doi: 10.1074/jbc.274.1.335. [DOI] [PubMed] [Google Scholar]
- 18.Schumann D, Chen CJ, Kaplan B, Shively JE. Carcinoembryonic antigen cell adhesion molecule 1 directly associates with cytoskeleton proteins actin and tropomyosin. J Biol Chem. 2001;276:47421–33. doi: 10.1074/jbc.M109110200. [DOI] [PubMed] [Google Scholar]
- 19.Kondo M, Cubillo E, Tobiume K, Shirakihara T, Fukuda N, Suzuki H, et al. A role for Id in the regulation of TGF-beta-induced epithelial-mesenchymal transdifferentiation. Cell Death Differ. 2004;11:1092–101. doi: 10.1038/sj.cdd.4401467. [DOI] [PubMed] [Google Scholar]
- 20.Gotzmann J, Mikula M, Eger A, Schulte-Hermann R, Foisner R, Beug H, et al. Molecular aspects of epithelial cell plasticity: implications for local tumor invasion and metastasis. Mutat Res. 2004;566:9–20. doi: 10.1016/s1383-5742(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 21.van Zijl F, Zulehner G, Petz M, Schneller D, Kornauth C, Hau M, et al. Epithelial–mesenchymal transition in hepatocellular carcinoma. Future Oncol. 2009;5:1169–79. doi: 10.2217/fon.09.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–73. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 23.Baek HJ, Lim SC, Kitisin K, Jogunoori W, Tang Y, Marshall MB, et al. Hepatocellular cancer arises from loss of transforming growth factor beta signaling adaptor protein embryonic liver fodrin through abnormal angiogenesis. Hepatology. 2008;48:1128–37. doi: 10.1002/hep.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baek HJ, Pishvaian MJ, Tang Y, Kim TH, Yang S, Zouhairi ME, et al. Transforming growth factor-beta adaptor, beta2-spectrin, modulates cyclin dependent kinase 4 to reduce development of hepatocellular cancer. Hepatology. 2011;53:1676–84. doi: 10.1002/hep.24128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mishra L, Cai T, Yu P, Monga SP, Mishra B. Elf3 encodes a novel 200-kD beta-spectrin: role in liver development. Oncogene. 1999;18:353–64. doi: 10.1038/sj.onc.1202313. [DOI] [PubMed] [Google Scholar]
- 26.Lee SY, Han HS, Lee KY, Hwang TS, Kim JH, Sung IK, et al. Sonic hedgehog expression in gastric cancer and gastric adenoma. Oncol Rep. 2007;17:1051–5. [PubMed] [Google Scholar]
- 27.Gunning P, Leavitt J, Muscat G, Ng SY, Kedes L. A human beta-actin expression vector system directs high-level accumulation of antisense transcripts. Proc Natl Acad Sci USA. 1987;84:4831–5. doi: 10.1073/pnas.84.14.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang J, Hardy JD, Sun Y, Shively JE. Essential role of biliary glycoprotein (CD66a) in morphogenesis of the human mammary epithelial cell line MCF10F. J Cell Sci. 1999;112:4193–205. doi: 10.1242/jcs.112.23.4193. [DOI] [PubMed] [Google Scholar]
- 29.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–9. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 30.Sobin LH, Wittekind C. TNM Classification of Malignant Tumors. 6. New York: Wiley; 2002. [Google Scholar]
- 31.Sadekova S, Lamarche-Vane N, Li X, Beauchemin N. The CEACAM1-L glycoprotein associates with the actin cytoskeleton and localizes to cell–cell contact through activation of Rho-like GTPases. Mol Biol Cell. 2000;11:65–77. doi: 10.1091/mbc.11.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Discher D, Parra M, Conboy JG, Mohandas N. Mechanochemistry of the alternatively spliced spectrin–actin binding domain in membrane skeletal protein 4.1. J Biol Chem. 1993;268:7186–95. [PubMed] [Google Scholar]
- 33.Discher DE, Winardi R, Schischmanoff PO, Parra M, Conboy JG, Mohandas N. Mechanochemistry of protein 4.1’s spectrin–actin-binding domain: ternary complex interactions, membrane binding, network integration, structural strengthening. J Cell Biol. 1995;130:897–907. doi: 10.1083/jcb.130.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hixson DC, Allison JP, Chesner JE, Leger MJ, Ridge LL, Walborg EF., Jr Characterization of a family of glycoproteins associated with the bile canalicular membrane of normal hepatocytes but not expressed by two transplantable rat hepatocellular carcinomas. Cancer Res. 1983;43:3874–84. [PubMed] [Google Scholar]
- 35.Borscheri N, Roessner A, Rocken C. Canalicular immunostaining of neprilysin (CD10) as a diagnostic marker for hepatocellular carcinomas. Am J Surg Pathol. 2001;25:1297–303. doi: 10.1097/00000478-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 36.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–7. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 37.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–35. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brummer J, Neumaier M, Gopfert C, Wagener C. Association of pp60c-src with biliary glycoprotein (CD66a), an adhesion molecule of the carcinoembryonic antigen family downregulated in colorectal carcinomas. Oncogene. 1995;11:1649–55. [PubMed] [Google Scholar]
- 39.Tamura K, Yokoyama S, Ieda J, Takifuji K, Hotta T, Matsuda K, et al. Hollow spheroids beyond the invasive margin indicate the malignant potential of colorectal cancer. BMJ Open. 2011;1:e000179. doi: 10.1136/bmjopen-2011-000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang Y, Katuri V, Dillner A, Mishra B, Deng CX, Mishra L. Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science. 2003;299:574–7. doi: 10.1126/science.1075994. [DOI] [PubMed] [Google Scholar]
- 41.Katuri V, Tang Y, Li C, Jogunoori W, Deng CX, Rashid A, et al. Critical interactions between TGF-beta signaling/ELF, and E-cadherin/beta-catenin mediated tumor suppression. Oncogene. 2006;25:1871–86. doi: 10.1038/sj.onc.1209211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao ZX, Jogunoori W, Choufani S, Rashid A, Blake T, Yao W, et al. Epigenetic silencing of beta-spectrin, a TGF-beta signaling/scaffolding protein in a human cancer stem cell disorder: Beck-with-Wiedemann syndrome. J Biol Chem. 2010;285:36112–20. doi: 10.1074/jbc.M110.162347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Y, Katuri V, Srinivasan R, Fogt F, Redman R, Anand G, et al. Transforming growth factor-beta suppresses nonmetastatic colon cancer through Smad4 and adaptor protein ELF at an early stage of tumorigenesis. Cancer Res. 2005;65:4228–37. doi: 10.1158/0008-5472.CAN-04-4585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


