Abstract
Introduction:
We compared short-term outcomes and costs between robotic-assisted nephroureterectomy (RANU) and laparoscopic radical nephroureterectomy (LNU) in a large population-based cohort of patients with upper-tract urothelial carcinoma (UTUC).
Methods:
Overall, 1914 patients with UTUC treated with RANU or LNU between 2008 and 2010 within the Nationwide Inpatient Sample were abstracted. Propensity-score matching was performed to account for inherent differences between patients undergoing RANU and LNU. Multivariable logistic regression models were fitted to compare postoperative complications, blood transfusions, prolonged length of stay, and costs between the 2 procedures.
Results:
Overall, a weighted estimate of 1199 (62.6%) and 715 (37.4%) patients received LNU and RANU, respectively. In multivariable analyses no significant differences were observed in postoperative transfusion and length of stay between the 2 surgical approaches (all p > 0.1). However, patients undergoing RANU were less likely to experience any complications compared to their counterparts undergoing LNU (p = 0.04). The utilization of RANU was associated with substantially higher costs compared to the laparoscopic approach. Our study is limited by its retrospective nature and the lack of adjustment for tumour stage and grade.
Conclusions:
Our results support the safety and feasibility of RANU for the treatment of UTUC. Indeed, the use of the robotic approach was associated with lower probability of experiencing perioperative complications compared to LNU. On the other hand, the utilization of RANU is associated with higher costs compared to LNU.
Introduction
Upper-tract urothelial carcinoma (UTUC) accounts for about 5% of all urothelial carcinomas.1 According to treatment guidelines, radical nephroureterectomy (RNU) represents the treatment of choice for localized disease.2 Based on equal efficacy and easier convalescence compared to the open procedure, many urologists have advocated minimally invasive approaches as the standard of care.2,3 In this context, laparoscopic nephroureterectomy (LNU) and robotic-assisted nephroureterectomy (RANU) are available; the robotic approach is being increasingly adopted for several other urologic malignancies.4 That being said, there is little data evaluating the safety and feasibility of RANU.5 To date, only few retrospective studies have been published, with limited sample size (ranging from 11 to 43), originating from tertiary referral centres, where results may not be representative of the American population at large.6–11
In the face of such little data, we wanted to examine short-term outcomes of RANU compared with LNU using a large contemporary cohort of patients representative of the United States population. Specifically, we focused on perioperative complications, blood transfusions, prolonged length of stay, in-hospital mortality and hospitalization costs.
Methods
Data source
Data from 2008 to 2010 from the U.S. Healthcare Cost and Utilization Project (HCUP) Nationwide Inpatient Sample (NIS) database were abstracted. The NIS is a 20% stratified probability sample that encompasses about 8 million acute hospital stays per year from 1045 hospitals in 46 states.
Sample population and surgical procedures
Patients with a primary diagnosis of renal pelvis neoplasm and ureter neoplasm were identified using the International Classification of Disease 9th revision, clinical modification (ICD-9-CM) diagnostic code: 189.1 and 189.2. Hospital sampling weights were used to estimate the total number of these procedures performed in the United States. Patients aged <18 years and those with missing age or hospital information were removed from the analyses (n = 147). Secondary diagnosis codes (ICD-9-CM 197.0, 197.7 and 198.x) were used to identify and exclude from our analyses patients with metastases. Relying on the ICD-9 procedure codes, patients who underwent RNU (code: 55.51) were abstracted. Subsequently, LNU and RANU were distinguished from open RNU via current procedure codes for laparoscopic and robot-assisted exploration (54.21 and 17.4x, respectively). Since the specific robotic-assisted modifier code was introduced on October 1, 2008, we restricted our analyses to patients diagnosed between October 2008 and December 2010. For the purpose of our analyses, we evaluated exclusively patients undergoing LNU or RANU. This resulted in a final population of 1914 patients.
Patient characteristics
For each patient, age at surgery, gender, race (white, black, other, unknown), and year of surgery were considered. Baseline Charlson Comorbidity Index (CCI) was calculated according to Charlson and colleagues,12 and adapted according to Deyo and colleagues (0 vs. 1 vs. ≥2).13 Patient insurance status was grouped as Medicare, Medicaid, private and other (including unknown insurance status).
Hospital characteristics
Hospital characteristics included hospital region and hospital teaching status, both of which were obtained from the American Hospital Association Annual Survey of Hospitals and defined by the U.S. Census Bureau.6 Hospital region included Northeast, Midwest, South and West. A hospital was considered a teaching hospital if it had an American Medical Association approved residency program, was a member of the Council of Teaching Hospitals, or had a ratio of 0.25 or higher of full-time equivalent interns and residents-to-non-nursing home beds.14 Annual hospital caseload was defined according to the number of RNU performed annually as previously described.15
Outcomes
ICD-9 codes were used to define complications, as previously described.16 We recorded specific ICD-9 complications (Table 1). We defined prolonged length of stay according to the median length of hospitalization as observed in the overall population before propensity-score matching (greater than 4 days). Total hospitalization costs were estimated from the NIS charge-to-cost converter and then adjusted to 2013 U.S. dollars.17 Data on the costs were available for 1209 out of 1914 patients (63.2%). A higher cost incurred during hospitalization was defined using the 75th percentile hospital costs in the entire population ($23 510).18
Table 1.
International Classification of Diseases, ICD-9 codes recorded
| Disease type | Code |
|---|---|
| Intraoperative | ICD-9 code: 998.2 |
| Urinary | ICD-9 code: 997.5 |
| Digestive | ICD-9 code: 997.4 |
| Respiratory | ICD-9 codes: 512.1; 997.3 |
| Hemorrhagic | ICD-9 codes: 998.11; 998.12 |
| Cardiac | ICD-9 code: 997.1 |
| Infectious | ICD-9 code: 998.59 |
| Vascular | ICD-9 codes: 997.2; 999.2 |
| Seromas | ICD-9 codes: 998.13; 998.51 |
| Wound complications | ICD-9 codes: 998.3; 998.83 |
| Others | ICD-9 codes: 998.4; 999.8 |
| Transfusions of packed cells or previously collected autologous blood | ICD-9 codes: 99.02; 99.04 |
ICD: International Classification of Diseases.
Statistical analysis
Descriptive statistics focused on frequencies and proportions for categorical variables. Means, medians, and ranges were reported for continuously coded variables. The independent t-test and Chi-square test were used to compare statistical significance of differences in means and proportions, respectively.
Our statistical approach consisted of different steps. First, due to inherent differences between patients undergoing RANU and LNU, an adjustment was performed using 1 to 1 propensity-score matching. This methodology is commonly used in observational studies to select control subjects who are matched with treated subjects on the controlled background covariates, which, if uncontrolled for, might lead to biased estimates of treatment effects. When matching is performed, the covariates of the control and treatment groups are balanced to reduce possible biases to a minimum.19 Propensity scores were computed by modelling a logistic regression with the dependent variable as the odds of undergoing RANU, and the independent variable as age at surgery, gender, race, CCI, hospital region, hospital teaching status, and insurance status. Subsequently, covariate balance between the matched groups were examined.20 Finally, multivariable generalized estimating equations (GEE) models were performed to assess the impact of the surgical approach (RANU vs. LNU) on perioperative outcomes and hospitalization costs, after accounting for age, gender, race, CCI, insurance status, hospital location, region, and teaching status.3
All tests were 2-side, with statistical significance set at p < 0.05. The Chi-square and Mann-Whitney test were used to assess differences in proportions and medians, respectively. All statistical tests were performed using the R statistical package (v2.15.2) or SPSS version 20 (SPSS, Chicago, IL).
Results
Baseline characteristics
Between October 2008 and December 2010, a weighted estimate of 1914 patients underwent LNU or RANU within the NIS (Table 2). Specifically, 1199 (62.6%) and 715 (37.4%) patients underwent LNU and RANU, respectively (Fig. 1). The use of RANU significantly increased over the study period. Specifically, RANU was 17% of all minimally-invasive cases in the last 3 months of 2008 and increased to 48% of all cases in the last 3 months of 2010 (p = 0.03).
Table 2.
Baseline characteristics of patients with upper-tract urothelial carcinoma stratified by surgical approach (LNU vs. RANU Nationwide Inpatient Sample 2008–2010
| Before propensity-score matching | After propensity-score matching | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Characteristics | LNU (n = 1199; 62.6%) | RANU (n = 715; 37.4%) | Standardized mean difference | p value† | LNU (n = 735; 50.3%) | RANU (n = 715; 49.7%) | Standardized mean difference | p value† |
| Age | ||||||||
| Mean (median) | 72.2 (75.0) | 70.7 (73.0) | 11.1 | 0.005 | 70.6 (74.0) | 70.7 (73.0) | 5.7 | 0.951 |
| IQR | 65.0–80.0 | 62.0–80.0 | 63.0–79.0 | 62.0–80.0 | ||||
| Gender, n (%) | ||||||||
| Male | 695 (58.0) | 451 (63.1) | 11.2 | 0.030 | 440 (59.9) | 451 (63.1) | 7.0 | 0.215 |
| Female | 504 (42.0) | 264 (36.9) | 295 (40.1) | 264 (36.9) | ||||
| CCI, n (%) | ||||||||
| 0 | 627 (52.3) | 434 (60.7) | 21.7 | <0.001 | 427 (58.1) | 434 (60.7) | 7.4 | 0.376 |
| 1 | 405 (33.8) | 222 (31.0) | 233 (31.7) | 222 (31.0) | ||||
| ≥2 | 167 (13.9) | 59 (8.3) | 75 (10.2) | 59 (8.3) | ||||
| Race, n (%) | ||||||||
| White | 877 (73.1) | 524 (73.3) | 3.0 | 0.001 | 540 (73.5) | 524 (73.3) | 3.4 | 0.069 |
| Black | 15 (1.3) | 22 (3.1) | 15 (2.0) | 22 (3.1) | ||||
| Other | 113 (9,4) | 39 (5.5) | 62 (8.4) | 39 (5.5) | ||||
| Unknown | 194 (16.2) | 130 (18.2) | 118 (16.1) | 130 (18.2) | ||||
| Insurance status, n (%) | ||||||||
| Medicare | 836 (69.7) | 469 (65.6) | 7.7 | 0.003 | 476 (64.8) | 469 (65.6) | 1.3 | 0.118 |
| Medicaid | 30 (2.5) | 33 (4.6) | 30 (4.1) | 33 (4.6) | ||||
| Private | 303 (25.3) | 179 (25.0) | 209 (28.4) | 179 (25.0) | ||||
| Other | 30 (2.5) | 34 (4.8) | 20 (2.7) | 34 (4.8) | ||||
| Hospital teaching status, n (%) | ||||||||
| Non-teaching | 438 (36.5) | 129 (18.0) | 53.5 | <0.001 | 130 (17.7) | 129 (18.0) | 1.8 | 0.891 |
| Teaching | 761 (63.5) | 586 (82.0) | 605 (82.3) | 586 (82.0) | ||||
| Hospital region, n (%) | ||||||||
| Northeast | 291 (24.3) | 167 (23.4) | 23.7 | <0.001 | 225 (30.6) | 167 (23.4) | 0.8 | <0.001 |
| Midwest | 347 (28.9) | 299 (41.8) | 217 (29.5) | 299 (41.8) | ||||
| South | 362 (30.2) | 187 (26.2) | 216 (29.4) | 187 (26.2) | ||||
| West | 199 (16.6) | 62 (8.7) | 77 (10.5) | 62 (8.7) | ||||
LNU: laparoscopic nephroureterectomy; RANU: robot-assisted nephroureterectomy; IQR: Interquartile range; CCI: Charlson Comorbidity Index:
Chi-square and Mann-Whitney for proportions and medians, respectively.
Fig. 1.
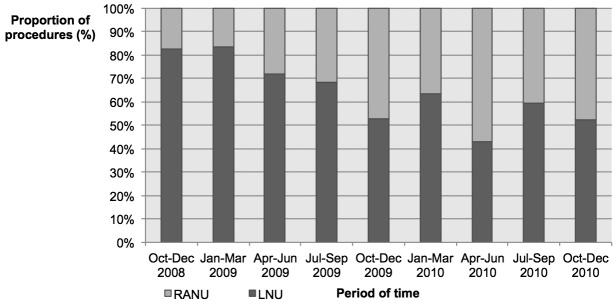
Use of laparoscopic radical nephroureterectomy (LNU) and robot-assisted radical nephroureterectomy (RANU) in patients with upper tract urothelial carcinoma (UTUC) included within the Nationwide Inpatient Sample (NIS) database between October 2008 and December 2010.
With respect to sociodemographic characteristics, RANU patients were significantly younger (mean age 70.7 vs. 72.2 years) and healthier (CCI ≥2: 13.9 vs. 8.3%, both p ≤ 0.01, Table 2) than their LNU counterparts, respectively. Moreover, men (63.1% vs. 58.0%, p = 0.03) were more frequently treated with RANU. There were also statistically significant differences in the use of RANU according to ethnicity and insurance status (all p ≤ 0.003). With respect to hospital characteristics, RANUs were more frequently performed at hospitals with a high hospital volume (3rd tertile, 43.5 vs. 26.8%, p < 0.001), a teaching status (82.0 vs. 63.5%, p < 0.001) and, surprisingly, in a rural setting (7.3 vs 3.0%, p < 0.001). Moreover, RANUs were more often performed in the Midwest (41.8 vs. 28.9%, p < 0.001) than LNUs. Following propensity-score matching, 735 LNU patients were matched to 715 RANU patients and all standardized mean differences of patient characteristics between the 2 groups were <10%, indicating a high degree of similarity in the distribution of both populations. All subsequent analyses were based on the post-propensity matched cohort.
Overall complications and hospitalization costs
Overall complications occurred less frequently in patients treated with RANU compared to LNU (11.9% vs. 18.2%, respectively; p < 0.001, Table 3). For example, digestive complications occurred in 7.4% in patients treated with RANU compared to 11.0% in patients treated with LNU (p = 0.02). No statistically significant differences were observed between RANU and LNU in the rates of blood transfusions (13.0% vs. 14.1%) and average length of stay (5.60 vs. 5.83 days, all p ≥ 0.5). However, patients treated with RANU had a lower rate of prolonged length of stay than LNU patients (39.9 vs. 49.0%, p < 0.001), as well as a lower rate of in-hospital mortality (0.0% vs. 1.4%, p = 0.002), respectively. Finally, the mean costs of hospitalization were significantly higher in patients treated with RANU compared to LNU ($23 235 vs. $17 637, respectively, p < 0.001).
Table 3.
Postoperative complications and cost stratified by surgical approach (laparoscopic nephroureterectomy [LNU] vs. robot-assisted nephroureterectomy [RANU]) for patients included in the Nationwide Inpatient Sample between 2008–2010
| Outcomes |
After propensity score matching
|
|||
|---|---|---|---|---|
| LNU (n = 735) | RANU (n = 715) | Overall (n = 1450) | p value | |
| Overall complications (%) | 134 (18.2) | 85 (11.9) | 219 (15.1) | 0.001 |
| Transfusions (%) | 104 (14.1) | 93 (13.0) | 197 (13.6) | 0.5 |
| Length of Stay, days | ||||
| Mean (median) | 5.83 (4) | 5.60 (4) | 5.71 (4) | 0.57# |
| IQR | (3–6) | (3–6) | (3–6) | |
| Length of stay >4 days&(%) | 360 (49.0) | 285 (39.9) | 645 (44.5) | <0.001 |
| Cost, $* | ||||
| Mean (median) | 17 637 (15 531) | 23 235 (20,959) | 20 236 (16 725) | <0.001# |
| IQR | (12 532–19 187) | (14 268–26,979) | (12 935–24 334) | |
| Higher cost incurred during hospitalization (%)** | 75 (14.9)* | 162 (37.9)* | 237 (25.5)* | <0.001 |
| In-hospital mortality | 10 (1.4) | 0 (0.0) | 10 (0.7) | 0.002 |
Defined as greater than the median length of stay in the pre propensity-score population (4 days);
Independent t-test for equality of means;
519 cases missing after matching. Values are rounded to the dollar and adjusted for U.S. 2013 inflation;
Defined as costs more or equal to the 75th percentile in the pre propensity-score population ($23,510). IQR: interquartile range; LNU: laparoscopic nephroureterectomy; RANU: robot-assisted nephroureterectomy.
Multivariable GEE analyses
After adjusting for all covariates and hospital clustering, patients undergoing RANU were significantly less likely to have any complication during hospitalization compared to those receiving LNU (odds ratio [OR]: 0.54, 95% confidence interval [CI]: 0.30–0.98; p = 0.04, Table 4). However, no statistically significant differences were observed with respect to blood transfusions and prolonged length of stay (both p ≥ 0.1). Finally, patients undergoing RANU procedures had a 4.1-fold risk of higher costs incurred during hospitalization (p = 0.02).
Table 4.
Generalized estimating equations for postoperative complications and costs stratified by surgical approach (laparoscopic nephroureterectomy [LNU] vs. robot-assisted nephroureterectomy [RANU]) for patients included in the Nationwide Inpatient Sample between 2008–2010
| After propensity score matching | ||
|---|---|---|
|
| ||
| Outcomes | RANU vs. LNU OR (95% CI) | p value |
| Postoperative complications | 0.54 (0.30–0.98) | 0.04 |
| Transfusions | 1.04 (0.51–2.10) | 0.9 |
| Length of stay >4 days | 0.62 (0.36–1.09) | 0.1 |
| Higher cost incurred during hospitalization* | 4.12 (1.69–10.07) | 0.02 |
LNU: laparoscopic nephroureterectomy; RANU: robot-assisted nephroureterectomy; OR: odds ratio, 95% CI : 95% confidence interval;
519 cases missing after matching. All models adjusted for age at surgery, gender, race, Charlson comorbidity index, insurance status, hospital location, region, and teaching status, as well as hospital clustering.
Discussion
Nephroureterectomy is the standard of care for UTUC. In the early 1990s, LNU was introduced.21 Compared to open nephroureterectomy, the LNU procedure is associated with fewer morbidities, shorter convalescence period,3 and comparable cancer control outcomes,22 which has rendered the approach the preferred treatment by many academic hospitals with high volume.3
In the last 10 years, robotic surgery has emerged as an alternative minimally invasive surgical treatment for many malignancies, including urological cancers.4,23–25 In prostate cancer, robotic-assisted radical prostatectomy has overtaken the traditional open approach: 64% vs. 36% as of the year 2009, respectively.25 In kidney cancer, robotic-assisted partial nephrectomy has also generated considerable interest, given its less prohibitive learning curve compared to the laparoscopic approach.24 Similarly, RANU has gained considerable interest in recent years for treatment of UTUC. RANU is associated with non-inferior morbidity and hospitalization after surgery, compared to LNU or open nephroureterectomy. For example, Hemal and colleagues, in a single-centre series with 15 patients, showed no complications following RANU and a mean hospital stay of 2.73 days.8 Similarly, Pugh and colleagues, in a multi-institutional study with 43 patients, showed a 14% complication rate following RANU and a median of hospital stay of 3 days.6
While such studies are undoubtedly meaningful and informative, all such reports were based on a small number of patients treated at tertiary care centres, where the feasibility of the procedure may be greatly different in the community. Furthermore, given the rarity of UTUC, it is unlikely that a randomized trial comparing RANU to the standard approach or LNU will ever be conducted in upcoming years. Therefore, large population-based assessments are needed to complement existing data from centres of excellence to confirm the safety and feasibility of RANU. It is under this context that we sought to evaluate short-term outcomes after RANU relative to LNU. These outcomes include overall complications, the rates of blood transfusions, length of hospitalization, as well as in-hospital mortality. In addition, we focused on the costs during hospitalization between RANU and LNU, as it has been adequately described in the literature that novel technologies that are marketed intensively are frequently associated with increased cost.26,27 Therefore, our goal was to compare in-hospital morbidity and mortality, as well as hospitalization costs between RANU and LNU in a national cohort of persons with UTUC treated in the United States. To to reduce to a minimum the potential effect of selection bias, we matched baseline patient and hospital characteristics of both treatment groups using a propensity-score methodology.
In the current study, our results demonstrated that RANU was associated with fewer complications after surgery than LNU-treated patients, and non-different hospitalization period and blood transfusion rates. Furthermore, our data appear to suggest lower in-hospital mortality for RANU compared to LNU. These trends remained true even after adjusting for known confounders, including hospital clustering. It has been previously stated that robotic-assisted approach offers several advantages, including 3-dimensional visualization, increased freedom of instrument movement, and enhanced ergonomics and surgeon comfort, all of which could contribute to lower intra- and postoperative outcomes compared to alternative treatments.4 On the other hand, it could be speculated that LNU remains technically more complex than RANU,28 which could result in seemingly higher complication rates, despite adjustment for hospital clustering.
Whereas complication rates were more favourable for RANU, the latter was associated with substantially higher hospital costs. For example, 38% of the hospitalizations for RANU generated costs above the 75th percentile of the pre-propensity score population compared to 15% for hospitalization for LNU (p < 0.001). It is not surprising that the robotic-assisted approach is more costly than other available alternatives. Previous authors have also noted such increased costs for robotic-assisted prostatectomies.4,25,29 Specifically, in the urologic setting, Yu and colleagues showed that robotic surgery was associated with higher costs compared to both the laparoscopic and open approaches.4 Unfortunately, most studies looking at perioperative costs associated with minimally invasive surgical approaches are limited to in-hospital stays. Consequently, a positive impact on savings after hospital discharge due to better in-hospital outcomes may be missed. For example, Lowrance and colleagues, in a population-based analysis of costs associated with hospitalization and 1 year postoperative care following minimally invasive and open prostatectomy, found no statistically significant difference in total mean costs between both procedures, after controlling for cofounding biases.30 Further studies relying on sophisticated decision modeling are needed to better delineate the difference in costs between procedures at long-term follow-up.
Utilization rate of RANU to treat UTUC achieved a remarkable jump from a mere 17% in 2008 to nearly 50% in 2010. This trend is comparable to the use of robotics in other malignancies.23,24 Robotic-assisted prostatectomy represents a noteworthy example of this phenomenon, where the utilization rate between 2005 and 2008 jumped by 60%, albeit with a decreasing incidence of prostate cancer.27 The trends observed for RANU are most likely attributed to the aggressive marketing strategies and patient expectations, despite limited level 1 evidence efficacy data supporting its comparative effectiveness relative to traditional treatment modalities.
Our results support the safety and feasibility of RANU relative to LNU in patients with UTUC. Indeed, RANU was associated with fewer in-hospital complications relative to LNU. However, the current analyses also suggest that the robotics approach is linked to non-negligible higher costs of resources during hospitalization. In this aspect, a more detailed analysis on the long-term costs and savings of RANU compared to alternative treatments may be necessary.
Several study limitations are worth mentioning. First, our results are limited by their retrospective nature. However, with the lack of prospective randomized trials assessing the safety and feasibility of RANU, retrospective observations from large population-based cohorts provide the higher level of evidence. Second, disease characteristics such as tumour stage and grade are not recorded in the NIS. Consequently, we cannot exclude that patients receiving a robotic approach represent individuals with less aggressive disease. On the other hand, the impact of disease aggressiveness on perioperative outcomes is controversial. Additionally, propensity-score matching was applied to account for differences in the baseline characteristics of patients treated with LNU or RANU. Third, another limitation of the NIS data is the lack of consistent surgeon identification. Accordingly, we were unable to adjust for the effect of surgeon volume and/or the learning curve. Fourth, because there were few complications in each category, we could not demonstrate specific complication rates. This is in accordance with the NIS confidentiality guidelines, which state that all cell numbers less than or equal to 10 should not be reported. inally, several sets of codes have been reported to identify complications in population-based cohorts. We relied on a set of ICD-9 codes previously published in several papers in the NIS, primarily in nephrectomy for kidney cancer, because of the similarity between both approaches and their complications.16
Conclusion
Our results support the safety and feasibility of RANU for the treatment of UTUC. Indeed, the use of the robotic approach was associated with lower probability of experiencing perioperative complications compared to LNU. On the other hand, the utilization of RANU is associated with higher costs compared to LNU.
Footnotes
Competing interests: Dr. Trudeau, Dr. Gandaglia, Dr. Shiffmann, Dr. Popa, Dr. Shariat, Dr. Montorsi, Dr. Perrotte and Dr. Sun all declare no competing financial or personal interests. Dr. Trinh received honorarium from Intuitive Surgical in the past. Dr. Karakiewicz is partially supported by the University of Montreal Health Centre Urology Specialists, Fonds de la Recherche en Santé du Quebec, the University of Montreal Department of Surgery and the University of Montreal Health Centre (CHUM) Foundation.
This paper has been peer-reviewed.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Rouprêt M, Babjuk M, Compérat E, et al. European Guidelines on Upper Tract Urothelial Carcinomas: 2013 Update. Eur Urol. 2013;63:1059–71. doi: 10.1016/j.eururo.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 3.Hanna N, Sun M, Trinh QD, et al. Propensity-score-matched comparison of perioperative outcomes between open and laparoscopic nephroureterectomy: A national series. Eur Urol. 2012;61:715–21. doi: 10.1016/j.eururo.2011.12.026. [DOI] [PubMed] [Google Scholar]
- 4.Yu H-Y, Hevelone ND, Lipsitz SR, et al. Use, costs and comparative effectiveness of robotic assisted, laparoscopic and open urological surgery. J Urol. 2012;187:1392–9. doi: 10.1016/j.juro.2011.11.089. [DOI] [PubMed] [Google Scholar]
- 5.Merseburger AS, Herrmann TR, Shariat SF, et al. EAU Guidelines on Robotic and Single-site Surgery in Urology. Eur Urol. 2013;64:277–91. doi: 10.1016/j.eururo.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 6.Pugh J, Parekattil1 Sijo, Willis Daniel, et al. Perioperative outcomes of robot-assisted nephroureterectomy for upper urinary tract urothelial carcinoma: A multi-institutional series. BJU Int. 2013;112:E295–E300. doi: 10.1111/bju.12163. [DOI] [PubMed] [Google Scholar]
- 7.Lim SK, Shin T-Y, Kim KH, et al. Intermediate-term outcomes of robot-assisted laparoscopic nephroureterectomy in upper urinary tract urothelial carcinoma. Clin Genitourin Cancer. 2013;11:515–21. doi: 10.1016/j.clgc.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 8.Hemal AK, Stansel I, Babbar P, et al. Robotic-assisted nephroureterectomy and bladder cuff excision without intraoperative repositioning. Urology. 2011;78:357–64. doi: 10.1016/j.urology.2010.12.075. [DOI] [PubMed] [Google Scholar]
- 9.Eandi JA, Nelson RA, Wilson TG, et al. Oncologic outcomes for complete robot-assisted laparoscopic management of upper-tract transitional cell carcinoma. J Endourol. 2010;24:969–75. doi: 10.1089/end.2009.0340. [DOI] [PubMed] [Google Scholar]
- 10.Won Lee J, Arkoncel FRP, Rha KH, et al. Urologic robot-assisted laparoendoscopic single-site surgery using a homemade single-port device: A single-center experience of 68 cases. J Endourol. 2011;25:1481–5. doi: 10.1089/end.2010.0656. [DOI] [PubMed] [Google Scholar]
- 11.Khanna R, Stein RJ, White MA, et al. Single institution experience with robot-assisted laparoendoscopic single-site renal procedures. J Endourol. 2012;26:230–4. doi: 10.1089/end.2011.0187. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 14. Healthcare Cost and Utilization Project: NIS Database Documentation-Description of Data Elements. http://www.hcup-us.ahrq.gov/db/nation/nis/nisdde.jsp. Accessed October 8, 2014.
- 15.Budaus L, Abdollah F, Sun M, et al. Annual surgical caseload and open radical prostatectomy outcomes: Improving temporal trends. J Urol. 2010;184:2285–90. doi: 10.1016/j.juro.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 16.Joudi FN, Allareddy V, Kane CJ, et al. Analysis of complications following partial and total nephrectomy for renal cancer in a population based sample. J Urol. 2007;177:1709–14. doi: 10.1016/j.juro.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 17. United States Department of Labor . Bureau of Labor Statistics of the U.S. Department of Labor. http://www.bls.gov/. Accessed October 8, 2014.
- 18.Ghani KR, Sukumar S, Sammon JD, et al. Practice patterns and outcomes for open and minimally invasive partial nephrectomy since the introduction of robotic partial nephrectomy: Results from the Nationwide Inpatient Sample. J Urol. 2013;189:e533. doi: 10.1016/j.juro.2013.02.2659. [DOI] [PubMed] [Google Scholar]
- 19.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–81. doi: 10.1002/(SICI)1097-0258(19981015)17:19<2265::AID-SIM918>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 20.Ho DE, Imai K, King G, et al. MatchIt: Nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:1–28. [Google Scholar]
- 21.Clayman RV, Kavoussi LR, Figenshau RS, et al. Laparoscopic nephroureterectomy: Initial clinical case report. J Laparoendosc Surg. 1991;1:343–9. doi: 10.1089/lps.1991.1.343. [DOI] [PubMed] [Google Scholar]
- 22.Ariane MM, Colin P, Ouzzane A, et al. Assessment of oncologic control obtained after open versus laparoscopic nephroureterectomy for upper urinary tract urothelial carcinomas (UUT-UCs): Results from a large French multicenter collaborative study. Ann Surg Oncol. 2011;19:301–8. doi: 10.1245/s10434-011-1841-x. [DOI] [PubMed] [Google Scholar]
- 23.Lowrance WT, Eastham JA, Savage C, et al. Contemporary open and robotic radical prostatectomy practice patterns among urologists in the United States. J Urol. 2012;187:2087–93. doi: 10.1016/j.juro.2012.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel HD, Mullins JK, Pierorazio PM, et al. Trends in renal surgery: Robotic technology is associated with increased use of partial nephrectomy. J Urol. 2013;189:1229–35. doi: 10.1016/j.juro.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 25.Sammon JD, Karakiewicz PI, Sun M, et al. Robot-assisted versus open radical prostatectomy: The differential effect of regionalization, procedure volume and operative approach. J Urol. 2013;189:1289–94. doi: 10.1016/j.juro.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Kolata G. Results unproven, robotic surgery wins converts. The New York Times. Feb 13, 2010. http://www.nytimes.com/2010/02/14/health/14robot.html?pagewanted=all&_r=0.
- 27.Barbash GI, Glied SA. New technology and health care costs — The case of robot-assisted surgery. N Engl J Med. 2010;363:701–4. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 28.Kho RM. Comparison of robotic-assisted laparoscopy versus conventional laparoscopy on skill acquisition and performance. Clin Obstet Gynecol. 2011;54:376–81. doi: 10.1097/GRF.0b013e31822b46f6. [DOI] [PubMed] [Google Scholar]
- 29.Kim SP, Shahb ND, Karnes RJ, et al. Hospitalization costs for radical prostatectomy attributable to robotic surgery. Eur Urol. 2013;64:11–6. doi: 10.1016/j.eururo.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Lowrance WT, Eastham JA, Yee DS, et al. Costs of medical care after open or minimally invasive prostate cancer surgery. Cancer. 2011;118:3079–86. doi: 10.1002/cncr.26609. [DOI] [PMC free article] [PubMed] [Google Scholar]


