Abstract
Introduction:
Results of clinical trials have demonstrated that circulating tumour cells (CTCs) are frequently detected in patients with urothelial tumours. The monitoring of CTCs has the potential to improve therapeutic management at an early stage and also to identify patients with increased risk of tumour progression or recurrence before the onset of clinically detected metastasis. In this study, we report a new effectively simplified methodology for a separation and in vitro culturing of viable CTCs from peripheral blood.
Method:
We include patients diagnosed with 3 types of urothelial tumours (prostate cancer, urinary bladder cancer, and kidney cancer). A size-based separation method for viable CTC - enrichment from unclothed peripheral blood has been introduced (MetaCell, Ostrava, Czech Republic). The enriched CTCs fraction was cultured directly on the separation membrane, or transferred from the membrane and cultured on any plastic surface or a microscopic slide.
Results:
We report a successful application of a CTCs isolation procedure in patients with urothelial cancers. The CTCs captured on the membrane are enriched with a remarkable proliferation potential. This has enabled us to set up in vitro cell cultures from the viable CTCs unaffected by any fixation buffers, antibodies or lysing solutions. Next, the CTCs were cultured in vitro for a minimum of 10 to 14 days to enable further downstream analysis (e.g., immunohistochemistry).
Conclusion:
We demonstrated an efficient CTCs capture platform, based on a cell size separation principle. Furthermore, we report an ability to culture the enriched cells – a critical requirement for post-isolation cellular analysis.
Introduction
Circulating tumour cells (CTCs), which are often detected as circulating epithelial cells, are extremely rare in healthy individuals, but detectable in the blood of patients with various solid tumours. CTCs are malignant cells in peripheral blood that originate from primary tumours or metastatic sites.
Results of clinical trials have demonstrated that CTCs are frequently detected in patients with urothelial tumours: prostate cancer, bladder and kidney cancer.1 The survival analysis for patients with metastatic disease suggests a prognostic role for CTCs in this setting. The assessment of CTCs can also provide information about a patient’s response to therapy. In patients with prostate cancer, those with ≥5 CTCs in 7.5 mL of blood have significantly worse overall survival than those with <5 CTCs. If the number of CTCs becomes <5 with therapy, the patient with an initially poor prognosis can have a survival similar to that of patients in the good prognosis (<5 CTCs) group.2
Rink and colleagues detected CTCs in 30% of patients with bladder cancer diagnosed as non-metastatic disease, and showed a significantly worse overall progression-free survival and cancer-specific survival in patients with high CTC numbers. CTCs have also been detected in patients with renal cell carcinoma in the peripheral blood by immunocytochemistry and polymerase chain reaction (PCR).3
The monitoring of CTCs has the potential to improve therapeutic management at an early stage and also to identify patients with increased risk of tumour progression or recurrence before the onset of clinically detected metastasis. Furthermore, the molecular profiling of CTCs can provide new insights into cancer biology and systemic treatment in neoadjuvant or adjuvant settings.
Detection of CTCs is not yet standardized in clinical practice because of the use of different enrichment and detection methods. The methodology most commonly used to detect CTCs is immunochemistry. This assay is based on immunocytochemical staining with monoclonal antibodies against epithelial or tumour-associated antigens. Immunocytochemical analysis is usually used in combination with density gradient centrifugation, immunomagnetic procedures or size filtration methods to enrich tumour cells prior to their detection. These methods provide the possibility of further morphological analysis of the detected CTCs.
Molecular detection of CTCs based on PCR amplification of either DNA or complementary DNA (mRNA) is hindered by the fact that the tumour cells of interest cannot be morphologically identified and isolated for further analyses. DNA-based methods rely on the detection of known mutations, amplifications or methylation patterns in the tumour cells.
It is also important to note that simple enumeration of CTCs will not contribute significantly to the development of improved or more personalized cancer treatments. Beyond an in vitro number count, an ex vivo functional study on patient-derived CTCs might promote an immediate treatment decision regarding drug resistance or prognosis.
In this study, we report on an effectively simplified methodology for a size-based separation and in vitro culturing of viable CTCs from peripheral blood.
Methods
Patients
In total, we reviewed 8 patients diagnosed with 3 types of urothelial tumors: 3 with prostate cancer, 3 with urinary bladder cancer and 2 with kidney cancer (Table 1). The final diagnoses were based on the histopathology results. After we received written informed patient consent, we collected clinical data from all participating patients. For each patient, about 8 mL of venous blood was drawn from the antecubital veins and placed into S-Monovette tubes (Sarstedt AG & Co., Numbrecht, Germany) containing 1.6 mg EDTA/mL blood as an anticoagulant. The samples were processed at room temperature using an isolation procedure and completed within 24 hours after the blood draw.
Table 1.
Individual patient characteristics
| Patient | Stadium | Tumour histology | Age | T | N | M | Gleason score/grade* | Surgery | PSA |
|---|---|---|---|---|---|---|---|---|---|
| Prostate cancer | |||||||||
| P1 | II | Adenocarcinoma | 52 | 2c | 0 | 0 | 3+4 | Operable | 3.3 ng/mL; 16.5% |
| P2 | II | Adenocarcinoma | 64 | 2c | 0 | 0 | 3+3 | Operable | 2.78 ng/mL; 12% |
| P3 | II | Adenocarcinoma | 55 | 2c | 0 | 0 | 4+4 | Operable | 8.2 ng/mL; 8.5% |
| Urinary bladder cancer | |||||||||
| B1 | 0a | Papillary carcinoma | 64 | a | 0 | 0 | 2 | Operable | n/a |
| B2 | I | Papillary carcinoma | 73 | 1 | 0 | 0 | 2 | Operable | n/a |
| B3 | 0a | Papillary carcinoma | 76 | a | 0 | 0 | 2 | Operable | n/a |
| Kidney cancer | |||||||||
| K1 | III | Clarocellulare carinoma | 66 | 3a | 0 | 0 | 3 | Operable | n/a |
| K2 | II | Clarocellulare carinoma | 70 | 2 | 0 | 0 | 2 | Operable | n/a |
Gleason score for prostate cancer; grade for Urinary bladder cancer and kidney cancer. PSA: prostate-specific antigen; n/a: not applicable.
The ethics committee of all participated Universities and Hospitals approved the study protocol according to the Declaration of Helsinki.
CTCs enrichment and culture
Recently, a new, effectively simplified size-based separation method for viable CTC - enrichment from unclothed peripheral blood has been introduced (MetaCell, Ostrava, Czech Republic). The size-based enrichment process is based on the filtration of peripheral blood through porous polycarbonate membrane (pores with 8 μm diameter). The minimum and maximum volume of the filtered peripheral blood may be adjusted up to 50 mL of fluid. Standardly, 8 mL peripheral blood from patients suffering with urothelial cancer was transferred into the filtration tube. The successive blood transfer in several steps was preferred to prevent the blood clogging on the membrane filtre. The peripheral blood flow was driven naturally by blood viscosity and supported by capillary action of the absorbent touching the membrane filter. Afterwards the membrane filtre kept in a plastic ring was transferred into the 6-well cultivation plate, then 4 mL RPMI media was added to the filtre top and the CTCs were cultured on the membrane in vitro under the conditions of standard cancer cell cultures (37°C, 5% atmosphere of CO2) and observed by inverted microscope. The CTCs grow in the FBS enriched RPMI medium (10%) for a minimum 14 days on the membrane (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5). The grown cells were analyzed by histochemistry (May-Grünwald staining, MGG) and immunohistochemistry using the specific antibodies to demonstrate the cell origin (pancytokeratin 1–FITC conjugated antibody [Sigma], cytokeratin7 antibody [Dako]) and unspecific, DAPI staining (Fig. 3).
Fig. 1.
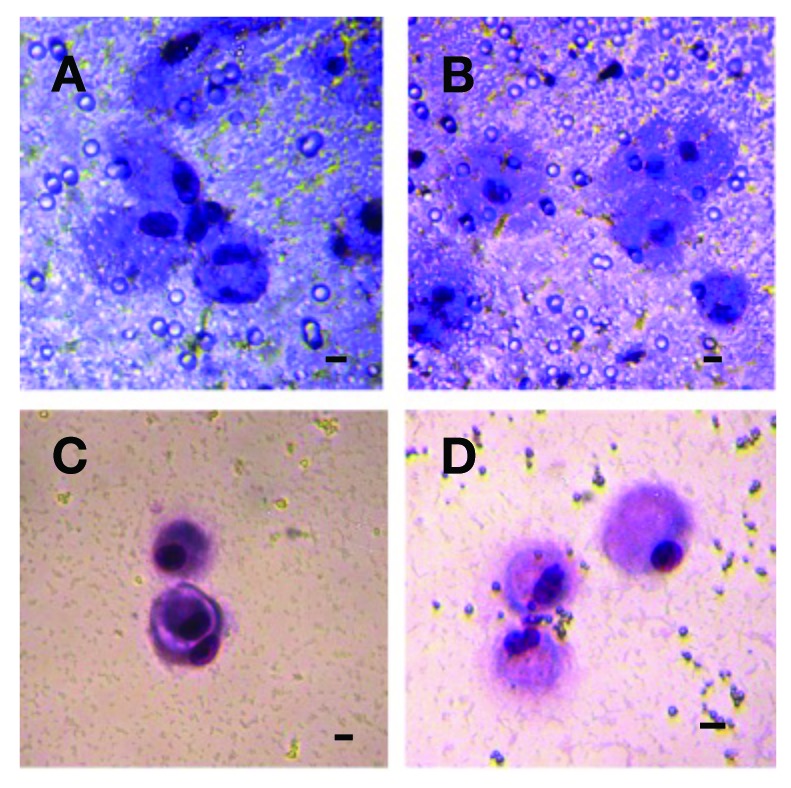
Circulating tumour cells (CTCs) isloated from a patient (P1) with prostate cancer grown in vitro 10 days. A, B: The CTCs detected on the membrane. C, D: The CTCs growing on the bottom of the culture plate after their escape from the separating membrane through the pores/counterstained by MGG-stain. A bar represents 10 μm.
Fig. 2.
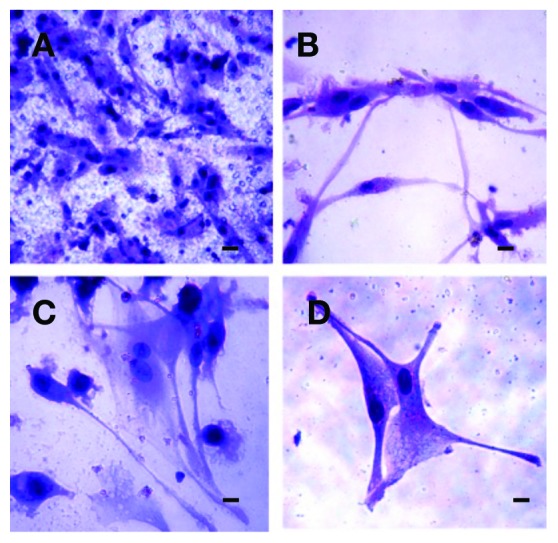
Circulating tumour cells (CTCs) isloated from a patient (P2) with prostate cancer grown in vitro 14 days. A: The CTCs growing on the membrane. B, C, D: The cells growing through the membrane to the bottom of the culture plate showing mesenchymal features/counterstained by MGG-stain. A bar represents 10 μm.
Fig. 3.
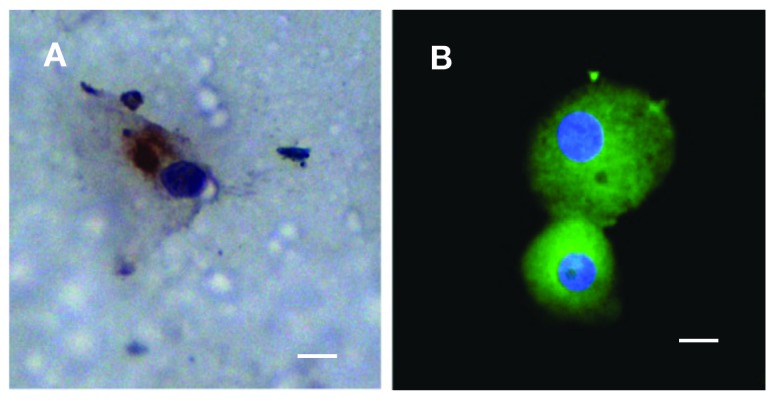
Circulating tumour cells (CTCs) captured from a patient (P1) with prostate cancer grown in vitro on the membrane. A: The CTCs counterstained by Pan-cytokeratin antibody (DAB-visualized). B: The CTCs captured through enrichment and subsequent cytospin procedure, counterstained by FITCPancytokeratin and DAP1. A bar represents 10 μm.
Fig. 4.
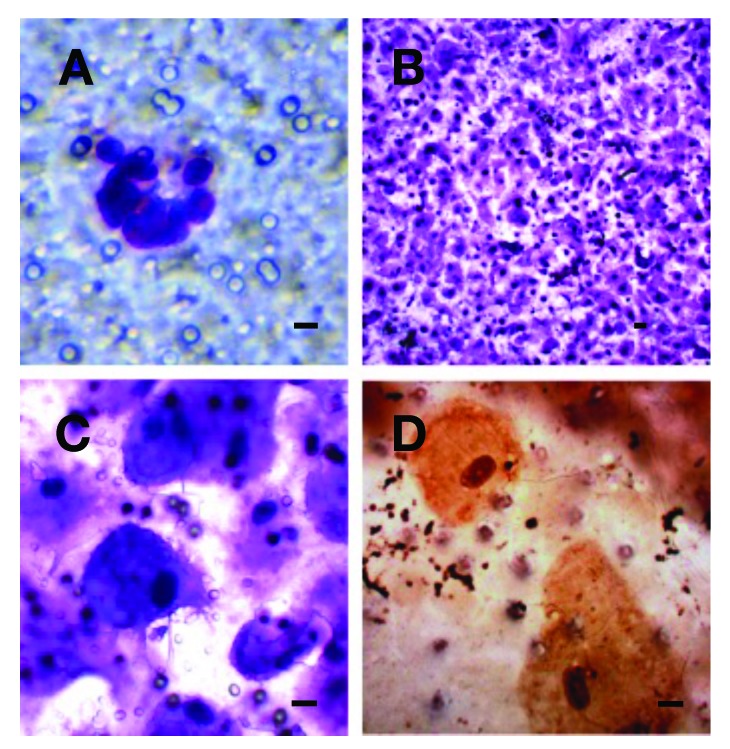
Circulating tumour cells (CTCs) from a patient (B1) with bladder cancer. A: The CTCs immediately after the separations. B: The CTCs on the separation membrane grown in vitro for 14 days creating a cell layer. C: The CTCs growing on the membrane. D: The CTCs growing on the membrane counterstained by CK-7 antibody (DAB-visualized). A bar represents 10 μm.
Fig. 5.
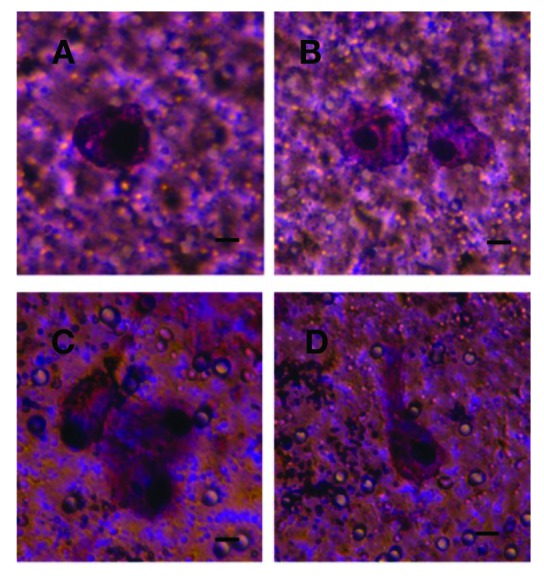
Circulating tumour cells captured from a patient (B2) with bladder cancer on the separation membrane grown in vitro for 14 days. A bar represents 10 μm.
Alternatively, the enriched CTCs fraction can be transferred from the membrane and cultured directly on any plastic surface or a microscopic slide. Microscopic slide culture is preferred if the immunohistochemistry/immunofluorescence analysis is planned. If an intermediate CTCs-analysis is warrented, the CTCs-fraction is transferred in phosphate buffered saline (1.5 mL) to the cytospin slide. The slide is then dried for 24 hours and immunohistochemically analyzed (Fig. 3, part B).
Cytological analysis
The cells were fixed and stained on the membranes and examined using light microscopy in 2 steps: (1) screening at ×20 magnification to locate cells and (2) observation at ×40 magnification for detailed cytomorphological analysis.
Isolated cells and/or clusters of cells of interest were selected, digitized, and examined by an experienced researcher and/or pathologist. CTCs were defined as cells presenting the following characteristics: (1) nuclear size equal or larger than 10 μm; (2) irregularity of the nuclear contour; (3) presence of a visible cytoplasm; (4) prominent nucleoli; (5) high nuclear-cytoplasmatic ratio (NC-ratio); (6) NC-ratio changes with the in vitro culture time; and (7) deformability/plasticity (growth through the membrane to the bottom and setting up new colonies).
Results
We report a successful implementation of CTCs isolation procedure in patients with prostate, urinary bladder and kidney cancer capturing cells with proliferation potential by capillary-action driven filtration. The viable CTC-cells captured on the membrane are enriched in a good fitness with a remarkable proliferation potential (Figs. 1–7). This has enabled us to set up in vitro cell cultures from the viable CTCs unaffected by any fixatives, antibodies or lysing solutions. The CTCs were cultured in vitro for a minimum of 10 to 14 days to enable further downstream analysis (e.g., immunohistochemistry).
Fig. 7.
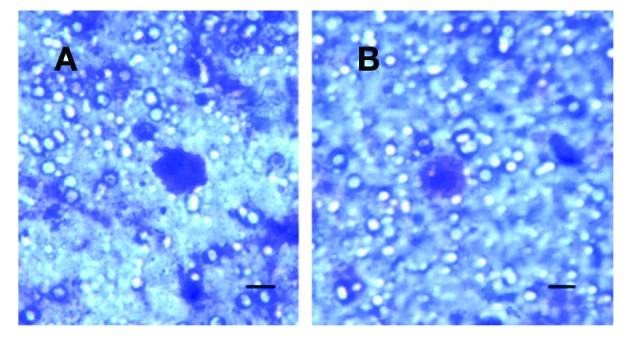
Circulating tumour cells captured from patients with kidney cancer (A: patient K1. B: patient K2) on the separating membrane, grown in vitro for 7 days. A bar represents 10 μm.
The size of the captured cells helped identify cancer cells even without any additional staining (e.g., MGG). The standard staining method has enabled us to encounter and analyze the nuclei inclusive nucleoli. Generally, we have found that if a nucleus is bigger than 10 μm, the cells do not have much cytoplasm immediately after the capture. If the cells are kept in the in vitro culture, the NC-ratio is not able to identify cancer cells.
Due to the cell size (>15 μm), nucleus size (>10 μm) and shape and expressive nucleoli visualized by MGG and simple DAPI-stain in the formerly fixed cells (Fig. 3), we were able to detect cancer cells not only on the separating membrane (Fig. 1, part A, Fig. 2, part A, Fig. 4, parts A, B), but also on the plastic bottom of the 6-well plate (Fig. 1, Fig 2, Fig 4, Fig 5, Fig. 6). The cells became immortal cell cultures on the plate bottom and presented partially changed phenotype towards fibroblast or endothelial-cell like phenotype when compared to the cells on the membrane (Fig. 2, Fig. 4).
Fig. 6.
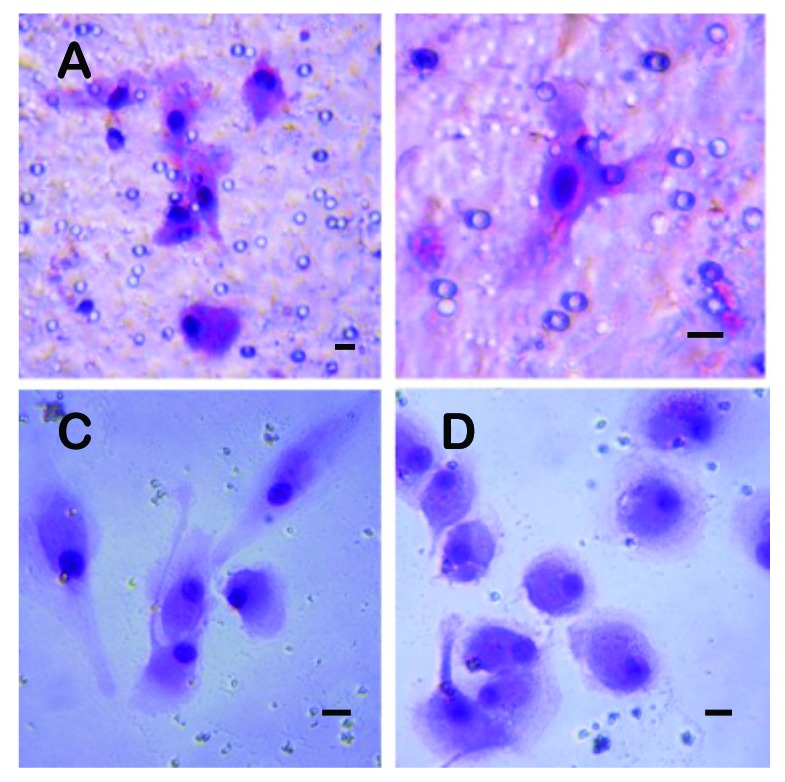
Circulating tumour cells (CTCs) captured from a patient (B3) with bladder cancer grown in vitro for 14 days. A, B: The CTCs growing on the separation membrane. C, D: The CTCs growing on the culture flask bottom after their escape from the membrane through the pores. A bar represents 10 μm.
These results indicate that the captured cancer cells display plasticity enabling them to overgrow the separating membrane. Reflecting the shape of the cancer cells in the “bottom fraction,” we may expect these cells to be a more flexible CTC-fraction. The immunohistochemical analysis has shown the abundance of the cytokeratin (pancytokeratin in prostate cancer and cytokeratin 7 in bladder cancer cells) in the “membrane” fraction as well as in the “invading” fraction demonstrating a carcinoma origin. We see enormous potential in the gene expression analysis, which, based on the RNA, could reveal the epithelial-mesenchymal character of the detected cancer cells.
If comparing simple CTC-counts in different patients with the same diagnosis, we do not report any significant difference, but we describe very high CTC-numbers in prostate cancer patient samples (more than 50 cells immediately after separation). This number is by far the highest when compared to urinary bladder cancer and kidney cancer. Only few cells could be detected in patients with kidney cancer.
Discussion
Solid tumours are heterogeneous, containing subpopulations of cancer cells with different invasive potential; consequently only a few tumour cells within the tumour mass will achieve successful metastatic implantation.4 Studies suggest that 0.01% of circulating tumour cells ultimately can produce a single bone metastasis, and at least 104 circulating tumour cells are required for the development of a metastatic locus.5,6
CTCs accompany tumour invasion into the bloodstream. Detection, monitoring, and molecular analysis of these rare cancer cells provide a powerful and non-invasive approach to detect early disease, assess prognosis and therapeutic response in established cancers, and target metastatic precursor cells.
CTCs detection appears to be an important new biomarker in cancer research. However, one of the main problems of CTCs detection lies in its low sensitivity with widely studied tools (RT-PCR, immunomagnetic detection). In this framework, new devices, such as the MetaCell assay, appear to help to remove this limitation.
The most extensively studied clinical role for CTCs is to use them as prognostic biomarkers. Generally, CTC-counts at a pre-defined time point (e.g., start of a new chemotherapy) are associated with a clinical end point. Most importantly, CTC counts outperformed prostate-specific antigen in predicting survival at all time points.7 Analysis of CTC-counts was shown to be of prognostic and predictive value in metastatic castration resistant prostate cancer (CRPC) in several clinical studies.8–13 Additionally, CTCs can also be assayed for various phenotypic markers, such as gene expression or protein markers.9,14–17 Scher and colleagues18 analyzed CTCs as a valid intermediate end point for overall survival in a prospective phase III trial of abiraterone acetate in docetaxel-refractory metastatic CRPC. The conversion of CTC counts (from >5 to <5 cells per 7.5 mL) in response to treatment was in relationship to overall survival. CTC kinetics fulfilled all of Prentice’s criteria for surrogacy for overall survival.19 This finding has the potential to influence clinical trials of new anticancer agents. CTC counts have also been able to predict the duration and magnitude of response to androgen-deprivation therapy in metastatic hormone sensitive prostate cancer.11
The research has been focused mainly on CTCs enumeration, which is in truth a simplistic, one-dimensional use for CTCs,7 mainly due to the technology used in the testing. The expectations are that the real clinical potential of CTCs will be discovered in molecular characterization of CTCs. For that purpose, new CTCs-isolation protocols and large studies around effective targeted therapies are required.
The detection of CTCs in the peripheral blood of cancer patients holds great promise, but remains technically challenging. The identification and characterization of CTCs require extremely sensitive and specific analytical methods that are usually combined with enrichment procedures, including density gradient centrifugation, immunomagnetic procedures with antibodies against either tumour-associated antigens.
Most of the assays are based on enrichment and subsequent identification of CTCs using monoclonal antibodies directed against epithelial epitopes. This approach is based on the fact that blood cells usually lack detectable expression of epithelial markers, being of mesenchymal origin. However, although the number of epithelial cells in the blood of healthy individuals and patients with a variety of non-malignant disease is low, they may be present in 0.3% of cases.20
Furthermore, using the EpCAM-based CellSearch (Veridex) methodology, Pantel and colleagues showed that circulating epithelial cells are found in 0% to 18.7% of patients with benign colonic disease; the EPISPOT Cytokeratin 19 assay found circulating epithelial cells in 8.3% to 28.6% of patients with benign colonic disease.21 This is in addition to the potential problem of CTC’s detection methods dependent on the tumour associated antigens that do not express EpCAM or cytokeratins.22 Importantly, all EpCAM-based enrichment systems share the same limitation; EpCAM can be downregulated during epithelial-to-mesenchymal transition (EMT), a process attributed to disseminating cancer cells.
Ideally, a CTCs capture technique should be highly sensitive, reproducible, and automated. It should have the ability to reliably capture a high percentage of CTCs present in a sample, while minimizing the number of false positive events and contamination from nonmalignant cells. The design of the test should be simple enough that it can be mass produced and performed in clinical laboratories with minimal interoperator variability. It should also have the ability to both quantify and characterize CTCs to limit operator bias.
Although a number of new innovative technologies to detect CTCs have recently been presented, the specificity and utility of these methods in large clinical studies have still to be demonstrated.
CTCs that emanate from solid tumours have a larger diameter and volume than other hematological cells found in the circulation. We used size-based separation because the most important advantage of filtration methods is the possibility to enrich “virgin CTC.” During the filtration process, interactions did not occur between antibodies and antigens on CTCs surface. This biological interaction is specific for immunomagnetic capture methods. The MetaCell device provides the possibility to enrich virgin CTCs suitable for next cultivation or single cell analysis. This aspect will have an important impact on the future design of clinical trials testing new drugs against targets expressed on metastatic cancer cells. In addition to the information on CTCs-counts, future trials with targeted therapies should also include the assessment of the specific therapeutic target on CTCs.
The primary challenge of MetaCell CTCs technology is to monitor minimal residual disease in patients without signs of overt metastasis. The monitoring of systemic therapies is probably the most exciting application of CTCs detection technologies. Identifying metastatic cell diversity through CTCs profiling could more effectively guide drug selection in late stage cancer patients, making it reasonable to speculate that patients whose blood contains CTCs with these diverse phenotypes could greatly benefit from optimized multidrug treatment regimens.
Conclusion
We demonstrated efficient CTCs capture platform based on a cell size separation principle. The system was used to isolate CTCs from urothelial cancer patient blood samples. Furthermore, we reported an ability to culture the captured cells, a critical requirement for post-isolation cellular analysis.
Footnotes
Competing interests: Dr. Kolostova and Dr. Bobek are consultants for MetaCell. Dr. Cegan declares no competing interest.
This paper has been peer-reviewed.
References
- 1.Msaouel P, Koutsilieris M. Diagnostic value of circulating tumor cell detection in bladder and urothelial cancer: Systematic review and meta-analysis. BMC Cancer. 2011;4:336. doi: 10.1186/1471-2407-11-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 3.Rink M, Chun FK, Minner S, et al. Detection of circulating tumour cells in peripheral blood of patients with advanced non-metastatic bladder cancer. BJU Int. 2011;107:1668–75. doi: 10.1111/j.1464-410X.2010.09562.x. [DOI] [PubMed] [Google Scholar]
- 4.Fidler IJ, Kripke ML. Metastasis results from preexisting variant cells within a malignant tumor. Science. 1977;197:893–5. doi: 10.1126/science.887927. [DOI] [PubMed] [Google Scholar]
- 5.Fidler IJ. Metastasis: Guantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2’-deoxyuridine. J. Natl. Cancer Inst. 1970;45:773–82. [PubMed] [Google Scholar]
- 6.Liotta LA, Saidel MG, Kleinerman J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 1976;36:889–94. [PubMed] [Google Scholar]
- 7.Friedlander TW, Fong L. The end of the beginning: Circulating tumor cells as a biomarker in castration-resistant prostate cancer. J Clin Oncol. 2014;32:1104–6. doi: 10.1200/JCO.2013.54.7307. . Epub 2014 Mar 10. [DOI] [PubMed] [Google Scholar]
- 8.Danila DC, Fleisher M, Scher HI. Circulating tumor cells as biomarkers in prostate cancer. Clin Cancer Res. 2011;17:3903–12. doi: 10.1158/1078-0432.CCR-10-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaffer DR, Leversha MA, Danila DC, et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:2023–9. doi: 10.1158/1078-0432.CCR-06-2701. [DOI] [PubMed] [Google Scholar]
- 10.Danila DC, Heller G, Gignac GA, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053–8. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 11.Goodman OB, Jr, Symanowski JT, Loudyi A, et al. Circulating tumor cells as a predictive biomarker in patients with hormone-sensitive prostate cancer. Clin Genitourin Cancer. 2011;9:31–8. doi: 10.1016/j.clgc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Goldkorn A, Ely B, Quinn DI, et al. Circulating tumor cell counts are prognostic of overall survival in Southwest Oncology Group trial S0421: A phase III trial of docetaxel with or without atrasentan for meta-static castration-resistant prostate cancer. J Clin Oncol. 2014;32:1136–42. doi: 10.1200/JCO.2013.51.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–9. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu T, Lu B, Tai YC, et al. A cancer detection platform which measures telomerase activity from live circulating tumor cells captured on a microfilter. Cancer Res. 2010;70:6420–6. doi: 10.1158/0008-5472.CAN-10-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin HK, Zheng S, Wiliams AJ, et al. Portable filter-based microdevice for detection and characterization of circulating tumor cells. Clin Cancer Res. 2010;16:1–8. doi: 10.1158/1078-0432.CCR-10-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danila DC, Anand A, Sung CC, et al. TMPRSS2-ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castrationresistantprostate cancer patients treated with abiraterone acetate. Eur Urol. 2011;60:897–904. doi: 10.1016/j.eururo.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y, Palma JF, Agus DB, et al. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin Chem. 2010;56:1492–5. doi: 10.1373/clinchem.2010.143297. [DOI] [PubMed] [Google Scholar]
- 18.Scher HI, Heller G, Molina A, et al. Evaluation of circulating tumor cell (CTC) enumeration as an efficacy response biomarker of overall survival (OS) in metastatic castration-resistant prostate cancer (mCRPC): Planned final analysis (FA) of COU-AA-301, a randomized double-blind, placebo-controlled phase III study of abiraterone acetate (AA) plus low-dose prednisone (P) post docetaxel. J Clin Oncol. 2011;29:293s. [Google Scholar]
- 19.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–40. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 20.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 21.Pantel K, Den´eve E, Nocca D, et al. Circulating epitelial cells in patients with benign colon diseases. Clin Chem. 2012;58:936–40. doi: 10.1373/clinchem.2011.175570. [DOI] [PubMed] [Google Scholar]
- 22.Mikolajczyk L, Millar S, Tsinberg P, et al. Detection of EpCAM-negative and cytokeratin-negative circulating tumor cells in peripheral blood. J Oncol. 2011;252361 doi: 10.1155/2011/252361. . Epub 2011 Apr 19. [DOI] [PMC free article] [PubMed] [Google Scholar]


