Abstract
Introduction:
We studied the feasibility of ex-vivo nephron-sparing surgery and autotransplantation for complex renal tumours. We also studied the role of laparoscopy in these situations.
Methods:
All patients who underwent renal autotransplantation for renal tumour at our centre were included in this retrospective study. Patient profiles were recorded in detail. Operative and postoperative details were also recorded.
Results:
Our series includes 3 patients. Two patients had complex renal cell carcinoma and 1 patient had bilateral large angiomyolipoma. In first 2 patients, laparoscopic approach was used for nephrectomy. Operative time for case 1, 2 and 3 was 5.5, 4.5, 8 (right side) and 6 (left side) hours, respectively. Cold ischemia time was 110, 90, 150 and 125 minutes, respectively. One patient required temporary postoperative hemodialysis.
Conclusion:
Ex-vivo nephron-sparing surgery and autotransplantation still remain a viable option for complex renal tumours. It offers satisfactory renal functional outcome with acceptable morbidity. The laparoscopic approach should be used whenever possible to reduce morbidity.
Introduction
Kidney autotransplantation was first performed by James Hardy in 1963 for the management of a high ureteral injury.1 After this landmark surgery, autotransplantation has been described for renal artery disease, complex urological reconstruction, upper ureteral tumour, extensive renal parenchymal tumour, and complex nephrolithiasis.2–6 Traditionally autotransplantation involves 2 separate incisions with morbidity and vascular complications. With technical advances in endourology and laparoscopy, indications for autotrans-plantation were reduced as of 1990. However, in the last decade there have been several reports of bench surgery and autotransplantation for complex renal tumours.7–9 Many of these reports have used combined laparoscopic and open approaches to reduce morbidity. In a small proportion of patients with complex renal tumours, this procedure preserved renal function with satisfactory oncological outcome. We present our experience with ex-vivo nephron surgery and autotranplantation for complex renal tumours. We also explore the role of laparoscopy in these complex procedures.
Methods
Case 1
A 64-year-old male presented with left renal cell carcinoma with metastasis in the opposite adrenal gland. In the past, he was treated with open right radical nephrectomy and left adrenalectomy for renal cell carcinoma with adrenal metastasis. His serum creatinine was 1.5 mg/dL during presentation. Computed tomography revealed 2 lesions in the solitary left kidney: one in the upper pole on the medial aspect measuring 4 × 3 cm and the other in the lower pole measuring 2 × 2 cm (Fig. 1a). The right adrenal lesion was removed by the transperitoneal laparoscopic approach. For the left renal tumour, we performed a transperitoneal laparoscopic left radical nephrectomy followed by bench dissection and autotransplantation (Fig. 1b). Both procedures were performed at the same time.
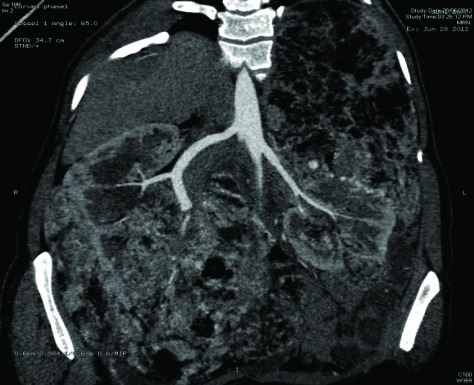
Fig. 1a.
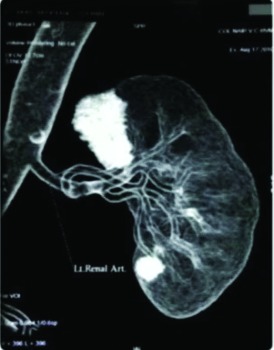
Case 1: Computed tomography revealing 2 lesions in the solitary left kidney: in the upper pole on the medial aspect measuring 4 × 3 cm and the other one in the lower pole measuring 2 × 2 cm.
Fig. 1b.
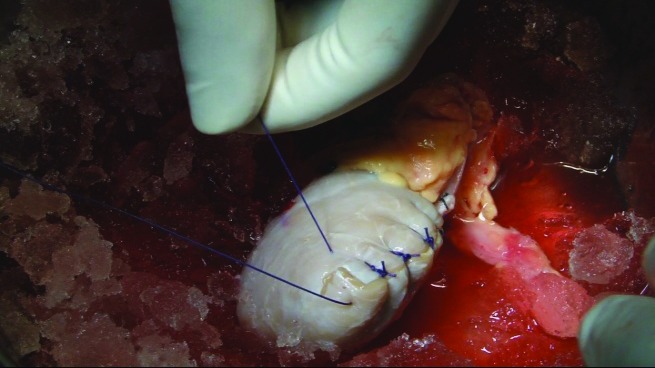
Case 1: For left renal tumour, transperitoneal laparoscopic left radical nephrectomy followed by bench dissection and autotransplantation.
Case 2
A 35-year-old female presented with bilateral renal cell carcinoma (Fig. 2a). For the right side upper polar tumour, transperitoneal laparoscopic nephron-sparing surgery was performed (Fig. 2b). Three weeks later the patient was operated for the left side complex renal tumour. Laparoscopic radical nephrectomy, bench surgery and tumour resection followed by autotransplantation of reconstructed kidney was performed (Fig. 2c).
Fig. 2a.
Case 2: Bilateral renal cell carcinoma.
Fig. 2b.
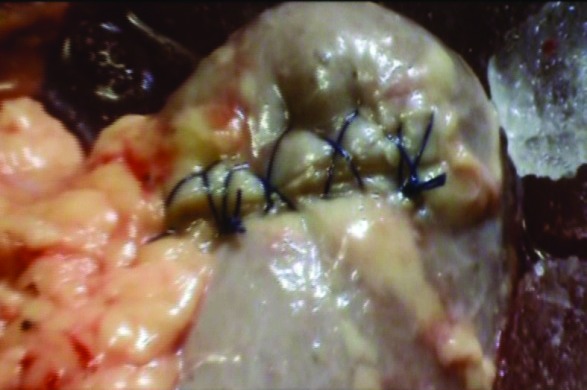
Case 2: For the right-sided upper polar tumour, transperitoneal laparoscopic nephron-sparing surgery was performed.
Fig. 2c.
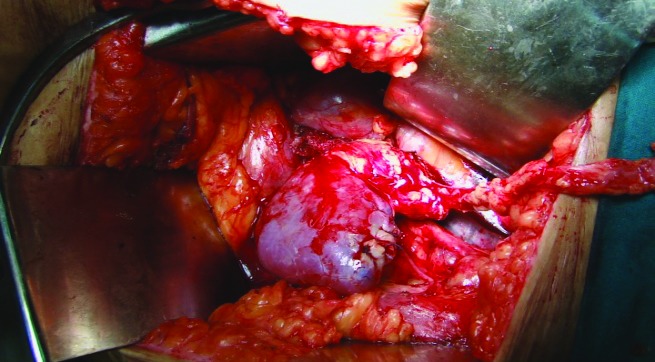
Case 2: Autotransplanted kidney with good perfusion.
Case 3
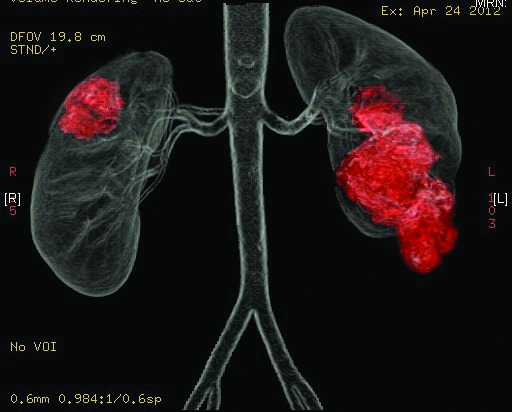
A 28-year-old female presented with giant bilateral angiomyolipoma measuring about 30 × 15 cm on the left side and 26 × 18 cm on the right side (Fig. 3a). Her renal parameters were within normal limits. Detailed evaluation ruled out tuberous sclerosis complex. Different options were discussed with the patient. Angioembolisation was not feasible in view of the large size of the tumour and the difficulty in identifying the feeding vessel. A bilateral open nephrectomy, ex-vivo nephron surgery and auto transplantation was performed in 2 separate sessions (Fig. 2, Fig. 3).
Fig. 3a.
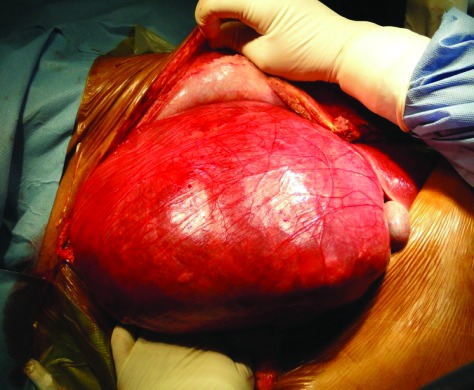
Case 3: Giant bilateral angiomyolipoma measuring about 30 × 15 cm on the left side and 26 × 18 cm on the right side.
Surgical technique
In the 2 patients with renal cell carcinoma, radical nephrectomy was performed by transperitoneal laparoscopic approach with the standard 4-port technique. The peritoneum was reflected along the line of Toldt. Dissection was continued outside the Gerota’s fascia. Uretogonadal pedicle was dissected from the iliac vessels to preserve adequate periureteral tissue. Hilar dissection was performed to achieve control over renal vessels. The kidney was freed from posterior and superior attachments to complete the procedure. Papeverine soaked gauze was kept around the renal artery; pneumoperitoneum was removed for 15 minutes to overcome the ischemic effects. The kidney was retrieved through a pfannenstiel incision after clipping the ureter, artery and vein sequentially. The drain was kept in the renal fossa and port closure was done. The kidney was kept in ice slush and perfused with a cold HTK solution on the back table. Bench surgery was performed to excise the tumour completely followed by callycorraphy and renorrhaphy using 3-0 and 2-0 polyglactin suture, respectively. Renal bed was prepared in the right iliac fossa by extending the pfannenstiel incision. Reconstructed kidney was autotransplanted by anastomosing the renal vein to the external iliac vein end-to-side and renal artery to the internal iliac artery end-to-end using 6-0 polypropylene suture. In both cases remnant kidney was anastomosed to the right iliac vessels. Ureteric reimplantation was performed by extravesical Lich-Gregoir technique. The drain was placed and the wound closed in layers.
In the patient with bilateral giant angiomyolipoma, open nephrectomy was performed with loin incision extending up to iliac fossa to facilitate autotransplant. First, the procedure was performed on the right side, 4 weeks later the procedure was repeated on the left side. After entering the peritoneal cavity, the colon was reflected from lateral attachments to bare the kidney and ureterogonadal pedicle. The ureterogonadal pocket was dissected up to the iliac vessels without disturbing the vascularity of the ureter. Dissection was continued outside the Gerota’s fascia to mobilize the kidney all around. Hilar dissection was performed to achieve control over renal vessels. Ureter was disconnected at the level of iliac vessels and diuresis was confirmed followed by ligation and disconnection of renal artery and vein sequentially. On the right side, the cuff of the vena cava was included in the renal vein. Each kidney weighed about 2500 g. Bench surgery was performed to excise the tumour completely and to reconstruct the kidney meticulously. Autotransplantation was performed extending the incision to the iliac fossa as described earlier.
Results
The patients’ clinical profiled are listed in Table 1. Operative and postoperative profiles are shown in Table 2. Case 1 required postoperative hemodialysis for 5 days. Renal parameters reached normal levels by postoperative day 12. Another 2 patients did not require hemodialysis during the postoperative period. There were no operative and postoperative complications. Follow-up imaging showed good perfusion of the autotransplanted renal unit. In case 3, the right autotransplanted kidney failed to show good perfusion; however the patient’s renal parameters reached normal limits.
Table 1.
Demographic profile
| Variable | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age/sex | 64/M | 35/F | 28/F |
| BMI (kg/m2) | 30.45 | 20.7 | 19.5 |
| Tumour location | Left upper and lower pole | Left lower and mid pole | Bilateral, multifocal |
| Tumour type | RCC | RCC | Angiomyolipoma |
| Preoperative serum creatinine (mg/dL) | 1.5 | 0.9 | 1.1 |
BMI: body mass index; RCC: renal cell carcinoma.
Table 2.
Operative and postoperative profile
| Variable | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Operative approach for nephrectomy | Transperitoneal laparoscopy | Transperitoneal laparoscopy | Open |
| Operative time (hrs) | 5.5 | 4.5 | Right-8, left- 6 |
| Cold ischemia time (min) | 110 | 90 | Right-150, left-125 |
| Blood loss (mL) | 250 | 200 | Right-500, Left-350 |
| Need for dialysis | Yes | No | No |
| Hospital stay (days) | 12 | 7 | Right-9, Left-8 |
| Follow-up duration (months) | 30 | 18 | 12 |
| Follow-up serum creatinine (mg/dL) | 1.7 | 1.2 | 1.3 |
Discussion
Radical nephrectomy for organ-confined renal cell carcinoma is associated with increased risk of chronic kidney disease and cardiovascular events.10,11 Nephron-sparing surgery has become the standard for managing small renal tumours. For tumours of the solitary kidney, multiple bilateral tumours and tumours in patients with impaired renal function, it becomes mandatory to preserve as many nephrons as possible. This goal can be achieved either by the laparoscopic or open approach; the latter is preferred for complex tumours. In a small proportion of patients with complex tumour, in situ tumour resection is not feasible. In such situations, ex-vivo tumour resection and autotransplantation are advocated. Previously complex tumours requiring the nephron-sparing approach have been managed by open nephrectomy, bench surgery, and autotransplantation. However, after 1990 this procedure was not common. Although reasons for this are not known, it could be because of the morbidity associated with a large incision and vascular complications associated with autotransplant. As of 2000, there has been a surge of interest in ex-vivo tumour resection and autotransplant for complex renal tumours, as well as for high ureteral injuries and ureteral tumour; this interest has created a need to preserve the kidney.7–8,12–14 Many of these reports have used the laparoscopic approach to perform nephrectomy thereby reducing the morbidity. This increase in interest could be due to increased experience with laparoscopic approach for radical nephrectomy and donor nephrectomy and increasing expertise in vascular anastomosis. Laparoscopic radical nephrectomy has been demonstrated to be safe, with an oncological outcome similar to the open approach with less morbidity.15 In 2000 Gill and colleagues reported on retropritoneoscopic nephrectomy and autotransplantation in 4 patients; one of these patients had a large proximal ueteric stricture.7 Meng and colleagues reported on transperitoneal laparoscopic nephrectomy, bench surgery and autotrans-plantation in 2 patients with renal tumour.8 They attributed the feasibility of this procedure to their expertise in laparoscopic donor nephrectomy. At our centre, we have performed more than 800 laparoscopic donor nephrectomies. We also have a lot of experience in renal transplantation which has helped us to embark on laparoscopic nephrectomy, ex-vivo nephron-sparing surgery and autotransplant for complex renal tumours requiring renal preservation.
In the first patient with 2 lesions in the left kidney and with a history of open adrenalectomy, open partial nephrectomy would have become very difficult. In the second patient with complex bilateral tumour, we chose to do ex-vivo excision in view of the young age and the multiplicity of the tumour. In the third patient with large bilateral angiomyolipoma, in situ partial nephrectomy was not possible in view of the multicentric large tumour and the difficulty in maintaining in situ cooling. Alternative approaches in the first and third patients were nephrectomy and renal replacement therapy. The drawbacks of renal replacement therapy are increased morbidity, mortality, and cost. Although the patient had bilateral large incisions, we avoided renal replacement therapy and its consequences. All 3 patients did not have renal replacement therapy, and currently live a good quality of life. As we mentioned earlier, the advantage of this procedure over renal replacement therapy is better quality of life and cost-effectiveness.
In cases 1 and 2, we performed renal transplant to contralateral iliac vessels by extending the retrieval site incision. However, in case 3 anastomosis was done to ipsilateral iliac vessels. Meng and colleagues also describe autotransplantation to the ipsilateral iliac vessels.8 Although there is a general belief that anastomosis to contralateral iliac vessels is technically easy, we did not find any difference between the two methods.
Recently, everolimus has been found to reduce the size of angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphanioleiomyomatosis-associated angiomyolipomata.16 This new treatment strategy appears promising and could be a potential treatment for tuberous sclerosis complex. In bilateral large angiomyolipomas, it may play a neo-adjuvant role and reduce the complexity of the surgical procedure. However, we did not get an opportunity to use this drug in our patient.
Conclusion
Ex-vivo nephron-sparing surgery for renal tumour is a viable option in extreme situations. Laparoscopic approach should be used whenever possible to reduce the morbidity of the procedure. Bench surgery and autotransplant offer several advantages over the anephric condition with renal replacement therapy and it should be considered after discussing the pros and cons with the patient.
Fig. 3b.
Case 3: Bilateral open nephrectomy, ex-vivo nephron surgery and auto transplantation was performed in 2 separate sessions.
Fig. 3c.
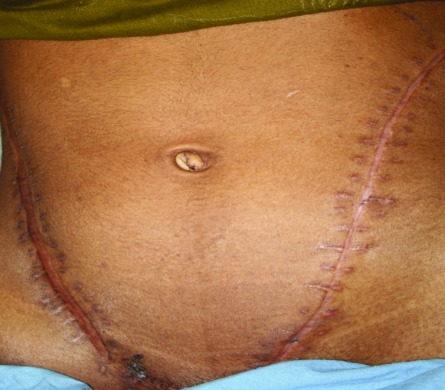
Case 3: Bilateral open nephrectomy, ex-vivo nephron surgery and auto transplantation was performed in 2 separate sessions.
Footnotes
Competing interests: Dr. Abraham, Dr. Siddaiah, Dr. Ramaswami, Dr. George, and Dr. Das all declare no competing financial or personal interests.
This paper has been peer-reviewed.
References
- 1.Hardy JD. High ureteral injuries. Management by autotransplantation of the kidney. JAMA. 1963;184:97–101. doi: 10.1001/jama.1963.03700150051008. [DOI] [PubMed] [Google Scholar]
- 2.Bodie B, Novick AC, Rose M, et al. Long-term results with renal autotransplantation for ureteral replacement. J Urol. 1986;136:1187–9. doi: 10.1016/s0022-5347(17)45278-5. [DOI] [PubMed] [Google Scholar]
- 3.Jackson CL, Novick AC. Renal autotransplantation: A reappraisal. World J Urol. 1988;6:129–35. doi: 10.1007/BF00326629. [DOI] [Google Scholar]
- 4.Novick AC, Jackson CL, Straffon RA. The role of renal autotransplantation in complex urological reconstruction. J Urol. 1990;143:452. doi: 10.1016/s0022-5347(17)39988-3. 457. [DOI] [PubMed] [Google Scholar]
- 5.Novick AC. Microvascular reconstruction of complex branch renal artery disease. Urol Clin North Am. 1984;2:465–75. [PubMed] [Google Scholar]
- 6.Novick AC. Role of bench surgery and autotransplantation in renal calculous disease. Urol Clin North Am. 1981;8:299–312. [PubMed] [Google Scholar]
- 7.Gill IS, Uzzo RG, Hobart MG, et al. Laparoscopic retroperitoneal live donor right nephrectomy for purposes of allotransplantation and autotransplantation. J Urol. 2000;164:1500–4. doi: 10.1016/S0022-5347(05)67015-2. [DOI] [PubMed] [Google Scholar]
- 8.Meng MV, Freise CE, Stoller ML. Laparoscopic nephrectomy, ex vivo excision and autotransplantation for complex renal tumors. J Urol. 2004;172:461–4. doi: 10.1097/01.ju.0000130668.94919.59. [DOI] [PubMed] [Google Scholar]
- 9. Ex-vivo partial nephrectomy and renal auto-transplantation for complex renal malignancies. www.specialisedservices.nhs.uk/document/10495. Accessed October 15, 2014.
- 10.Fergany AF, Hafez KS, Novick AC. Long-term results of nephron sparing surgery for localized renal cell carcinoma: 10-year followup. J Urol. 2000;163:442. doi: 10.1016/S0022-5347(05)67896-2. [DOI] [PubMed] [Google Scholar]
- 11.Herr HW. Partial nephrectomy for unilateral renal carcinoma and a normal contralateral kidney: 10-year followup. J Urol. 1999;161:33. doi: 10.1016/S0022-5347(01)62052-4. [DOI] [PubMed] [Google Scholar]
- 12.Meng MV, Freise CE, Stoller ML. Expanded experience with laparoscopic nephrectomy and auto-transplantation for severe ureteral injury. J Urol. 2003;169:1363. doi: 10.1097/01.ju.0000054927.18678.5e. [DOI] [PubMed] [Google Scholar]
- 13.Fabrizio MD, Kavoussi LR, Jackman S, et al. Laparoscopic nephrectomy for autotransplantation. Urology. 2000;55:145. doi: 10.1016/S0090-4295(99)00367-2. [DOI] [PubMed] [Google Scholar]
- 14.Jarrett TJ, Potter SR, Girrotto JA, et al. Laparoscopic assisted autotransplantation for treatment of transitional cell carcinoma of the mid ureter. J Urol. 2001;165:1625. doi: 10.1016/S0022-5347(05)66367-7. [DOI] [PubMed] [Google Scholar]
- 15.Portis AJ, Yan Y, Landman J, et al. Long-term followup after laparoscopic radical nephrectomy. J Urol. 2002;167:125. doi: 10.1016/S0022-5347(05)65277-9. [DOI] [PubMed] [Google Scholar]
- 16.Bissler JJ, Kingswood JC, Radzikowska E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381:817. doi: 10.1016/S0140-6736(12)61767-X. [DOI] [PubMed] [Google Scholar]


