Abstract
The recent development of infectious retroviral pseudotypes bearing hepatitis C virus (HCV) glycoproteins represents an opportunity to study the functionally active form of the HCV E1 and E2 glycoproteins. In the culture supernatant of cells producing HCV retroviral pseudotypes, the majority of E2 was not associated with infectious particles and failed to sediment on sucrose gradients. The E2 that was incorporated into infectious particles appeared as a triplet of diffuse bands at 60, 70, and 90 kDa. Similarly, three major forms of E1 were incorporated into the pseudotype particles, migrating at 33, 31, and 25 kDa. Endoglycosidase H (endo-H) treatment of particles demonstrated that the incorporated E1 was partially or completely sensitive to digestion. In contrast, the majority of the incorporated E2 was endo-H resistant. Purified pseudotype particles were found to contain both disulfide-linked aggregates and nonaggregated E1 and E2. HCV pseudotypes generated from cells expressing E1E2p7 showed similar heterogeneity in the incorporated glycoproteins and were of comparable infectivity to those generated by expression of E1E2. Our results demonstrate the heterogenous nature of E1 and E2 incorporated into retroviral pseudotypes and highlight the difficulty in identifying forms of the HCV glycoproteins that initiate infection.
Hepatitis C virus (HCV) is an enveloped virus classified in the Hepacivirus genus of the family Flaviviridae (22). The single-stranded, positive-sense RNA genome contains a single open reading frame that is translated to yield the viral proteins in the form of a polyprotein. A cellular signal peptidase releases the putative structural proteins (core, E1, E2, and p7) from this polyprotein, consistent with their translocation across the endoplasmic reticulum (ER) membrane. E1 and E2 are type I integral transmembrane proteins that undergo extensive N-linked glycosylation and are likely to function in virus attachment and cell entry. The p7 protein is a 63-amino-acid hydrophobic polypeptide predicted to contain two transmembrane-spanning regions and localized to both the plasma membrane and ER of expressing cells (3). Cleavage at the E2-p7 junction is inefficient, resulting in the production of an E2-p7 species of unknown function (21, 24). While dispensable for the replication of HCV subgenomic replicons, p7 is critical for infectivity in the chimpanzee (32) and has recently been shown to function as an ion channel (18, 28).
Due to the lack of an in vitro system that efficiently supports the full virus life cycle, most studies of the HCV structural proteins have used heterologous expression systems (reviewed in references 11 and 27). Both E1 and E2 glycoproteins (gp's) have been reported to localize to the ER, with no cell surface expression detectable. The glycans associated with these proteins are not modified by Golgi enzymes and are sensitive to endoglycosidase H (endo-H), suggesting static retention of E1 and E2 in an early compartment of the secretory pathway (15). These observations are consistent with a model of HCV assembly at the ER membrane and are supported by the presence of ER retention signals within the transmembrane domains of both gp's (5, 7, 17, 25).
Expression studies in vitro have revealed two forms of the gp's: aggregates covalently linked by disulfide bonds, and noncovalently associated E1E2 heterodimers. Aggregated gp's have been suggested to result from a nonproductive folding pathway, whereas noncovalently associated heterodimers may represent a prebudding form of the gp's (9, 12, 13). Currently, there is little information on the disulfide bonding and glycosylation status of E1 and E2 in native HCV particles. In hepatoma cells supporting full-length HCV RNA replication, only noncovalently associated E1E2 was observed, whereas transient plasmid-based expression of the same proteins resulted in the formation of disulfide-linked aggregates (reference 30 and unpublished observations).
Our laboratory (19) and others (2, 10) recently reported HCV gp expression at the surface of transiently transfected cells, allowing the genesis of retroviral particles bearing the HCV gp's. These pseudotype particles are infectious for liver-derived cells and represent a tool to study a functional HCV gp complex. To identify the functional form of the HCV gp's, we characterized the E1 and E2 incorporated into infectious pseudotype particles. The incorporated gp's were found to be heterogeneous with respect to their molecular mass, disulfide linkages, and glycosylation.
MATERIALS AND METHODS
Antibodies and reagents.
Rat monoclonal antibodies (MAbs) 10/76b, 11/20, 3/11, 6/53, 7/16, and 6/1a were previously described (16). Anti-E1 MAb 725P was purchased from Maine Biotechnology (Portland, Maine), A4 was a kind gift from H. Greenberg (Stanford University, Stanford, Calif.), and H52 and H53 were from Jean Dubuisson (Institut Pasteur de Lille, Lille, France). Immunoblot assays for E1 were performed with a mixture of A4 and 725P and for E2 with a mixture of 3/11, 6/53, and 6/1a. Horseradish peroxidase-conjugated anti-mouse (Pierce, Rockford, Ill.) and anti-rat (Jackson, West Grove, Pa.) immunoglobulin G were used according to the manufacturers' recommendations. Endo-H and N-glycosidase F were used according to the manufacturer's instructions (Calbiochem, San Diego, Calif.). Dulbecco's modified Eagle medium (DMEM) and fetal bovine serum (FBS) were from Invitrogen (Carlsbad, Calif.). Plasmid pNL4.3.Luc.R−E− (8) was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Plasmid construction.
Sequences encoding E1E2 (polyprotein residues 170 to 746) and E1E2p7 (residues 170 to 809) of the H77 HCV sequence were amplified by PCR and ligated with pcDNA3.1(-)neo (Invitrogen) using standard procedures. Inserts were sequenced to confirm identity. The endogenous signal sequence constituting the 21 most-C-terminal amino acid residues of Core were included to allow translocation across the ER membrane. Expression vectors encoding Core-E1E2p7 (polyprotein residues 1 to 809), E1E2p7NS2 (residues 170 to 1026), and Core-E1E2p7NS2 (residues 1 to 1026) were generated similarly.
Generation of HIV-HCV pseudotype particles.
Human immunodeficiency virus (HIV)-HCV pseudotypes were generated as previously described (19). Briefly, 293T cells were cotransfected with an E1E2 expression plasmid and pNL4.3.Luc.R−E−, using Lipofectamine 2000 (Invitrogen). Lipid-DNA complexes were removed 4 to 6 h later and replaced with DMEM supplemented with 3% FBS. At 48 h posttransfection, the culture medium was collected, clarified by low-speed centrifugation for 15 min, and then stored at 4°C before analysis. Transfected cell monolayers were washed with phosphate-buffered saline (PBS) and resuspended in lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1% NP-40, Complete protease inhibitors [Roche Biochemicals, Indianapolis, Ind.]) on ice for 30 min. Lysates were clarified by centrifugation at 4°C for 15 min at maximum speed in a microcentrifuge, and the resulting supernatants were removed, aliquoted, and stored frozen at −80°C.
Infectivity assay.
Retroviral pseudotype infectivity was measured as previously described (19). Briefly, Hep3B cells were seeded at 8 × 103 cells per well of a 96-well plate the day prior to infection. For infection, medium was removed and pseudotype virus stock, diluted in DMEM supplemented with 3% FBS and 4 μg of Polybrene (Sigma, St. Louis, Mo.)/ml, was added. After overnight incubation, the inoculum was removed and replaced with DMEM supplemented with 3% FBS. At 72 h postinfection, the medium was removed and the cells were lysed with 40 μl of cell lysis buffer (Promega, Madison, Wis.) per well. Luciferase activity was assayed by the addition of 35 μl of lysate to 50 μl of luciferase substrate and measured for 10 s in a luminometer (Lumat LB 9507).
Sucrose gradients.
Linear sucrose gradients were formed on a Gradient Master 107ip (Biocomp Inc., New Brunswick, Canada) according to the manufacturer's instructions. For analysis of sedimentation, 7-to-47% (wt/vol, in PBS) sucrose gradients were centrifuged in an SW41 rotor at 35,000 rpm for 3 h at 4°C. Fractions were collected from the top of the gradient and trichloroacetic acid (TCA) precipitated prior to separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Since the sucrose concentration in fractions from a 7-to-47% gradient was inhibitory in the Hep3B infectivity assay, linear 5-to-20% (wt/vol, in PBS) sucrose gradients were used for infectivity studies. These gradients were centrifuged in an SW41 rotor for 15 min at 35,000 rpm at 4°C. For pelleting virus through a sucrose cushion, 10 ml of cell culture medium was layered onto 1.5 ml of 20% (wt/vol, in PBS) sucrose before centrifugation at 25,000 rpm in an SW41 rotor for 3.5 h. The medium and cushion were discarded, and the virus pellet was resuspended in 100 μl of PBS on ice.
p24 ELISA.
After pelleting through a sucrose cushion, the pseudotype virus was inactivated by resuspension in PBS supplemented with 1% Empigen and incubated at 56°C for 30 min. A commercially available HIV type 1 p24 antigen enzyme-linked immunosorbent assay (ELISA) protocol (Aalto Bio Reagents, Dublin, Ireland) was used to quantify the p24 content of samples.
RESULTS
The majority of extracellular E2 is not associated with infectious particles.
Due to the lack of an efficient cell culture system to propagate HCV, the nature of functional HCV E1E2 gp's is unclear. The recent observation that retroviruses can incorporate HCV gp's, and that the resulting viruses infect human liver-derived cells in a pH-dependent manner, suggests that these particles contain functional HCV gp's (2, 19, 34). We therefore isolated HIV-HCV particles and characterized their incorporated E1 and E2.
The clarified cell culture medium from transfected 293T cells producing HIV-HCV pseudotype particles was analyzed by velocity sedimentation on a 5-to-20% sucrose gradient. Fractions were collected and analyzed by immunoblotting for HCV E2 and p24, the HIV type 1 capsid protein (Fig. 1A). Surprisingly, the majority of E2 did not sediment, remaining in the loading zone and in the top-most gradient fraction. In contrast, the majority of p24 sedimented, consistent with its incorporation into particles released into the medium, with only a small amount of p24 detectable in the top fraction of the gradient. Similar results were obtained if the pseudotype virus stocks were stored at 4°C, −80°C, or analyzed immediately after harvesting. The same fractions were analyzed for infectivity (Fig. 1B). Since the majority of E2 protein failed to sediment, to confirm that particle infectivity was HCV gp dependent, all fractions were preincubated with MAb 11/20 specific for HCV E2 or an irrelevant control MAb, 10/76b. Infectivity for Hep3B cells cosedimented with the p24-containing gradient fractions and was specifically inhibited by 11/20 (Fig. 1B).
FIG. 1.
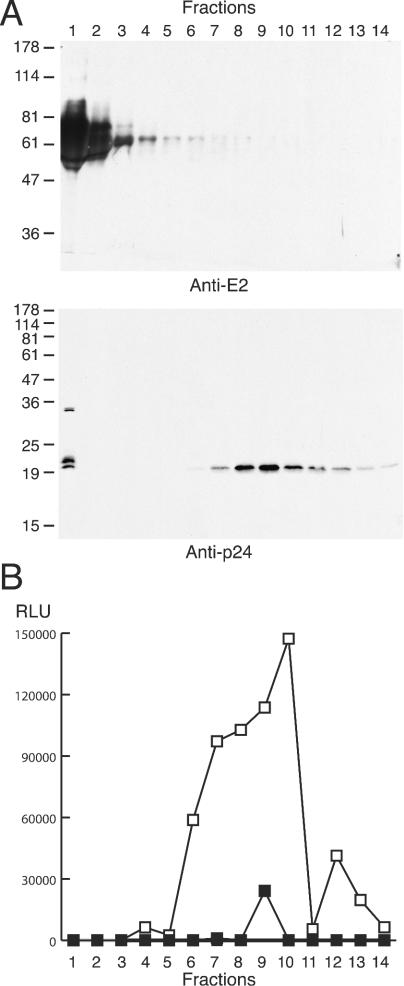
The majority of released E2 does not cosediment with particles or infectivity. Cell culture medium from 293T cells producing HIV-HCV pseudotype particles was applied to a 5-to-20% sucrose gradient, fractions of equal volume were collected from the top of the gradient (1 = top and 14 = bottom), and aliquots were either precipitated with TCA for immunoblotting with anti-E2 or anti-p24 (8 or 12% polyacrylamide gels, respectively) (A) or assayed for infection of Hep3B cells (B). Fractions were incubated either with anti-E2 (11/20; ▪) or an irrelevant MAb (10/76b; □) for 1 h at 37°C, prior to infection. The migration of molecular mass markers is indicated (in kilodaltons). RLU, relative light units.
Since the majority of the E2 released from HIV-HCV pseudotype-producing cells was not associated with infectivity, it was necessary to remove the nonsedimenting E2 to characterize the gp's incorporated into infectious particles. Since the nonsedimenting E2 barely entered the sucrose gradient, this material could be soluble or of low density. We therefore assessed whether pelleting the virus through a 20% sucrose cushion could remove the nonsedimenting species. After pelleting, the virus was resuspended in PBS and analyzed by sedimentation on a 7-to-47% sucrose gradient (Fig. 2). As previously shown, the majority of E2 in the medium failed to sediment along the gradient, though a sedimenting species could be detected upon overexposure of the immunoblot (Fig. 2, upper left panel). Pelleting through the cushion allowed the removal of the nonsedimenting E2 and concentrated a sedimenting species (Fig. 2, upper right panel). A fraction of the total p24 was observed in the top-most fraction, even after pelleting through the cushion, possibly representing soluble p24 that was released from virions disrupted during resuspension. The E2 protein that sedimented on the gradient migrated as a diffuse smear with several distinct forms (∼60, 70, and 90 kDa). Cosedimentation of p24 and E2 was not complete, with the peak of E2 in fraction 10 and p24 most abundant in fractions 10 and 11.
FIG. 2.
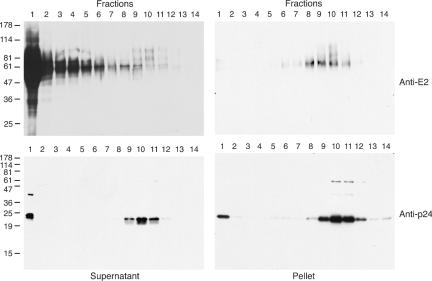
Nonsedimenting E2 is removed by pelleting through a sucrose cushion. HIV-HCV pseudotype particles, either in cell culture medium (supernatant) or purified by pelleting through a sucrose cushion (pellet), were sedimented upon 7-to-47% sucrose gradients. Fractions were collected from the top of the gradient (1 = top, 14 = bottom), precipitated with TCA, and analyzed by immunoblotting with anti-E2 or anti-p24 (8 or 12% polyacrylamide gels, respectively). The migration of molecular mass markers is indicated (in kilodaltons).
To assess whether the nonsedimenting E2 species affected infection by HIV-HCV particles, the top fraction of a 5-to-20% sucrose gradient, run in parallel to that depicted in Fig. 1, was added to an infectivity assay using pseudotype particles purified by pelleting through a sucrose cushion. No infectivity was associated with this gradient fraction, and its addition had no effect on the infection by pelleted virus (data not shown). The nature of this nonsedimenting E2 species remains obscure, but since it was separable from, and did not affect the infectivity of, HIV-HCV pseudotype particles, it has not been characterized further.
Characterization of incorporated E1E2 gp's.
Multiple virus- and plasmid-based expression systems have been used to demonstrate the localization of HCV E1 and E2 to the ER. Consistent with this location, their N-linked glycans are sensitive to digestion by endo-H. To assess the glycosylation status of the incorporated HCV gp's, pseudotype particles were generated using an E1E2 expression plasmid, pelleted through a sucrose cushion, denatured, and either mock treated or digested with endo-H or N-glycosidase F. Similar digestions were performed on transfected cell lysates. E1 and E2 were analyzed by SDS-PAGE and immunoblotting (Fig. 3).
FIG. 3.
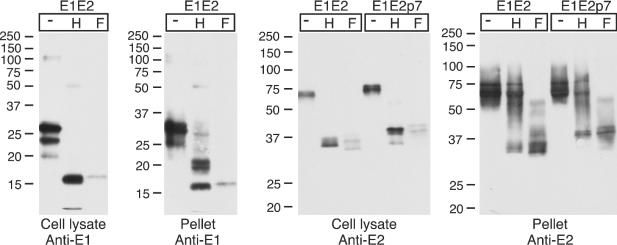
Endoglycosidase sensitivity of cell-associated and pseudotype-incorporated gp's. 293T cells were transfected to generate HIV-HCV pseudotype particles with plasmids expressing either E1E2 or E1E2p7. Cell lysates or cushion-pelleted particles were denatured prior to mock digestion (−) or digestion with endo-H (H) or N-glycosidase-F (F), applied to a reducing SDS-PAGE, and immunoblotted with anti-E1 or anti-E2 antibodies (12 or 10% polyacrylamide gels, respectively). The migration of molecular mass markers is indicated (in kilodaltons).
E1 in cell lysates appeared as a major band, migrating at ∼27 kDa, with further species migrating at 24 and 20 kDa. The 27-kDa protein probably represents a fully glycosylated E1 species (four glycans added), with the 24-kDa protein partially glycosylated (three glycans added). The 20-kDa species may represent a partially deleted form of E1 resulting from excision of a cryptic intron (14). All of the E1 species in the cell lysate were sensitive to endo-H, the majority comigrating with the 17-kDa N-glycosidase F-deglycosylated species after digestion. An E1-related species, migrating at ∼5 kDa, likely represents the intron-excised E1 after deglycosylation. Following N-glycosidase F digestion, the signal for both E1 and E2 by immunoblotting was reduced, possibly due to reduced recognition of the deglycosylated form by the MAbs used for immunoblotting. E1 incorporated into the HIV-HCV particles migrated more slowly than that detected in the cell lysate, with species migrating at 33, 31, and 25 kDa. Each of these species was, at least partially, sensitive to endo-H digestion. Following endo-H treatment, the 17-kDa fully deglycosylated form (comigrating with N-glycosidase F-treated E1) was detected, along with a doublet of bands at 21 and 19 kDa. These bands may represent E1 with two and one endo-H-resistant glycans added. The minor E1-related product migrating at ∼5 kDa, corresponding to the intron-excised E1 after deglycosylation, was also incorporated into pseudotype particles (apparent upon longer exposures) (data not shown). E2 in the cell lysate was sensitive to digestion with endo-H. Doublets of bands were visible after both endo-H and N-glycosidase F digestion. These may be the result of a proteolytic cleavage or the presence of a glycan resistant to digestion with both endo-H and N-glycosidase F (the latter will not cleave oligosaccharides containing α1-3-linked core fucose). In contrast to the cell-associated form of E2 and the particle-incorporated E1, the majority of incorporated E2 was completely resistant to endo-H.
Disulfide-linked aggregates have been suggested to result from a nonproductive folding pathway for E1 and E2, while noncovalently linked E1E2 heterodimers are thought to be a prebudding form of the HCV gp's. To determine if disulfide-linked aggregates were incorporated into HIV-HCV pseudotype particles, viruses were pelleted through a sucrose cushion and separated by SDS-PAGE under reducing or nonreducing conditions, and E1 or E2 was detected by immunoblotting (Fig. 4).
FIG. 4.
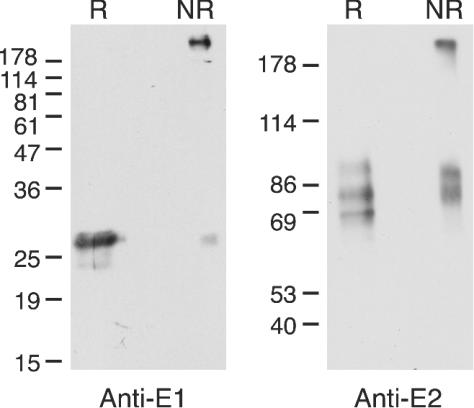
Disulfide-linked aggregates of both E1 and E2 are incorporated into pseudotype particles. HIV-HCV pseudotype particles were purified by pelleting through a sucrose cushion before analysis by reducing (R) or nonreducing (NR) SDS-PAGE and immunoblotting with anti-E1 or anti-E2 antibodies (12 or 8% polyacrylamide gels, respectively). The migration of molecular mass markers is indicated (in kilodaltons).
Under nonreducing conditions, incorporated E1 and E2 were detected both as slowly migrating (∼200 kDa) and more rapidly migrating, nonaggregated species. These slowly migrating species were absent after reduction, indicating their stabilization by disulfide bonds. These data are consistent with the incorporation of both disulfide-linked aggregates and nonaggregated E1 and E2 into HIV-HCV pseudotype particles. Under nonreducing conditions, nonaggregated E2 migrated as a doublet, in contrast to the reduced E2, which migrated as three bands within a diffuse smear. This difference in migration indicated that intramolecular disulfide bonds contribute to the secondary structure of the pseudotype particle-incorporated, nonaggregated E2.
In summary, these data suggest that the pseudotype particle-associated E1 and E2 passed through the Golgi apparatus, consistent with retroviral budding from the plasma membrane. However, the incorporated HCV gp's were heterogeneous in nature, consisting of disulfide-linked aggregates and nonaggregated species, with diverse N-linked glycans.
Effect of flanking polyprotein sequences on pseudotype infectivity.
Recent studies have generated HIV-HCV pseudotypes using plasmid expression constructs encoding E1E2. During virus infection, however, the gp's are expressed as part of the HCV polyprotein, flanked by the Core and p7 proteins. Indeed, the p7 protein is often observed in the context of E2-p7, a relatively stable protein that may have a function distinct from that of E2. The recently demonstrated ion channel activity of p7 (18, 28) is reminiscent of the M2 protein of influenza virus, which acts to protect the hemagglutinin protein from the acidic environment of the Golgi during transport to the cell surface (26, 33). Since HCV gp-mediated entry requires, like influenza virus, a low-pH step, we hypothesized that p7 might play a similar role to M2 and, hence, coexpression of p7 with E1E2 may enhance pseudotype particle infectivity.
To examine the effect of p7 upon E1E2 function, we generated pseudotype particles by expressing E1E2p7. Since E2 and E2-p7 comigrate on SDS-PAGE, deglycosylation is necessary to resolve these two species (21). As seen for E1E2-bearing particles, when E1E2p7 was used as the expression construct, a doublet of E2-related bands was detected after both endo-H and N-glycosidase F treatments (Fig. 3). Comparison of the deglycosylated E2 species generated from plasmids expressing E1E2 and E1E2p7 showed that E2 generated in the presence of p7 migrated more slowly. This observation is consistent with an inefficient cleavage at the E2-p7 junction and expression and incorporation of an E2-p7 species into pseudotype particles. Some cleavage at the E2-p7 site was detectable, especially after endo-H treatment of the cell lysate, where a band comigrating with endo-H-treated E2 from the E1E2 plasmid was visible. Migration of E1 was unaffected by the presence of p7 in the construct (data not shown).
To compare the infectivity of particles generated from cells expressing E1E2 or E1E2p7, viruses were pelleted through a sucrose cushion and assayed for p24 antigen. Quantification of the particle-associated fraction allowed normalization of particle numbers and, hence, the evaluation of differences in incorporation of E1 and E2. Samples containing equivalent particulate p24 were analyzed by immunoblotting, and the level of incorporated E1 and E2 was found to be reduced ∼3-fold upon p7 coexpression (Fig. 5). Infection of Hep3B cells with equal amounts of pseudotype particles (based on particulate p24 content) showed comparable levels of infectivity (within a threefold difference) (Fig. 5B). Similar results were seen when Huh7.5 cells were used to assay infectivity (data not shown). These data suggest that E2-p7 is incorporated into pseudotype particles, and its presence does not modify the particle infectivity.
FIG. 5.
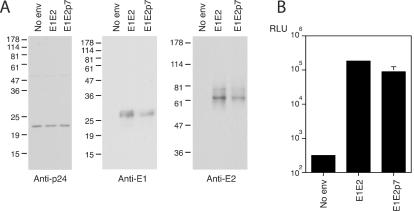
Analysis of pseudotype particles generated using the E1E2p7 expression construct. (A) Incorporation of HCV gp's, normalized to particulate p24. Immunoblotting was performed with anti-p24, anti-E1, or anti-E2 antibodies (12, 12, or 8% polyacrylamide gels, respectively). The migration of molecular mass markers is indicated (in kilodaltons). (B) Infectivity of pseudotypes generated using E1E2 or E1E2p7 for Hep3B cells. Hep3B cells were infected with transfected cell medium containing 1.2 ng of particulate p24, and 72 h postinfection cells were lysed and assayed for luciferase activity. This is a representative of three experiments, with comparable data observed. Values are the means of triplicate wells, with standard deviations indicated. RLU, relative light units.
The Core protein has been shown to interact with E1 (23) and might conceivably be incorporated into pseudotype particles. In addition, while the mechanisms are unknown, sequences C terminal of the structural proteins are required for the production of infectious flavivirus particles (1, 20). To test the effect of Core and NS2 coexpression on pseudotype infectivity, plasmids encoding Core-E1E2p7, E1E2p7NS2, and Core-E1E2p7NS2 were used to generate pseudotype particles. Expression of the gp's from these vectors was reduced compared to that of the E1E2 and E1E2p7 constructs; hence, the amount of E1 and E2 incorporated into pseudotype particles was reduced (data not shown). When the infectivity of these particles was assayed, the reduced levels of incorporation were reflected in lower levels of luciferase activity.
During analysis of the E1 and E2 present within cell lysates and particles, an approximately 100-kDa protein species was detectable when E1E2, and more so when E1E2p7, was expressed and immunoblots were overexposed (Fig. 6). This species was most apparent when blots were probed for the presence of E1; however, a comigrating band was observed when probed with anti-E2 MAbs, migrating just above the diffuse E2 bands (data not shown). This species could be detected when the MAbs were used individually. This protein was not detectable, however, upon nonreducing SDS-PAGE, suggesting that it is a component of the high-molecular-mass, disulfide-linked complex (Fig. 6, middle panel). After deglycosylation with endo-H, this band was missing and a faster-migrating band became apparent, indicating that the species was sensitive to endo-H digestion (Fig. 6, right panel). This band may represent an E1E2 form that is resistant to SDS treatment or an uncleaved E1E2 species, demonstrating a further level of heterogeneity within the incorporated gp's.
FIG. 6.
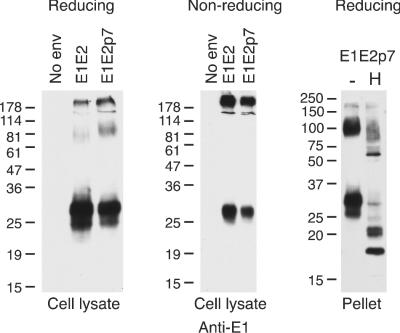
A 100-kDa species is present in cell lysates and pseudotype particles. Particles were generated using E1E2 or E1E2p7 expression constructs. Cell lysates were separated under reducing (left panel) or nonreducing (middle panel) conditions on 12% polyacrylamide gels, followed by immunoblotting with anti-E1. Particles were purified by pelleting through a sucrose cushion and then denatured and either mock treated (−) or treated with endo-H (H). Samples were separated by reducing SDS-PAGE and immunoblotted with anti-E1. The migration of molecular mass markers is indicated (in kilodaltons).
DISCUSSION
The HCV gp's E1 and E2 have been reported to associate in two ways: covalently linked via disulfide bonds in high-molecular-weight aggregates, or noncovalently in a heterodimeric complex (9, 12, 13). In most expression systems, E1 and E2 localize to the ER, retaining endo-H-sensitive N-linked glycans characteristic of the early compartments of the secretory transport system. Due to the lack of an efficient cell culture system for the propagation of HCV, there is a paucity of information regarding the native forms of E1 and E2 present in virions. Such information would help our understanding of the mechanisms of virus-cell attachment and membrane fusion and may guide the development of vaccines to generate immunity against functional forms of the HCV gp complex. The recent development of retroviral particles pseudotyped with the HCV gp's (2, 10, 19) represents an opportunity to study the functional E1E2 complex.
Surprisingly, the majority of E2 released from 293T cells did not cosediment with infectious pseudotype particles. The release of this nonsedimenting material may be related to the cell surface expression of E2 in the transfected 293T cells. The length of the E1 and E2 transmembrane domains may be better suited to spanning the ER membrane, rather than the plasma membrane, leading to an unstable complex when expressed at the cell surface. A secreted form of E2 was detected when E2 was expressed alone and was attributed to a reduced stability in the membrane in the absence of E1 (6). An alternative explanation is that the nonsedimenting E2 is incorporated in particles associated with a low-density component that prevents them from entering a sucrose gradient. A nonsedimenting E2 species could be detected when E1E2 of genotypes 1b and 2a were expressed and when E1E2 was expressed in the absence of HIV proteins (data not shown). Since the nonsedimenting E2 was not infectious and failed to affect infection by pseudotype particles, its biological relevance is unclear. Pelleting pseudotype particles through a sucrose cushion allowed us to separate the nonsedimenting E2 species from the infectious particles and to characterize the gp's incorporated into infectious particles. Analysis of gradient fractions for E2 and p24, using gradients of various sucrose concentrations, failed to show a complete cosedimentation, with the peak of p24 antigen always further down the gradient than E2 (Fig. 2). This suggests that not all of the particles contain E2 and that HCV gp's may preferentially incorporate into slower-sedimenting particles.
Analysis of the glycosylation status of E1 and E2 incorporated into pseudotype particles indicated that the majority of E2 was endo-H resistant. In contrast, when E1 was treated with endo-H, a fully deglycosylated form was observed (comigrating with N-glycosidase-F-treated E1), as well as 21- and 19-kDa species. These latter two forms probably correspond to the addition of two and one endo-H-resistant glycans, respectively. These observations are consistent with the budding of HIV-HCV particles through the plasma membrane, as previously proposed (19). After digestion with N-glycosidase F, doublets of E2-related bands were observed. We cannot rule out the possibility that the N-glycosidase F digestion reactions were not all complete, although E1 in the same lysates was completely deglycosylated under these conditions. An alternative explanation is that E2 undergoes a proteolytic cleavage or that the doublet may be derived by alternative splicing due to the presence of cryptic splice sites that are utilized during the nuclear expression of the gp's. Dumonceaux and colleagues recently reported the expression of an E1 species containing an in-frame deletion due to excision of an intron sequence during nuclear transcription in a plasmid-based expression system (14). We were able to detect a 20-kDa E1 species in cell lysates which may correspond to the intron-excised E1 (Fig. 3). After digestion with endo-H, the deleted E1 migrated at ∼5 kDa and was incorporated into the pseudotype particles. Since HCV is not expected to be transcribed in the nucleus and the splice acceptor site is not conserved among other HCV strains (P. Balfe, personal communication), the biological relevance of this E1 species is questionable.
Comparison of reduced and nonreduced samples indicated that incorporated E1 and E2 were in both high-molecular-mass forms dependent upon the integrity of disulfide bonds and also in non-disulfide-linked species (Fig. 4). The high-molecular-weight forms likely represent the disulfide-linked aggregates that have been observed in other expression systems and that have been suggested to result from a nonproductive folding pathway (4). Such complexes were found in particles purified from a chronically HCV-infected patient (29), suggesting that they may represent a natural form of E1 and E2 in the virion. In this latter study, however, the infectivity of the recovered particles was not demonstrated.
The p7 protein has recently been demonstrated to possess ion channel activity (18, 28). This activity is reminiscent of the M2 protein of fowl plague virus, which can act to protect the viral hemagglutinin protein from the acidic environment of the trans-Golgi network during its transportation through the secretory pathway, avoiding the low-pH-induced conformational changes that occur during influenza virus entry (26, 33). Since HCV gp-mediated entry is, like influenza virus, acid dependent and the pseudotype-incorporated E1 and E2 proteins pass through the Golgi (as indicated by the endo-H resistance of E1- and E2-associated glycans), we hypothesized that p7 might play a role similar to that of M2 in increasing the Golgi pH. Coexpression of p7 with E1E2 might therefore give rise to pseudotype particles of greater infectivity. This was not the case, however, as particles generated with E1E2 or E1E2p7 were of similar infectivity. It is possible that an ion channel activity of p7 has a role to play in the biogenesis of the authentic HCV particle. An E1- and E2-related protein of ∼100 kDa was detected in both cell lysates and pseudotype particles and could be visualized with antibodies specific for both E1 and E2. This species was more abundant when E1E2p7 was expressed, suggesting that its formation may be favored by the presence of p7. This band may represent an SDS-resistant heterodimer of E1E2, an uncleaved E1E2 species, or an assembly intermediate for the HCV gp's.
Thus, the gp's incorporated into HIV-HCV pseudotype particles exhibit heterogeneity with regard to their glycosylation pattern and disulfide linkages. Initially, we intended to utilize the HIV-HCV pseudotype particles to assess the antigenicity of the functional E1 and E2 gp's at neutral and low pH; however, the diversity of incorporated gp's makes these assays difficult to interpret. Such heterogeneity is perhaps unsurprising, given the incorporation of both functional and nonfunctional HIV gp120-41 molecules into HIV particles (31). It was speculated that nonfunctional envelope gp's represent an immunodominant antigen during HIV infection, biasing the humoral immune response towards antibodies that have minimal reactivity to functional gp's and, hence, have poor neutralizing activity. It remains to be determined whether nonfunctional forms of the HCV gp's are incorporated into pseudotypes or virions in vivo and, indeed, which form of the HCV gp's is responsible for mediating pseudotype infectivity.
Acknowledgments
We thank Harry Greenberg and Jean Dubuisson for generously providing antibodies.
This work was supported by PHS grant CA57973 and the Greenberg Medical Research Institute.
REFERENCES
- 1.Agapov, E. V., C. L. Murray, I. Frolov, L. Qu, T. M. Myers, and C. M. Rice. 2004. Uncleaved NS2-3 is required for production of infectious bovine viral diarrhea virus. J. Virol. 78:2414-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartosch, B., J. Dubuisson, and F. L. Cosset. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 197:633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrere-Kremer, S., C. Montpellier-Pala, L. Cocquerel, C. Wychowski, F. Penin, and J. Dubuisson. 2002. Subcellular localization and topology of the p7 polypeptide of hepatitis C virus. J. Virol. 76:3720-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choukhi, A., S. Ung, C. Wychowski, and J. Dubuisson. 1998. Involvement of endoplasmic reticulum chaperones in the folding of hepatitis C virus glycoproteins. J. Virol. 72:3851-3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocquerel, L., S. Duvet, J. C. Meunier, A. Pillez, R. Cacan, C. Wychowski, and J. Dubuisson. 1999. The transmembrane domain of hepatitis C virus glycoprotein E1 is a signal for static retention in the endoplasmic reticulum. J. Virol. 73:2641-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocquerel, L., J. C. Meunier, A. Op de Beeck, D. Bonte, C. Wychowski, and J. Dubuisson. 2001. Coexpression of hepatitis C virus envelope proteins E1 and E2 in cis improves the stability of membrane insertion of E2. J. Gen. Virol. 82:1629-1635. [DOI] [PubMed] [Google Scholar]
- 7.Cocquerel, L., J. C. Meunier, A. Pillez, C. Wychowski, and J. Dubuisson. 1998. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J. Virol. 72:2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 9.Deleersnyder, V., A. Pillez, C. Wychowski, K. Blight, J. Xu, Y. S. Hahn, C. M. Rice, and J. Dubuisson. 1997. Formation of native hepatitis C virus glycoprotein complexes. J. Virol. 71:697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummer, H. E., A. Maerz, and P. Poumbourios. 2003. Cell surface expression of functional hepatitis C virus E1 and E2 glycoproteins. FEBS Lett. 546:385-390. [DOI] [PubMed] [Google Scholar]
- 11.Dubuisson, J. 2000. Folding, assembly and subcellular localization of hepatitis C virus glycoproteins. Curr. Top. Microbiol. Immunol. 242:135-148. [DOI] [PubMed] [Google Scholar]
- 12.Dubuisson, J., H. H. Hsu, R. C. Cheung, H. B. Greenberg, D. G. Russell, and C. M. Rice. 1994. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J. Virol. 68:6147-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dubuisson, J., and C. M. Rice. 1996. Hepatitis C virus glycoprotein folding: disulfide bond formation and association with calnexin. J. Virol. 70:778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dumonceaux, J., E. G. Cormier, F. Kajumo, G. P. Donovan, J. Roy-Chowdhury, I. J. Fox, J. P. Gardner, and T. Dragic. 2003. Expression of unmodified hepatitis C virus envelope glycoprotein-coding sequences leads to cryptic intron excision and cell surface expression of E1/E2 heterodimers comprising full-length and partially deleted E1. J. Virol. 77:13418-13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duvet, S., L. Cocquerel, A. Pillez, R. Cacan, A. Verbert, D. Moradpour, C. Wychowski, and J. Dubuisson. 1998. Hepatitis C virus glycoprotein complex localization in the endoplasmic reticulum involves a determinant for retention and not retrieval. J. Biol. Chem. 273:32088-32095. [DOI] [PubMed] [Google Scholar]
- 16.Flint, M., C. Maidens, L. D. Loomis-Price, C. Shotton, J. Dubuisson, P. Monk, A. Higginbottom, S. Levy, and J. A. McKeating. 1999. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J. Virol. 73:6235-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flint, M., and J. A. McKeating. 1999. The C-terminal region of the hepatitis C virus E1 glycoprotein confers localization within the endoplasmic reticulum. J. Gen. Virol. 80:1943-1947. [DOI] [PubMed] [Google Scholar]
- 18.Griffin, S. D., L. P. Beales, D. S. Clarke, O. Worsfold, S. D. Evans, J. Jaeger, M. P. Harris, and D. J. Rowlands. 2003. The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug, amantadine. FEBS Lett. 535:34-38. [DOI] [PubMed] [Google Scholar]
- 19.Hsu, M., J. Zhang, M. Flint, C. Logvinoff, C. Cheng-Mayer, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. USA 100:7271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kümmerer, B. M., and C. M. Rice. 2002. Mutations in the yellow fever virus nonstructural protein NS2A selectively block production of infectious particles. J. Virol. 76:4773-4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, C., B. D. Lindenbach, B. M. Pragai, D. W. McCourt, and C. M. Rice. 1994. Processing in the hepatitis C virus E2-NS2 region: identification of p7 and two distinct E2-specific products with different C termini. J. Virol. 68:5063-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: viruses and their replication, p. 991-1041. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Field's virology, 4th ed., vol. 1. Raven Press, New York, N.Y.
- 23.Lo, S. Y., M. J. Selby, and J. H. Ou. 1996. Interaction between hepatitis C virus core protein and E1 envelope protein. J. Virol. 70:5177-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizushima, H., M. Hijikata, S. Asabe, M. Hirota, K. Kimura, and K. Shimotohno. 1994. Two hepatitis C virus glycoprotein E2 products with different C termini. J. Virol. 68:6215-6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mottola, G., N. Jourdan, G. Castaldo, N. Malagolini, A. Lahm, F. Serafini-Cessi, G. Migliaccio, and S. Bonatti. 2000. A new determinant of endoplasmic reticulum localization is contained in the juxtamembrane region of the ectodomain of hepatitis C virus glycoprotein E1. J. Biol. Chem. 275:24070-24079. [DOI] [PubMed] [Google Scholar]
- 26.Ohuchi, M., A. Cramer, M. Vey, R. Ohuchi, W. Garten, and H. D. Klenk. 1994. Rescue of vector-expressed fowl plague virus hemagglutinin in biologically active form by acidotropic agents and coexpressed M2 protein. J. Virol. 68:920-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Op De Beeck, A., L. Cocquerel, and J. Dubuisson. 2001. Biogenesis of hepatitis C virus envelope glycoproteins. J. Gen. Virol. 82:2589-2595. [DOI] [PubMed] [Google Scholar]
- 28.Pavlovic, D., D. C. Neville, O. Argaud, B. Blumberg, R. A. Dwek, W. B. Fischer, and N. Zitzmann. 2003. The hepatitis C virus p7 protein forms an ion channel that is inhibited by long-alkyl-chain iminosugar derivatives. Proc. Natl. Acad. Sci. USA 100:6104-6108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petit, M. A., C. Jolivet-Reynaud, E. Peronnet, Y. Michal, and C. Trepo. 2003. Mapping of a conformational epitope shared between E1 and E2 on the serum-derived human hepatitis C virus envelope. J. Biol. Chem. 278:44385-44392. [DOI] [PubMed] [Google Scholar]
- 30.Pietschmann, T., V. Lohmann, A. Kaul, N. Krieger, G. Rinck, G. Rutter, D. Strand, and R. Bartenschlager. 2002. Persistent and transient replication of full-length hepatitis C virus genomes in cell culture. J. Virol. 76:4008-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poignard, P., M. Moulard, E. Golez, V. Vivona, M. Franti, S. Venturini, M. Wang, P. W. Parren, and D. R. Burton. 2003. Heterogeneity of envelope molecules expressed on primary human immunodeficiency virus type 1 particles as probed by the binding of neutralizing and nonneutralizing antibodies. J. Virol. 77:353-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakai, A., M. S. Claire, K. Faulk, S. Govindarajan, S. U. Emerson, R. H. Purcell, and J. Bukh. 2003. The p7 polypeptide of hepatitis C virus is critical for infectivity and contains functionally important genotype-specific sequences. Proc. Natl. Acad. Sci. USA 100:11646-11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi, K., and R. A. Lamb. 1994. Influenza virus M2 protein ion channel activity stabilizes the native form of fowl plague virus hemagglutinin during intracellular transport. J. Virol. 68:911-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang, J., G. Randall, A. Higginbottom, P. Monk, C. M. Rice, and J. A. McKeating. 2004. CD81 is required for hepatitis C virus glycoprotein-mediated viral infection. J. Virol. 78:1448-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]


