Abstract
Mortality due to AIDS-related Cryptococcal meningitis (CM) is often >50% in low-middle income countries. Dissemination of CM can result in intracranial mass lesions known as cryptococcoma. Patients who develop cryptococcomas often have worse outcomes when compared to patients with cryptococcosis without cryptococcoma. We describe a cryptococcoma in the central nervous system (CNS) in a Ugandan patient with AIDS, and review the diagnosis and management with special focus on difficulties encountered in low or middle-income countries.
Keywords: Cryptococcus meningitis, Cryptococcoma, Immunosuppressed Host, Central nervous system, Human Immunodeficiency Virus
1. Introduction
Human Immunodeficiency Virus (HIV)-associated Cryptococcal meningitis (CM) is an opportunistic fungal infection of the brain and spinal cord and a common cause of adult meningitis in sub-Saharan Africa due to the high prevalence of HIV [1,2]. With a mortality rate of 17–36%, CM is responsible for approximately 25% of HIV/AIDS related deaths in sub-Saharan Africa [3]. Cryptococcomas are rare mass lesions caused by the dissemination of Cryptococcus neoformans and establishment of focal tissue infection. The formation of a cryptococcoma is dependent on an inflammatory response to C. neoformans and so is more often seen in immunocompetent individuals [4] or those that have initiated antiretroviral therapy (ART) [5]. Cryptococcomas occur most often in the CNS but may occur in other locations such as the lungs [1]. The presence of a cryptococcoma is often only apparent when focal neurologic findings persist; outcomes are dismal [6]. As with CM, combination amphotericin-based antifungal therapy and relief of increased intracranial pressure are mainstays of treatment of cerebral cryptococcomas [6]. We present a case of a Ugandan male who developed a cryptococcoma and passed away. Diagnosis and treatment of HIV-associated cryptococcomas are more challenging in resource-limited environments where CM is most common. For this reason, our case and discussion will focus on the care and treatment of a cryptococcoma in resource-limited settings.
2. Case
A 45 year-old HIV positive male was admitted at Mulago National Referral Hospital in Kampala, Uganda with 1 week of vomiting and fever. He had a 2 months history of cough and a 1 month history of headache. The patient was diagnosed with HIV 1 month prior to admission with a CD4 count of 4 cells/mcL and had not yet initiated ART or prophylactic medications. A lumbar puncture was performed with an opening pressure of 140 mm H2O. CSF analysis revealed WBC of less than 5 cells/mcL, protein of 60 mg/dL, and cryptococcal antigen lateral flow assay (CrAg LFA; Immy Inc., Norman, Oklahoma) titer of 1:8000. The patient was started on 50 mg of amphotericin B, 1200 mg fluconazole, and 100 mg sertraline daily (day 0). Sertraline was started as part of the Adjunctive Sertraline for the Treatment of HIV-Associated CM (ASTRO; ClinicalTrials.gov: NCT01802385) trial during open-label dosing finding pilot. In accordance with WHO guidelines for administration of amphotericin, the patient was also given IV fluids, oral KCl, and magnesium electrolyte supplementation [7]. Upon completing 14 days of amphotericin, the patient׳s symptoms had improved so he was discharged on 800 mg fluconazole and 200 mg on day +14.
The patient was readmitted to the hospital for vomiting and dehydration +28 days after his initial admission for the treatment of CM; he was aggressively rehydrated with IV fluids. The patient was also started on anti-retroviral therapy (Zidovudine, lamivudine/3TC, Efavirenz), and Cotrimoxazole at this time. During this hospital stay the patient was found to have mild anemia (hemoglobin 9.9 g/dL) and mild renal insufficiency (creatinine 1.49 g/dL). The patient׳s overall condition improved with the administration of fluids and antiemetics. He was discharged at +35 days under stable condition.
The patient returned as an outpatient +45 days after his last admission with continued general body weakness and dehydration and was readmitted to the hospital as an abdominal ultrasound revealed lymphadenopathy. The patient was started on isoniazid, rifampin, ethambutol, pyrazidamide, and vitamin B6 for presumptive abdominal tuberculosis and discharged on day +46.
At approximately day +70, the patient was seen in clinic after having a generalized tonic-clonic seizure. A physical and neurological exam revealed right-sided hemiparesis. A brain Computerized Tomography (CT) revealed a central nervous system (CNS) mass lesion in the occipital lobe and the patient was readmitted for further evaluation and management. The frequency of the patient׳s seizures and the intensity of the patient׳s right-sided hemiparesis rapidly worsened and he became progressively confused while in the hospital; the patient׳s Glasgow Coma Scale reduced from 14/15 to 12/15.
Upon admission the patient was restarted on 50 mg amphotericin daily for the treatment of a presumed cryptococcoma. A repeat head CT (obtained 6 weeks after the initial CT – Fig. 1a) revealed a massive hypodense lesion in the occipital lobe with an 18.1 mm midline shift (Fig. 1b). Toxoplasma titers were obtained and were positive for IgG (316.5 IU/mL) and within the upper limit of normal for IgM (0.189). As no improvement had been observed from day +70 to day +78, neurosurgery was consulted to consider possible surgical treatment. In accordance with neurosurgery׳s recommendations, the patient was started on ceftriaxone for empirical treatment of a possible bacterial brain abscess; no improvement was noted. On day +81, prednisone was started for ongoing cerebral edema. During this time, the patient continued to become more lethargic and anorexic. On day +83 of readmission, metronidazole was added to the ceftriaxone for aspiration pneumonia. The patient passed away that same night on day +83.
Fig. 1.
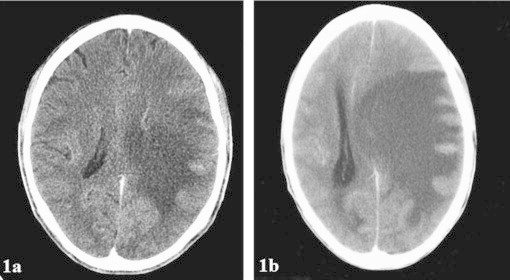
Brain CT images showing evolution of cryptococcoma. 1a (left) The patient׳s CT image from October 2013, and 1b (right) the patient׳s CT image from December 2013.
A post-mortem autopsy was performed the following morning and a large cryptococcoma was noted. Quantitative CSF cultures were performed, which demonstrated the ongoing presence of viable C. neoformans (1430 cfu/mL). After consultation between the medical and pathology teams, the cause of death was listed as cerebral cryptococcoma with viable organisms.
3. Discussion
We present a case of a Ugandan male infected with HIV who contracted CM, developed a cryptococcoma, and passed away. The occurrence of a cryptococcoma within the brain parenchyma is rare [8,9]. When this does occur, however, the most common sites are the basal ganglia, thalamus, and cerebellum [10]. Diagnosis and treatment of cryptococcomas are quite difficult. Our purpose for presenting this case is to discuss these difficulties with a specific focus on their relation to low and middle-income countries where most cryptococcomas are seen.
The initial diagnosis of CM is direct. In a patient with no previous history of CM, the CrAG LFA is extremely accurate [11]. However, the diagnosis is much more difficult when the patient has a previous history of CM and presents with symptoms of a recurrence. As a result, there are multiple possibilities in such a case.
First, a patient may have lingering elevated intracranial pressures related to the previous cryptococcal infection as the large capsule of C. neoformans is known to block CSF drainage from the arachnoid villi [5]. Blockage is likely to happen in persistent cryptococcal infections in which sterility of cerebrospinal fluid has never been achieved. Persons with very low culture growth are often asymptomatic and may decline lumbar punctures prior to documented CSF sterility [5]. Documenting sterility is a critical step before reverting to the fungistatic fluconazole dose following the consolidation phase. The Infectious Diseases Society of America (IDSA) defines persistent infection as positive CSF cultures after 4 weeks of proven antifungal therapy at established effective doses [6]. Positive CSF culture growth is needed to confirm the infection. Changing cryptococcal antigen titers, positive India ink examination results, and evidence of CSF inflammation are often misleading. Our patient was found to have a titer of 1:8000 with an initial CSF fungal burden of 210,000 cfu/mL.
Another possible source of these symptoms can be immune reconstitution inflammatory syndrome (IRIS) if the patients have recently started on or improved adherence to their ART. The diagnosis of IRIS is usually one of exclusion that should begin with ruling-out relapsed CM. In patients with pure IRIS (e.g. not a combination of CM relapse and IRIS) no growth occurs on CSF fungal culture. A CSF culture may take up to 1–2 weeks to confirm whether or not growth is present. Crag titers generally decline over time (though not in a predictable fashion) and are not reliable in cases of suspected IRIS [5]. CSF WBC count may be a useful predictor of differentiating IRIS from relapse. A study has demonstrated the median CSF WBC counts at time of CM-IRIS to be higher (median 50, IQR: 5–117, max 212 cells/mcL) than in CM-relapse (median 10, range 5–30 cells/mcL, P=0.044) [13].
Third, the patient may have relapse related to poor fluconazole compliance or fluconazole resistance. Fluconazole minimum inhibitory concentrations (MIC) are not routinely done and were not done on our patient due to the unavailability of resources needed for the test. Shelbourne et al. reported that persons with relapse returned to the hospital later than patients with CM-IRIS (165 days vs. 59 days, P=0.029) after CM diagnosis, were less likely to have initiated ART (33% vs. 100%, P<0.001), and were less likely to have virological and immunological response to ART (medium decrease in HIV-1 RNA level, −0.15 in relapse in relapse vs. −2.27 log 10 copies/mL in CM-IRIS; P<0.001; median increase in CD4+ cell count, 4 vs. 93 cells/mcL; P=0.002) [14]. As such, whether or not the patient has begun ART and/or timing from initial CM episode may be helpful in differentiating relapsed CM from IRIS.
Finally, the patient may also present with a cryptococcoma. This has been associated with persistent low fungal burden in the CSF because the walled off mass is thought to continuously shed the cryptococcus microorganisms into CSF. Imaging may not initially be indicative of cryptococcoma making diagnosis difficult. When a lesion is seen, its appearance may be ambiguous. While considering alternative diagnoses such as toxoplasmosis or pyogenic abscess formation, one must strongly consider cryptococcomas in a patient with recent CM.
Cryptococcoma formation may be a consequence of rapid immune recovery due to HAART introduction [15]. For instance, a case of intramedullary cryptococcoma has been reported in an HIV-infected patient who suffered from a meningeal cryptococcosis 3 years previous and one separate case of intracranial cryptococcoma in a patient undergoing HAART [15]. C. neoformans meningitis with associated cerebral abscess lesions and other focal localization of the yeast infection were extremely infrequent in the pre-HAART era [15]. It is possible that as the immune system improves, the body׳s response to inflammation is able to wall off the cryptococcal infection, forming a cerebral abscess or cryptococcoma. Our patient initially presented to us with a low CD4+ count of 4 cells/mL. He was initiated on ART 4 weeks after initial presentation. It is possible that his improvement in the immune status did predispose him to developing a cryptococcoma, given his rapid progression of symptoms after beginning ART. Formal study of risk factors for the development of a cryptococcoma has not been done and may well never be done, as this is a rare finding.
Case reports of cryptococcomas have reported patients that presented with either sterile CSF or low fungal burden compared to the patients’ initial CM episode at hospital presentation [15–17]. At our patient׳s initial hospital presentation, the CSF fungal burden was 210,000 cfu/mL. The patient had not cleared the CSF at the 14th dose of amphotericin (standard therapy). After 4 weeks of amphotericin and fluconazole, CSF culture for our patient grew 230 cfu/mL. At autopsy, the CSF grew 1430 cfu/mL, a relatively low count. This is in keeping with what has been reported by other groups.
Conclusive guidelines as to the effective management of cryptococcomas are still lacking. At this point, amphotericin based antifungal therapy and relief of increased intracranial pressure are mainstays of treatment. Amphotericin B and fluconazole, despite still being inadequate in supply [18], are now more available in the developing world in some locations. In our setting, patients’ acceptance to diagnostic and therapeutic lumbar punctures has improved and routine measurement of renal function including electrolytes in the developing world is more accessible. Treatment of cerebral cryptococcoma is generally difficult. Previously reported cases of cryptococcomas were successfully treated using antifungal therapy and surgical removal of the cryptococcoma [19]. Voriconazole, miconazole, and neurosurgery have all been reported as successful salvage therapies [15,17], though systematic studies have not been conducted. Interestingly, Adalimumab, a recombinant human immunoglobulin G1 monoclonal antibody specific for human TNF alpha, has also been used to successfully treat a cryptococcoma [16].
While some specialized settings in low and middle-income countries may have access to a wide array of diagnostic tests, limitations in the availability of neuroimaging, neurosurgical services, basic laboratory infrastructure, and medical mycology laboratories with the ability to accurately culture C. neoformans, may make diagnosis of a cryptococcoma extremely difficult. Further, even well-established drugs such as amphotericin B deoxycholate and flucytosine are not available in many resource-poor settings – if they are available, proper IV fluid supplementation and electrolyte supplementation and monitoring are often not available. More advanced and/or expensive therapies such as liposomal amphotericin B, voriconazole, and neurosurgery are rarely feasible options for physicians in many low and middle-income countries outside of major urban centers.
In the national referral hospital for Uganda – our patient was initiated on prophylactic antibiotics, ceftriaxone for a possible bacterial infection, and high dose fluconazole and amphotericin B. The patient also received 6 therapeutic lumbar punctures during the hospitalization to reduce his ICP. Neurological surgery was consulted but surgery was not considered a realistic option and numerous medications mentioned above were not available to attempt clearance of the infection.
In conclusion, we presented a case of a Ugandan male with HIV and CM who developed a cryptococcoma that may have been related to immune reconstitution. Recurrent symptoms after treatment of CM are perplexing as the potential diagnoses are numerous and the diagnostic testing imperfect – even in resource-rich settings. When caring for a patient with persistent or recurrent meningitis symptoms in a resource poor setting, like our case, clinicians must carry a high index of suspicion for cryptococcoma and imaging studies are of the utmost importance. Comparison of subsequent CSF fungal growth with prior counts is also helpful and will narrow the diagnostic possibilities; however, these tests may not yield results in time for a gravely ill patient. In the absence of clear guidelines, we believe that treatment with a combination of long term amphotericin and high dose fluconazole may yield better outcomes in the patients if completed with proper monitoring of treatment toxicity as well as treatment progress.
Conflict of interest
The authors have no conflict of interest.
Acknowledgments
The authors are supported by the National Institute of Neurologic Diseases and Stroke, the National Institute of Allergy and Infectious Diseases and Fogarty International Center (R01NS086312-01, R01AI078934-04, and R25TW009345-02). We thank Dr. Reuben Kiggundu for patient care.
References
- 1.Jackson A, Hosseinipour MC. Management of cryptococcal meningitis in sub-Saharan Africa. Curr HIV/AIDS Rep. 2010;7(3):134–142. doi: 10.1007/s11904-010-0052-6. [DOI] [PubMed] [Google Scholar]
- 2.Stevens DA, Denning DW, Shatsky S, Armstrong RW, Adler JD, Lewis BH. Cryptococcal meningitis in the immunocompromised host: intracranial hypertension and other complications. Mycopathologia. 1999;146(1):1–8. doi: 10.1023/a:1007031514495. [DOI] [PubMed] [Google Scholar]
- 3.Kambugu A, Meya DB, Rhein J, O׳Brien M, Janoff EN, Ronald AR. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin Infect Dis. 2008;46(11):1694–1701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clin Infect Dis. 2000;31(2):499–508. doi: 10.1086/313992. [DOI] [PubMed] [Google Scholar]
- 5.Musubire AK, Boulware DR, Meya DB, Rhein J. Diagnosis and management of cryptococcal relapse. J AIDS Clin Res. 2013;Sup 3(3):S3–003. doi: 10.4172/2155-6113.s3-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children, 2011. Available from: www.who.int/hiv/pub/cryptococcal_disease2011. [PubMed]
- 8.Ho T-L, Lee H-J, Lee K-W, Chen W-L. Diffusion-weighted and conventional magnetic resonance imaging in cerebral cryptococcoma. Acta Radiol. 2005;46(4):411–414. doi: 10.1080/02841850510021201. [DOI] [PubMed] [Google Scholar]
- 9.Vender JR, Miller DM, Roth T, Nair S, Reboli AC. Intraventricular cryptococcal cysts. Am J Neuroradiol. 1996;17(1):110–113. [PMC free article] [PubMed] [Google Scholar]
- 10.Tien R, Chu P, Hesselink J, Duberg A, Wiley C. Intracranial cryptococcosis in immunocompromised patients: CT and MR findings in 29 cases. Am J Neuroradiol. 1991;12(2):283–289. [PMC free article] [PubMed] [Google Scholar]
- 11.Jarvis JN, Percival A, Bauman S, Pelfrey J, Meintjes G, Williams GN. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin Infect Dis. 2011;53(10):1019–1023. doi: 10.1093/cid/cir613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulware DR, Bonham SC, Meya DB, Wiesner DL, Park GS, Kambugu A. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis. 2010;202(6):962–970. doi: 10.1086/655785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shelburne SA, Darcourt J, White AC, Greenberg SB, Hamill RJ, Atmar RL. The role of immune reconstitution inflammatory syndrome in AIDS-related Cryptococcus neoformans disease in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40(7):1049–1052. doi: 10.1086/428618. [DOI] [PubMed] [Google Scholar]
- 15.Sabbatani S, Manfredi R, Pavoni M, Consales A, Chiodo F. Voriconazole proves effective in long-term treatment of a cerebral cryptococcoma in a chronic nephropathic HIV-negative patient, after fluconazole failure. Mycopathologia. 2004;158(2):165–171. doi: 10.1023/b:myco.0000041904.71381.e3. [DOI] [PubMed] [Google Scholar]
- 16.Sitapati AM, Kao CL, Cachay ER, Masoumi H, Wallis RS, Mathews WC. Treatment of HIV-related inflammatory cerebral cryptococcoma with adalimumab. Clin Infect Dis. 2010;50(2):e7–e10. doi: 10.1086/649553. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein L, Jacoby I. Successful treatment of cerebral cryptococcoma and meningitis with miconazole. Ann Intern Med. 1980;93(4):569–571. doi: 10.7326/0003-4819-93-4-569. [DOI] [PubMed] [Google Scholar]
- 18.Cameron A, Roubos I, Ewen M, Mantel-Teeuwisse AK, Leufkens HG, Laing RO. Differences in the availability of medicines for chronic and acute conditions in the public and private sectors of developing countries. Bull World Health Organ. 2011;89(6):412–421. doi: 10.2471/BLT.10.084327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, You C, Liu Q, Liu Y. Central nervous system cryptococcoma in immunocompetent patients: a short review illustrated by a new case. Acta Neurochir. 2010;152(1):129–136. doi: 10.1007/s00701-009-0311-8. [DOI] [PubMed] [Google Scholar]


