Abstract
Purpureocillium lilacinum and Beauveria bassiana were isolated from lung sampled at necropsy of a 12 year-old female loggerhead sea turtle (Caretta caretta) that had displayed abnormal buoyancy. Histopathologic evaluation revealed pleuritis and pneumonia with non-melanized, septate hyphae and fruiting structures identical to those of P. lilacinum. This case emphasizes the importance of a histological correlate to fungal culture when environmental fungi are isolated and demonstrates the infrequent phenomenon of fruiting or conidial production in tissue.
Keywords: Caretta caretta, Fruiting body, Histopathology, Pneumonia, Purpureocillium lilacinum, Turtle
1. Introduction
Purpureocillium lilacinum (Thom) [1] formerly Paeciliomyces lilacinus (Thom) [2] is a non-melanized, ubiquitous, environmental, filamentous mold. Infection caused by this species, now referred to as purpureocilliosis, is sporadic and usually limited to immunosuppressed humans or animals. P. lilacinum has been most frequently described as the cause of hyalohyphomycosis in humans [3], a dog [4], a cat [5], and reptiles [6]. In humans, purpureocilliosis is associated with disease in immunocompromised individuals, especially organ transplant patients [3,7]. Disease due to P. lilacinum is increasing in importance as the population of immunosuppressed individuals grows and immunosuppressive drugs become more effective and more frequently prescribed. It is of particular concern in patients with indwelling devices or intraocular lens implants, which contribute to it being considered an emerging fungal pathogen [3,8]. In rare cases, it is diagnosed in humans and animals without underlying immunosuppressive disease or demonstrable evidence of immunosuppression [9,10].
In Order Chelonia, P. lilacinum has been associated with disease in a number of species, including Chinese soft-shelled turtles (Trionyx sinens) [11], an Aldabra tortoise (Dipsochelys dussemieri) [12], Fly River turtles (Carettochelys insculpta) [13], a Greek tortoise (Testudo graeca), and an ornate slider (trachemys ornate) [6]. Susceptibility of sea turtles to P. lilacinum has been represented by a case of disseminated granulomas in a hawksbill sea turtle (Eretmochelys imbricata) [14], as the cause of mycotic pneumonia in a group of commercially raised green sea turtles (Chelonia mydas) [15], and as the cause of mycosis in loggerhead sea turtles [16]. The disease has also been described in the reptilian orders Crocodylia [17] and Squamata [6].
2. Case
A 12 year-old female loggerhead sea turtle (Caretta caretta) cared for at a public aquarium was found dead in its exhibit after a history of abnormal buoyancy and decreased appetite of several weeks duration, and a necropsy was performed. The sea turtle had been maintained for 5.5 years in a multi-taxa exhibit tank (7.75 m diameter×1.2 m deep) that held 72,000 l of synthetic saltwater at 25 to 26.1 °C and 20 ppt salinity. The most notable macroscopic postmortem finding was that of firm, hemorrhagic lungs with scattered, firm, yellow, well demarcated foci of pneumonia (Fig. 1). Tissue samples of lung and other coelomic viscera obtained at necropsy were fixed by immersion in 10% formalin and submitted to the Connecticut Veterinary Medical Diagnostic Laboratory (Storrs, CT, USA), where they were trimmed and routinely processed for paraffin embedment and histologic sectioning. Swabs of frozen tissue samples of lung were streaked onto Sabouraud dextrose agar and inhibitory mold agar with gentamicin (0.05 g/L), and cultures were incubated at 30 °C. Histopathologic evaluation revealed severe, multifocal and focally extensive, heterophilic and granulomatous pneumonia and pleuritis, with multiple heterophilic granulomas extending into ectatic faveolar spaces and expanding the pleura. In several regions, faveolar septae and pleura were expanded by infiltrates of degenerate heterophils together with proteinaceous edema and fibrin (Fig. 2). Innumerable, intertwined, non-melanized, septate fungal hyphae were located in granulomas and lining the luminal surfaces of affected faveolar septae. Hyphae were 2.5 to 4 µm wide, had parallel walls, and were readily demonstrable using silver stains. Diagnostic fruiting structures, e.g. conidiophores, conidiogenous cells and conidia, were evident in histologic sections of the lung in foci of pneumonia and pleuritis (Fig. 3). Bacteria were present along with hyphae in faveolae in some instances, and bacterial cultures of samples of lung (performed at a different veterinary diagnostic laboratory) yielded moderate growth of Klebsiella oxytoca and alpha-hemolytic Streptococcus, and light growth of Proteus mirabilis and Enterococcus sp. There was concurrent ulcerative bacterial cystitis and renal lesions, which included interstitial fibrosis, tubular degeneration and necrosis, and membranous glomerulopathy.
Fig. 1.
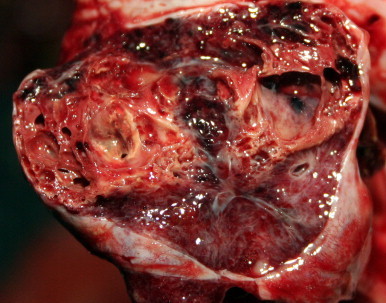
Gross appearance of pneumonia in a loggerhead sea turtle with purpureocilliosis. There is a granulomatous focus surrounded by firm, yellow fibrous tissue in which are variably sized spaces. This image was adjusted for contrast, brightness and color balance by applying the levels and sharpen functions of Adobe Photoshop CS5 to the original image as a whole. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
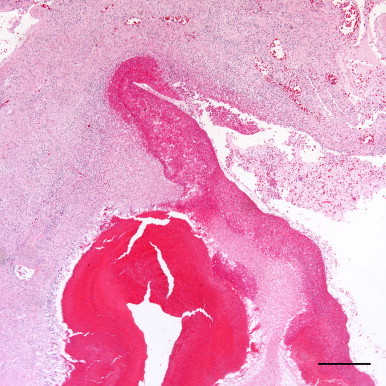
A heterophilic granuloma extends into an ectactic faveolar space, while the adjacent faveolar space, also ecstatic, is lined on one margin by a thick serofibrinous and heterophilic exudate. Hematoxylin and eosin. Bar=500 µm. This image was adjusted for contrast, brightness and color balance by applying the levels and sharpen functions of Adobe Photoshop CS5 to the original image as a whole.
Fig. 3.
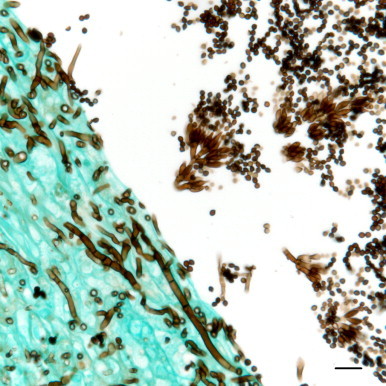
Fungal fruiting structures composed of dense clusters of phialidic conidiogenous cells supported on metulae with chains of oval to subglobose conidia borne from these conidiogenous cells are present in the faveolar space; filamentous fungal hyphae with septae and parallel walls invade the adjacent faveolar wall. Grocott’s methenamine silver. Bar=10 µm. This image was adjusted for contrast, brightness and color balance by applying the levels and sharpen functions of Adobe Photoshop CS5 to the original image as a whole.
Fungal cultures of swabs from the lung grown on Sabouraud dextrose agar and inhibitory mold agar with gentamicin (0.05 g/L) yielded two hyaline molds after 4 days of incubation at 30 °C. These were subsequently referred to the Fungus Testing Laboratory (University of Texas Health Science Center at San Antonio, San Antonio, TX) for species identification and were accessioned into their culture collection as isolate numbers UTHSC 10–30 and 10–31. They were identified as Beauveria bassiana and P. lilacinum, respectively, based upon their macroscopic and microscopic features on potato flakes agar and carnation leaf agar (prepared in-house) after 6 days incubation at 25 °C. B. bassiana displayed small, white, granular colonies and produced its smooth, subglobose conidia (2–3 μm) in discrete masses along the hyphae from denticles on a geniculate rachis. Phenotypic features used to identify P. lilacinum included mauve-colored colonies with a pale reverse, finely-roughened conidiophores giving rise to dense clusters of phialidic conidiogenous cells supported on metulae and chains of oval to subglobose conidia borne from these conidiogenous cells. Morphologic features of fruiting structures of the fungus identified in foci of pneumonia and pleuritis were identical to those of the P. lilacinum isolate recovered from the lung, thereby confirming its etiologic role in the pulmonary lesions (Fig. 3).
3. Discussion
In this case of mycotic pneumonia and pleuritis, fruiting structures diagnostic for P. lilacinum and comparable to those in culture were recognized in histologic tissue sections in anatomic association with pulmonary lesions. Morphology on fungal culture has previously been combined with morphologic features observed histologically to identify P. lilacinum infections in humans [10], turtles [14] and a cat [5]. Identification of P. lilacinum based on its microscopic morphology alone, either in the host or in culture, is difficult since P. lilacinum could be misidentified as either a Penicillium or a Paecilomyces species, such as P. variotii, due to similarities in reproductive structures. The color of the colony on a plant-based medium such as potato flakes agar (PFA) clearly distinguishes these genera/species, however, which for P. lilacinum, Penicillium spp., and most Paecilomyces spp. would be violet to mauve, some shade of green, or tan to brownish, respectively. Because all of the above are common environmental fungi that can be present as laboratory contaminants, the clinical significance of their isolation is often questioned. This concern is further intensified when the anatomic location of the sample is inherently non-sterile and available to contamination or colonization by an environmental mold, e.g. the skin, mouth or nasal cavity. Histopathologic identification of morphologically compatible fungal structures in lesions can be the key to ascribing significance to isolates from clinical samples and is indispensible in determining whether an isolate represents a contaminant, colonization or bona fide infection [18].
The phenomenon of fruiting or conidial production in tissue is rare in human fungal infections, where it is mostly seen with chronic cavitary pulmonary aspergillosis [19]; its occurrence in fungal infections in animals is less well known. In general, it is rare that histopathologic examination alone can provide the genus and species of a fungus [18]. This is especially true in cases where hyaline filamentous hyphae are seen in tissue, which makes this case particularly unique and re-emphasizes the need for thorough histopathologic examination toward the most exacting description possible in the context of the anatomic and pathologic features of the case. Although no fruiting structures were observed for a Beauveria species in the multiple histologic sections examined, this does not rule out the possibility that not-fruiting fungal hyphae of B. bassiana could also have been present. It is not implausible that an infection by two fungi could have occurred in this turtle. The occurrence of dual fungal infections in human medicine has been reported to be as high as 20% [18]. Like P. lilacinum, B. bassiana is ubiquitous in the environment and capable of being isolated from indoor air; it too produces hyaline, septate hyphae, which are indistinguishable from those produced by P. lilacinum. Additionally, B. bassiana has been identified as an agent of mycotic pleuritis and pulmonary disease in a turtle, e.g. a captive pond slider (Trachemys scripta) [20]. In cases of dual fungal infections, it may be that only the most abundant fungus is recognized; if the second fungus is isolated in culture but not recognized in tissue section, then histopathologic and culture results will appear discrepant [18]. PCR was attempted on formalin-fixed, paraffin-embedded tissue from affected areas of lung, but the tissue was unsuitable for fungal PCR testing because DNA was not amplifiable from any tissue block using primers and PCR conditions that amplify a fragment of a common housekeeping gene (data not shown). Given these data and the possibility that it could have been an environmental contaminant, the B. bassiana isolate was considered less significant to the origin or progression of the pulmonary lesions in this case.
Fungal infection is often associated with poor cellular immunity and can be predisposed by environmental conditions such as overcrowding, high population density, stress, poor water quality and, in the case of cold stunned turtles, a drop in environmental and, hence, body temperature. In this case, however, the turtle was well acclimated to its surroundings, and all water quality parameters were within normal limits. It is unknown if the concurrent cystitis and renal lesions affected the overall immune status of this turtle. A complete immunologic evaluation was not performed on this sea turtle; so, the immune status of this individual before death is open to speculation. On the other hand, P. lilacinum has been described in humans and animals without underlying predisposing factors or immunosuppression [9,10].
In summary, this case demonstrates the infrequently encountered phenomenon of fruiting or conidial production in tissue, and it reiterates the importance of having a histological correlate to fungal culture when environmental fungi are isolated. Even though fungal culture remains the gold standard for diagnosis of purpureocilliosis, since P. lilacinum is a common environmental fungus, the results of culture in the absence of a histologic correlate should be interpreted with care. Poikilotherms represent a broad group of animals of veterinary concern in which environmental factors, specifically temperature, exert significant influence on immunologic processes. As a result, these animals can be susceptible to infection by opportunistic environmental fungi, some of which may be emerging in their public and veterinary health relevance.
Conflict of interest statement
There are none.
Acknowledgements
We would like to thank Ione Jackman and Denise Long of the Connecticut Veterinary Medical Diagnostic Laboratory (Storrs, CT) for histological preparations, Elizabeth Thompson of the Fungus Testing Laboratory (San Antonio, TX) for fungus culture, and Jack Schneider of the Maritime Aquarium at Norwalk (Norwalk, CT) for support of this collaboration.
References
- 1.Luangsa-Ard J., Houbraken J., van Doorn T., Hong S.B., Borman A.M., Hywel-Jones N.L. Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol Lett. 2011;321:141–149. doi: 10.1111/j.1574-6968.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- 2.Samson R.A. Paecilomyces and some allied Hyphomycetes. Stud Mycol. 1974;6:58–62. [Google Scholar]
- 3.Antas P.R.Z., Brito M.M.S., Peixoto E., Ponte C.G.G., Borba C.M. Neglected and emerging fungal infections: a review of hyalohyphomycosis by Paecilomyces lilacinus focusing on disease burden, in vitro antifungal susceptibility and management. Microbe Infect. 2012;14(1):1–8. doi: 10.1016/j.micinf.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Littman M.P., Goldschmidt M.H. Systemic paecilomycosis in a dog. J Am Vet Med Assoc. 1987;191:445–447. [PubMed] [Google Scholar]
- 5.Rosser E.J. Cutaneous paecilomycosis in a cat. J Am Anim Hosp Assoc. 2003;39:543–546. doi: 10.5326/0390543. [DOI] [PubMed] [Google Scholar]
- 6.Paré J.A., Jacobson E.R. Mycotic diseases of reptiles. In: Jacobson E.R., editor. Infectious diseases and pathology of reptiles. Taylor and Francis; Boca Raton, FL: 2007. pp. 534–535. [Google Scholar]
- 7.Van Schooneveld T., Freifeld A., Lesiak B., Alkali A., Sutton D.A., Iwen P.C. Paecilomyces lilacinus infection in a liver transplant patient: case report and review of the literature. Transpl Infect Dis. 2007;2007(10):117–122. doi: 10.1111/j.1399-3062.2007.00248.x. [DOI] [PubMed] [Google Scholar]
- 8.Tan T.Q., Ogden A.K., Tillman J., Demmier G.J., Rinaldi M.G. Paecilomyces lilacinus catheter-related fungemia in an immunocompromised pediatric patient. J Clin Microbiol. 1992;30(9):2479. doi: 10.1128/jcm.30.9.2479-2483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey J., D’Amico R., Sutton D.A., Rinaldi M.G. Paecilomyces lilacinus vaginitis in an immunocompetent patient. Emerg Infect Dis. 2003;9(9):1155–1158. doi: 10.3201/eid0909.020654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ono N., Sato K., Yokomise H., Tamura K. Lung abscesses caused by Paecilomyces lilacinus. Resp. 1997;66:85–87. doi: 10.1159/000029345. [DOI] [PubMed] [Google Scholar]
- 11.Li X., Zhang C., Fang W., Lin F. White-spot diseases of Chinese soft-shelled turtles (Trionyx sinens) caused by Paecilomyces lilacinus. J Zhejiang Univ Sci B. 2008;9(7):578–581. doi: 10.1631/jzus.B0720009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heard D.J., Cantor G.H., Jacobson E.R., Purich B., Ajello L., Padhe A.A. Hyalohyphomycosis caused by Paecilomyces lilacinus in an Aldabra tortoise. J Am Vet Med Assoc. 1986;9:1143–1145. [PubMed] [Google Scholar]
- 13.Lafortune M., Wellehan J.F.X., Terrell S.P., Jacobson E.R., Heard D., Kimbrough J.W. Shell and systemic hyalohyphomycosis in Fly River turtles (Carettochelys insculpta) caused by Paecilomyces lilacinus. J Herp Med Surg. 2005;15:15–19. [Google Scholar]
- 14.Posthaus H., Krampe M., Pagan O., Guèho E., Suter C., Bacciarini L. Systemic paecilomycosis in a hawksbill turtle (Eretmochelys imbricata) J Mycol Med. 1997;7:223–226. [Google Scholar]
- 15.Jacobson E.R., Gaskin J.M., Shields R.P., White F.H. Mycotic pneumonia in mariculture-reared green sea turtles. J Am Vet Med Assoc. 1979;175:929–933. [PubMed] [Google Scholar]
- 16.Hernandez-Divers, SJ, Norton, T, Hernandez-Divers, SM, Strunk, A, Sanchez, S, Currin, P, Garcia, AP. Endoscopic diagnosis of pulmonary granulomas due to Paecilomyces in a juvenile sea turtle. In: Caretta caretta, proceedings of the association of reptile and amphibian veterinarians, Reno, NV; 2002. pp. 3–4.
- 17.Maslen M., Whitehead J., Forsyth W.M., McCracken H., Hocking A.D. Systemic mycotic disease of captive crocodile hatchling (Crocodylus Porosus) caused by Paecilomyces lilacinus. J Med Vet Mycol. 1988;26:219–225. doi: 10.1080/02681218880000311. [DOI] [PubMed] [Google Scholar]
- 18.Guarner J., Brandt M.E. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247–280. doi: 10.1128/CMR.00053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anila K.R., Somanathan T., Mathews A., Jayasree K. Fruiting bodies of Aspergillus: an unusual finding in histopathology. Lung India. 2013;30(4):357–359. doi: 10.4103/0970-2113.120623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González Cabo J.F., Espejo Serrano J., Bárcena Asensio M.C. Mycotic pulmonary disease by Beauveria bassiana in a captive tortoise. Mycoses. 1995;38:167–169. doi: 10.1111/j.1439-0507.1995.tb00043.x. [DOI] [PubMed] [Google Scholar]


