Abstract
We report a 55 year old woman with post-ET PV for 12 years, who experienced resolution of severe constitutional symptoms within 3 days, a marked reduction in splenomegaly and a rapid decline in the JAK2V617F allele burden during combination therapy with interferon-alpha2a and ruxolitinib. Within 4 weeks the patient achieved complete hematological remission with normalization of peripheral blood counts and within 10 months the JAK2V617F-allele burden was reduced from 90% to 28%. Such a rapid decline in the JAK2V617F allele burden is highly unusual in PV-patients during low-dose IFN-alpha2 monotherapy and this finding warrants a prospective study with combination therapy.
Keywords: Polycythemia vera, JAK2V617F-allele burden, Combination therapy, Interferon, Ruxolitinib
Highlights
-
•
Combination therapy with low-dose Interferon (IFN) and ruxolitinib (rux) is feasible.
-
•
IFN and rux promptly alleviates symptoms and rapidly induces complete hematological remission.
-
•
IFN and rux reduces allelic burden more effectively than seen in conventional IFN or rux regimens.
-
•
The combinatorial approach was successful in a patient with prior side-effects to IFN.
1. Case report
A 55-year old woman with PV diagnosed in the precursor stage of ET 12 years ago was referred due to intolerance to hydroxyurea (HU) (fever and exanthema) and pegylated interferon-alpha2b (PEG-Intron) (exanthema). Since 2009 the patient had suffered two episodes of transitory cerebral ischemia (TCI). Accordingly, permanent treatment with clopidogrel had been instituted. Several CT-scans were normal. At the time of referral, the patient received treatment with anagrelide and clopidogrel. On admission, the patient׳s hemoglobin concentration was 11.9 g/dL, the leukocyte count was 21.9×109/L and the platelet count was elevated at 526×109/L. CRP and plasma lactate dehydrogenase (LDH) levels were normal. A bone marrow biopsy was compatible with a diagnosis of PV with grade 1 reticulin fibrosis. A peripheral blood-smear showed no leucoerythroblastosis. The patient still needed phlebotomies to keep the hematocrit below 0.42, a total of four being performed within the last six months prior to referral. An abdominal ultrasound revealed a spleen length of 20 cm giving rise to intermittent spleen pain and abdominal discomfort. Because of hypermetabolic symptoms, pronounced abdominal discomfort, and intolerance of PEG-Intron and HU, treatment with Rux was initiated at a dose of 20 mg twice daily and anagrelide was discontinued. Within the first 3 days of Rux therapy, the patient experienced remarkable clinical improvement with resolution of severe fatigue, night sweats, abdominal pain and pruritus, which had negatively influenced the patient׳s quality of life during the past 2–3 years. Four days after starting Rux, the patient acutely experienced TCI-like symptoms with transient decrease of strength in the left arm and abnormal sensations in the left half of the tongue and neck. The symptoms were very similar to those which the patient had experienced during previous attacks of TCIs. The blood values disclosed a slight decrease in the leukocyte count (17.6×109/L) but an increase in the platelet count (573×109/L). A CT-scan was normal. Because the platelets were elevated, Rux therapy was combined with PEG-IFN-alpha2a (Pegasys) at a low dose of 45 µg every second week. After 1 month a complete hematologic remission (CHR) was achieved (Fig. 1) and after 2.5 months an abdominal ultrasound revealed a reduction in spleen length from 20 cm to 13.8 cm. After 6 months the spleen was no longer palpable.
Fig. 1.
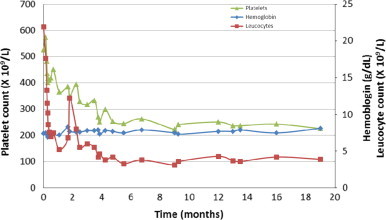
Hemoglobin, leukocyte and platelet levels during combination therapy with Ruxolitinib and Peg-IFN-alpha2a.
The combination therapy was exceedingly well tolerated without any side effects to either drug. Remarkably, despite an advanced PV-stage the JAK2V617F allele burden was rapidly lowered from 90% mutated alleles at referral to 59% at 6 months, 28% at 10 months and 12% at 16 months of therapy (Fig. 2).
Fig. 2.
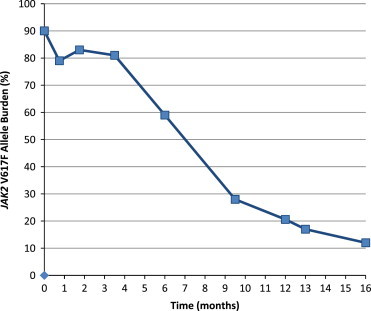
JAK2 V617F allele burden over time.
Furthermore, the patient had both her carotid arteries scanned by ultrasound because of the TCI-symptoms, and the physician (not knowing that the patient had started therapy) described an improvement of the blood-flow (initially turbulent flow, but approximately one year later the flow was more laminar).
2. Discussion
This case report – being the first on combination therapy with IFN and a JAK1-2 inhibitor – has convincingly shown that this combinatorial approach is highly efficacious in a PV-patient with advanced disease and a large tumor burden, as evidenced by pronounced splenomegaly and a high JAK2V617F-allele burden. Most importantly, the treatment was associated with a rapid decline in the JAK2V617F-allele burden within a few months, which has been argued not to be possible due to inhibition of IFN-signaling during JAK-inhibitor treatment. Of note, serial ultrasound examinations of the carotid arteries showed an improvement of the blood-flow. Whether this improvement was attributed to normalization of the hematocrit, leukocyte and platelet-counts or might be due to the anti-inflammatory potential of Rux within the carotid artery wall diminishing inflammation (atherosclerosis being a chronic inflammatory disease) is elusive but certainly warrants further investigation.
The potent efficacy of this combination therapy in our patient may be explained by several mechanisms [1]. Firstly, using Rux with a half-life of approximately 3 h may leave a time-window of a several hours daily in which efficient IFN-signaling is possible. Also the possibility exists that – at a certain level of JAK-inhibition by Rux – the IFN-signaling is merely modulated rather than totally abolished. Secondly, the potent anti-inflammatory effect of JAK1-2 inhibition may have reduced or eliminated the (transient) systemic inflammation response, mediated by the release of inflammatory cytokines in the context of the IFN- mediated tumor killing. Thirdly, by reducing the release of TNF-alpha – a cytokine, which has been shown to facilitate clonal evolution – concomitant JAK1-2 inhibition with Rux might have improved the tumor-reducing effect of IFN. Fourthly, the possibility exists that IFN might actually have augmented the effects of JAK1-2 inhibition, as IFN blocks the intramedullary release of cytokines from bone marrow stroma. These cytokines have been shown to protect JAK2V617F-positive tumor cells from the JAK1-2 inhibitor-induced tumor killing [2]. Fifthly, most recently, chronic inflammation with oxidative stress and generation of reactive oxygen species (ROS) has been argued to be of major importance for clonal evolution and disease progression in MPNs [1–3]. Indeed, MPNs have been shown most recently to be associated with pronounced oxidative stress and ROS accumulation and most lately the JAK2V617F mutation per se has been demonstrated to generate ROS [4]. Since IFN-signaling is impaired by oxidative stress it is most intriguing to consider, if a combinatorial approach with a JAK1-2 inhibitor indeed might improve IFN-signaling – otherwise potentially impaired by oxidative stress mediated by the MPN-clone itself [2,4].
Accordingly, considering all the above anti-inflammatory actions, a combinatorial approach with IFN and a JAK1-2 inhibitor may prove to be more efficacious than single-agent therapy [5]. Furthermore, IFN-a2 also activates dormant stem cells [6] and mobilizes them to be targets for potent JAK1-2 inhibition. Thus, by concurrently depleting dormant JAK2V617F MPN propagating stem cells with IFN-alpha [7] and targeting the proliferating downstream progeny with JAK1-2 inhibitors [6,8], a combination of IFN and a JAK1-2 inhibitor may be a highly efficacious treatment modality in MPNs with superior tumor control and less IFN side-effects [1,2].
Our patient tolerated the combination therapy exceedingly well without side effects or myelosuppression, which otherwise might be a concern, taking into consideration that both JAK inhibition and IFN may be associated with myelosuppression. However, in the context of treating MPN patients with elevated cell counts (the pancytopenic myelofibrosis patient with severe myelofibrosis is not a candidate for IFN and accordingly neither for combination therapy) myelosuppression is not likely to occur provided that low-dose IFN (e.g., Pegasys 45 µg subcutaneously once weekly) and low-dose Rux (e.g., ruxolitinib 10 mg twice daily) are being used. Otherwise, combination therapy with IFN+Rux is not expected to be associated with any particular risk or side effects. In fact, the flue-like symptoms during the initial phase of IFN treatment, being likely associated with “a systemic inflammation response”, may actually vanish, when IFN-a2 is combined with a potent anti-inflammatory agent such as Rux.
In conclusion, describing a single case observation of a PV-patient we have for the first time delivered the “proof of concept” that combination therapy with Rux and “low-dose” IFN is safe, tolerable and highly efficacious in PV, as evidenced by a rapid reduction in the JAK2V617F-allele burden in concert with normalization of blood counts and resolution of pronounced splenomegaly and constitutional symptoms. This observation warrants prospective trials of this combinatorial approach in patients with PV and hyperproliferative MF to assess, if combination therapy induces a more rapid decline in the JAK2V617F-allele burden compared to monotherapy with either drug. In addition, studies are urgently needed to elucidate, if combination therapy – by alleviating potential side effects of IFN, which otherwise might disqualify for further IFN- treatment, might actually rescue IFN-intolerant or IFN-non-responsive patients, thereby improving their quality of life and likely maintaining the goal of achieving major molecular remission and hopefully “minimal residual disease” as well [1,2,9,10].
Acknowledgments
All authors contributed to the writing and editing of the article. Hans Hasselbalch has received a research Grant from Novartis. Novartis had no knowledge regarding this case report and consequently had no influence on the manuscript.
References
- 1.Hasselbalch H.C. Perspectives on the impact of JAK-inhibitor therapy upon inflammation-mediated comorbidities in myelofibrosis and related neoplasms. Expert Rev Hematol. 2014;7:203–216. doi: 10.1586/17474086.2013.876356. [DOI] [PubMed] [Google Scholar]
- 2.Hasselbalch H.C. The role of cytokines in the initiation and progression of myelofibrosis. Cytokine Growth Factor Rev. 2013;24:133–145. doi: 10.1016/j.cytogfr.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Hasselbalch H.C. Chronic inflammation as a promotor of mutagenesis in essential thrombocythemia, polycythemia vera and myelofibrosis. A human inflammation model for cancer development? Leuk Res. 2013;2013;3737:214–220. 214–220. doi: 10.1016/j.leukres.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Marty C., Lacout C., Droin N., Le Couédic J.-P., Ribrag V., Solary E. A role for reactive oxygen species in JAK2 V617F myeloproliferative neoplasm progression. Leukemia. 2013;27:2187–2195. doi: 10.1038/leu.2013.102. [DOI] [PubMed] [Google Scholar]
- 5.Verstovsek S., Mesa R.A., Gotlib J., Levy R.S., Gupta V., DiPersio J.F. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799–807. doi: 10.1056/NEJMoa1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Essers M.A.G., Offner S., Blanco-Bose W.E., Waibler Z., Kalinke U., Duchosal M.A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- 7.Kiladjian J.-J., Cassinat B., Chevret S., Turlure P., Cambier N., Roussel M. Pegylated interferon-alfa-2a induces complete hematologic and molecular responses with low toxicity in polycythemia vera. Blood. 2008;112:3065–3072. doi: 10.1182/blood-2008-03-143537. [DOI] [PubMed] [Google Scholar]
- 8.Hasan S., Lacout C., Marty C., Cuingnet M., Solary E., Vainchenker W. JAK2V617F expression in mice amplifies early hematopoietic cells and gives them a competitive advantage that is hampered by IFNα. Blood. 2013;122:1464–1477. doi: 10.1182/blood-2013-04-498956. [DOI] [PubMed] [Google Scholar]
- 9.Silver R.T., Kiladjian J.-J., Hasselbalch H.C. Interferon and the treatment of polycythemia vera, essential thrombocythemia and myelofibrosis. Expert Rev Hematol. 2013;6:49–58. doi: 10.1586/ehm.12.69. [DOI] [PubMed] [Google Scholar]
- 10.Larsen T.S., Moller M.B., de S.K., Norgaard P., Samuelsson J., Marcher C. Minimal residual disease and normalization of the bone marrow after long-term treatment with alpha-interferon2b in polycythemia vera. A report on molecular response patterns in seven patients in sustained complete hematological remission. Hematology. 2009;14:331–334. doi: 10.1179/102453309X12473408860587. [DOI] [PubMed] [Google Scholar]


