Abstract
Drosophila melanogaster has been a classic model organism for the studies of genetics. More than 15,000 Drosophila genes have been annotated since the entire genome was sequenced; however, many of them still lack functional characterization. Various gene-manipulating approaches in Drosophila have been developed for the function analysis of genes. Here, we summarize some representative strategies utilized for Drosophila gene targeting, from the unbiased ethyl methanesulfonate (EMS) mutagenesis and transposable element insertion, to insertional/replacement homologous recombination and site-specific nucleases such as the zinc-finger nuclease (ZFN), the transcription activator-like effector nuclease (TALEN) and the CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 system. Specifically, we evaluate the pros and cons of each technique in a historical perspective. This review discuss important factors that should be taken into consideration for the selection of a strategy that best fits the specific needs of a gene knockout project.
Keywords: Drosophila, CRISPR, Cas9, Transposons, Homologous recombination, Gene knock-out, Genome editing
Background
Drosophila melanogaster is a well-studied model organism. Because 75% of human disease genes have counterparts in the Drosophila genome [1, 2], fruit flies has been used as a genetic model to study physiological mechanisms and pathological conditions such as aging [3], diabetes [4], neurodegenerative disorders [5], and cancer [6]. With more than 15,000 annotated genes [7], merely 37% of Drosophila genes that have obvious phenotypes are further studied, while the remaining still awaits future investigation [7, 8]. Conventional unbiased forward genetic screens with the employment of ethyl methanesulfonate (EMS) [9] or X-rays [10] have successfully uncovered numerous mutants based on various visible phenotypes. Random-insertion of P-elements is another common method to generate mutants by creating deletions following the excision of P-elements. As for targeted mutagenesis, fly biologists have mainly relied on two forms of homologous recombination-based gene targeting: insertional (“Ends-In”) [11] and replacement (“Ends-Out) [12], for the past decade. Although the aforementioned approaches are powerful in identifying novel functions of the annotated genes, large-scale genetic screenings via these methods may be both labor-intensive and time-consuming.
In recent years, several sequence-guided DNA endonucleases have been applied to generating targeted mutations in model organisms including Drosophila [13]. These site-specific nucleases are programmable, that is, they can induce DNA double-strand breaks (DSBs) that stimulate non-homologous ends-joining (NHEJ) and/or subsequent homologous recombination (HR) at targeted loci [14], therefore generate a frame-shift mutation or a replacement with an extragenous null allele. This type of gene knockout techniques includes the zinc-finger nuclease (ZFN) [13], the transcription activator-like effector nuclease (TALEN) [15], and the CRISPR (clustered regularly interspaced short palindromic repeats)/Cas9 systems [16–18]. Particularly, CRISPR/Cas9 has drawn the attentions of Drosophila biologists because it greatly reduces the time and financial requirement, and makes genome-wide gene knockout projects much more practical. Below we will summarize these important approaches in gene disruption, discuss the pros and cons of each technique, and end with emphasis on the applications and future challenges of CRISPR/Cas9 system.
EMS mutagenesis
The use of potential DNA alkylating agents, especially EMS, has been a standard approach for mutagenesis during the classic era of forward genetic screenings in model organisms. EMS induces new mutations, mostly GC to AT transitions [19], once every ~400 kb at random sites of Drosophila genome [20]. As EMS shows no preference for coding sequence, it is able to disrupt genes unbiasedly and to create mutations of various nature in one gene. Besides, the relative low-cost makes EMS the most commonly used reagent to survey all genes in a mutagenesis scheme aiming to saturate the whole Drosophila genome [9]. Although the ease of use is appealing, several potential pitfalls of EMS mutagenesis should be taken into consideration. First, its phenotype-based approach narrows the investigations to certain discernible phenotypes, such as the changes of eye colors, organ sizes or wing shapes. In this way, mutations generated by EMS may be either overlooked because of the subtle phenotypes or unable to recover due to the lethality. Second, most cases of EMS-based mutagenesis result in point mutations. For a given gene, a single point mutation may only generate a hypomorphic allele with subtle phenotypes. Therefore, the effects of a gene may be underestimated by simply analyzing the hypomorphic mutants, which may lead to an underestimation in the effects of a gene. Third, mapping the mutations induced by EMS is fairly challenging since the mutated loci are not tagged with recognizable sequences, unlike some other approaches such as P-element-mediated disruptions (the details will be discussed later). Besides, EMS often mutates only one strand of the DNA and leaves the unmutated complementary strand, thus causing genetic mosaic in the progeny after rounds of DNA replications [9]. In order to limit the coverage of the genome and to ensure that the phenotype of interest can be transmitted to future generations, screenings following EMS mutagenesis commonly focus on individual chromosomes or chromosome arms. Since the location of an EMS mutation now can be better determined by using high-resolution, high-throughput SNP mapping, identifying the hits from the EMS screening has become relatively promising and more appealing to researchers [21]. Lastly, as EMS may produce multiple hits in the gene of interest [20], verifying the mutation responsible for the scored phenotype requires laborious complementation tests or full-genome sequencing. The time and effort has been considerably reduced nowadays in order to plot the molecular nature of an EMS mutation since the Drosophila genome sequence was released and next-generation sequencing became available [20]. Undoubtedly, EMS mutagenesis is still considered a powerful tool for Drosophila gene manipulating.
Transposon-mediated mutagenesis
Transposons are pieces of DNA that are mobile in the genomes. Taking the nature of mobility, several transposons have been employed for gene disruption and modification in Drosophila, including P-elements, piggyBacs and Minos. Traditionally, P-elements are extensively used for forward mutagenesis either by direct gene disruptions following the insertions or via the imprecise excisions after the second translocation events. As the class II transposon, a P-element moves in the genome through a DNA-based "cut and paste" mechanism and tends to insert near actively transcribed genes, where the chromatin structure is relatively loose [22]. Each P-element encodes a protein called P transposase, which recognizes the inverted repeats of P-element and mobilize itself from the original site. An engineered exogenous P-element for mutagenesis contains an internal deletion that abolishes the translation of the transposase, but such P-elements inserted in the genome can also be mobilized by addition of the transposase gene in the other chromosome via a cross [23]. The mobilized P-elements sometimes remove the flanking DNA, creating a deletion where P-elements have originally been integrated [24]. Thus, despite P-element insertions may not completely disrupt the function of genes; loss-of-function alleles are often generated by the imprecise excision (Figure 1).
Figure 1.
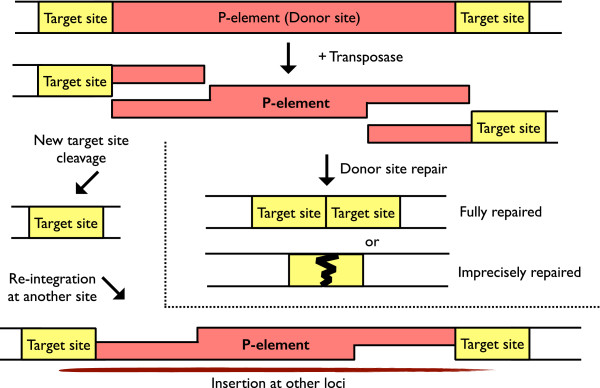
The scheme of P-element transposition. The illustrations show a model of P-element mediated transposition. First, the transposase binds to sequences within both P-element termini and initiates a DSB at each end. The excised P-element could be translocated into a new target site to disrupt another gene (left). Gap repair can then generate duplicated target sites with the P-element sequences at each end. The 3′ extensions left at the donor site can be used for repair either from homologous sequences located in the other copy as a fully repaired gene which contains two adjacent P-element target site (top right), or by non-homologous end-joining for imprecise repair, which could generate products that contain varying lengths of P-element-derived sequences as the imprecisely repaired condition (bottom right).
The transposon-based gene disruption has several advantages that outrank EMS mutagenesis. First, the occurrence of second-site mutations in each individual is relatively low in the P-element system. Visible markers, such as white+, may be engineered to be a part of the transposon, and multi-transposition events can be easily selected out by the presence or absence of markers at the early stages of screening. Second, P-elements show high frequency of mobilization, and they can be modulated via the expression of active transposases that are controlled temporally and spatially. Third, the screen efficiency for the transposon-based mutagenesis largely depends on the marker(s) that can be scored. For example, P-element insertions with white+, as mentioned above, allow molecular mapping by scoring the eye color. Additionally, the insertion sites could be mapped by calculating the recombination rate between different P-element insertions [25], or directly identified by techniques such as inverse PCR [26] or splinkerette PCR [27].
The main disadvantage of P-element-mediated gene disruption is its preference for inserting into “hotspots”, which largely reside in the promotor regions of some genes [28]. Regardless of original insertion loci and the nature of sequence composition, 30–40% of the P-elements land in the same 200–400 genomic hotspots, making the genome-wide, saturated disruption scheme by P-elements less feasible. Interestingly, the preference for hotspots appears to be unique to P-elements and is evidently absent in other transposons, such as piggyBac or Minos, since piggyBac favorably targets TTAA sequences while Minos inserts at random [29]. Although P-elements have once been considered a powerful tool for genome-wide gene disruption, single P-element insertions are only able to disrupt 25% of Drosophila essential genes and about 40% of the total annotated genes, according to the estimation from the Berkeley Drosophila Genome Project (BDGP) [30, 31]. Using piggyBac and Minos has significantly improved the mutagenesis rate to 60% of the genome [32]. However, the low frequency in piggyBac-mediated imprecise excision has hindered them from further creating mutations in the genes of interest directly. Subsequently, the development of Minos-Mediated Integration Cassette (MiMIC) for gene-targeting has improved this weakness. In addition to the backbone of Minos, MiMIC is modified to carry a gene trap cassette flanked by two inverted ΦC31 attP sites. With Minos showing no insertion site preference, targeting every Drosophila gene is feasible and therefore this transposon could potentially accomplish unbiased genome-wide mutagenesis [33]. Taking advantage of ΦC31 attP sites, gene trap cassettes in MiMIC can be easily swapped with any DNA sequence flanked with attB sites by ΦC31 recombinase, allowing further gene modifications and genome editing [33]. For examples, a florescence reporter can be introduced to tag the protein expression and the effects of MiMIC insertions can be reverted after excising the whole cassettes.
Homologous recombination
Mutagenesis via chemicals or transposons is used to create mutations at random sites of the genome, and it has to be followed with screenings to identify phenotypes of interests. This type of classical forward genetic approach has proved its way in identifying novel genes while, in the opposite way, a gene can be specifically mutagenized in a reverse genetic approach once the genome sequence is available. The classical reverse genetic approach for Drosophila utilizes the homologous recombination (HR) to replace genomic DNA. HR regularly occurs at low frequencies in normal cells and it repairs DSBs after DNA damages thus increases genetic variation during meiosis [34]. Some microbes also employ homologous recombination to exchange genetic material between different strains [35].
The HR-mediated gene disruption first requires a fly line harboring a donor DNA that is derived from a P-element cassette, carrying DNA sequences homologous to the target locus flanked FLP Recombinase Target sites (FRTs) and I-SceI recognition site(s). Two enzymes, the site-specific recombinase FLP and endonuclease I-SceI [36, 37], are subsequently introduced into the Drosophila line in order to create DSBs in an inserted donor transgene. The recombination between two FRT sites excises the cassette out from the original site and the cleavage at the I-SceI recognition site(s) creates a DSB, which induces the homology repair machinery that results in HR between the donor DNA and the targeted chromosomal sequence [38]. The arrangement of homologous sequences and I-SceI sites in the donor DNA defines two targeting strategies, Ends-In and Ends-Out, creating a tandem duplication or deletion of the target gene, respectively (Figure 2). Although the Ends-Out targeting is successfully used in mice for gene deletion, the frequency of Ends-Out targeting is relatively low in Drosophila [39, 40]. Ideally, candidates are selected out by losing FRT sites; however, high false positives are also documented due to damaged FRTs that are not ruled out by the scoring method and interfere with the efficiency of gene targeting [39]. A modified donor DNA expressing reaper, a cell death gene, can eliminate progenies that did not undergo recombination events and therefore speed up the screening process [39]. Addition of positive selection markers, such as the eye-specific 3xP3-RFP to facilitate the screening, can also increase the recovery of correct knockout events [41]. On the other hand, genome editing can be fulfilled by introducing the donor DNA, carrying a desired modification to the site of recombination via the Ends-In targeting strategy [42]. Combined the integration of attP site into the gene of interest, repeated genome editing of that gene can be accomplished through the following runs of ΦC31-dependent recombination events [43]. Although it is labor-intensive, this Ends-In homologous recombination has been a reliable method for Drosophila genome editing.
Figure 2.
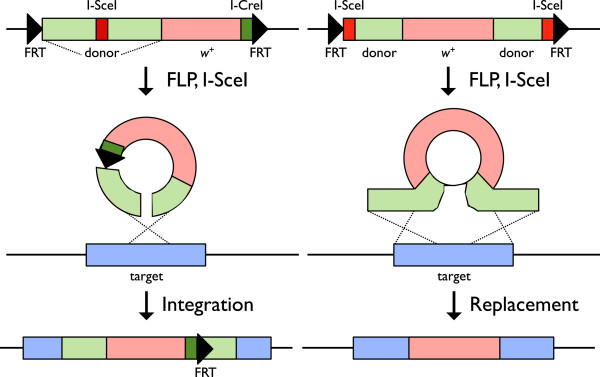
The comparison of Ends-In and Ends-Out homologous recombination. Ends-In and Ends-Out are two paradigms for gene targeting. The major difference is whether the DSB is located within the region of homology (Ends-In) or at the ends (Ends-Out). The figure compares the basic outcomes of these two methods. With ends-in (left), a break is made within the region of homology. Recombination with the target results in a tandem duplication of all the homologous sequence carried on the donor, separated by any sequences that are between the FRT sites (in this case, the white + and I-CreI site). In contrast, Ends-Out provides a simple replacement event between the genome and the homologous sequence. The result is to interrupt the targeted gene with a modified, heterologous sequence, such as the w + marker (right).
Because of the limitations in the replication and transposition of P-elements, the size of donor DNA is limited to approximately 30 kb. The P[acman] vector harbors dual replication origins for maintaining large DNA sequences and for inducing to high copy numbers. Therefore, P[acman] can be used to construct donor DNA up to 100 kb via recombineering-mediated gap repair using Bac or P1 genomic clones as templates [43]. Additionally, ΦC31 integrase-mediated transgenesis significantly increases the efficiency of integrating P[acman] plasmid into the fly genome. Further modifications have advanced the applications of P[acman] in gene targeting and alterations. For examples, the employment of the Gateway system not only facilitates the tagging, but also provides a platform to knock out genes systematically; the introduction of selection markers, such as the eye-specific 3xP3-RFP that mentioned above, eases the screening process; and the combination of Cre-loxP systems allows the removal of the targeting cassettes and therefore reverts the mutations [44].
Although I-SceI-mediated homologous recombination opens the door for scientists to disrupt target genes specifically, the low recombination frequency and numerous false-positives have limited this technique from large screenings. Newer methods, including ZFN, TALEN and CRISPR/Cas9, involve the creating DSBs by nucleases directly in genomic DNA, different from the DSBs generated by SceI that are created in extrachromosal donor DNA. In the presence of genomic DSBs, different repair machineries are employed based on the availability of the homologous template. NHEJ functions by directly joining the two ends of a DSB is the favored way when a homologous template is absent [45]. Because NHEJ-dependent repair ignores DNA deletion or insertion in DSB sites, frame-shift mutations are sometimes created [46]. Alternatively, DSBs can also be repaired through HR either relying on the other copy of undamaged chromosome in a diploid organism or using an exogenous DNA fragment as a template. In the latter case, a donor DNA sequence with homology arms is specifically introduced into the target gene, which results in gene modification in a base pair-precise manner [47].
ZFN and TALEN
ZFN and TALEN are artificial chimeric enzymes that contain a DNA binding domain and a Fok1 DNA-cleavage domain (Figure 3A-B). With the DNA cleavage action itself showing no sequence preference, the specificity of ZFN and TALEN is determined by their DNA-binding domains, which can be engineered to recognize specific sequence in the gene of interest. Each Zinc-finger domain consists of approximately 30 amino acids that bind to 3 base pairs of nucleotides. Instead, TALEN uses the DNA-binding domains from transcription activator-like effectors (TALEs) and recognizes a single base pair. Therefore, linking multiple Zinc-fingers or TALE repeats are required to create a long DNA recognition sequence and increase the specificity of gene targeting. The mutagenesis efficiency of ZFN-induced gene targeting in Drosophila has been estimated to be as high as 1–10% at several loci including ry, coil and pask genes [47, 48]. A test at the y locus via ZFN-targeting has proved that DSBs can enhance the efficiency of gene targeting over simply homologous recombination [47]. ZFN system has evidently facilitated researchers in the aspect of manipulating the genes of interest; however, in Drosophila and other organisms such as the zebrafish [49] and Caenorhabditis elegans [50], the efficiency of TALEN is superior to that of ZFN. Considering the fact that each TALE targets a single nucleotide, it provides better design flexibility than that of a Zinc-finger. Moreover, the length of spacers between DNA-binding sites is less flexible in ZFN than those in TALEN that show less effect on nuclease activity with either an extra or deleted base pair. The specificity of ZFN depends on the affinity between the zinc-finger domain and the target site; therefore, ZFN has to be designed for each specific target. The short binding sites of ZFN increase the possibility of off-target cleavage, which might result in elevated DSBs events in the cells and cause cell death [51]. Even though TALEN outranks ZFN in many ways, it is speculated that TALEN does not release the ends immediately after cleavage and may interfere with the onset of DNA repair [52].
Figure 3.
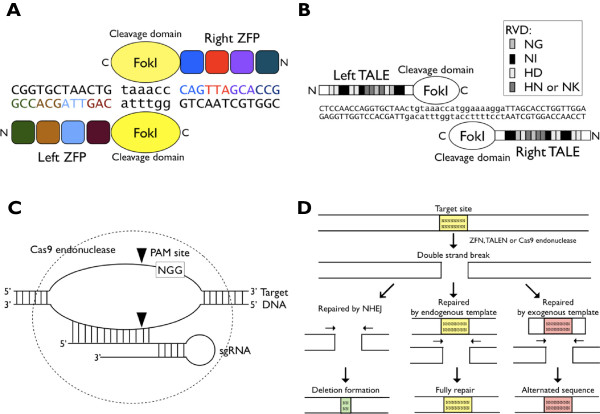
Site-specific endonucleases. Three classic site-specific endonucleases including ZFN, TALEN and CRISPR/Cas9 are shown. (A) ZFN simply consists of a Zinc-finger protein (ZFP) fused to Fok1 endonuclease. The sequence composition of the α-helix in the zinc-finger determines the nucleotide binding specificity of the ZFP. As a result, a ZFP chain can be created by joining a few ZFPs together and generating high specificity, allowing Fok1 endonuclease to accurately cleave DNA at the target site. (B) The C-terminal end of a TALE contains a Fok1 endonuclease for DNA cleavage. The central part of the TALE contains a number of almost similar repeats that mediate specific binding to target loci in the genome, and each of these repeats specifically binds to one base of the target DNA via two amino acids named repeat variable di-residues (RVDs), including NG, NI, HD and HN (or NK) for recognizing one of the four different nucleotides: T, A, C and G, respectively [53, 54]. (C) Cas9 forms a sequence-specific endonuclease when complexed with the sgRNA. The Cas9/sgRNA complex then recognizes the targeted sequence, 20-bp in length, ending with two guanines (NGG) called the PAM site. Cleavage occurs on both strands upstream of the PAM sites. (D) The DSB is first induced by ZFN, TALEN or Cas9 endonuclease and then repaired by three possible mechanisms. When repaired by NHEJ, random deletions would occur at the site (left). When the repair is done by the endogenous template within the genome, the sequence would be fully repaired (middle). If an exogenous modified template is added, the sequence could be altered after repair, which is regarded as the gene editing (right).
Cas9, sgRNA mediated high specificity for gene interruption
Although ZFN and TALEN have demonstrated significant advances in the technology of gene targeting, they still require a new design for each gene, unaccommodating for systematic gene-disruption. CRISPR/Cas9 uses a small guide RNA (sgRNA) to target DNA, dramatically lowers the difficulties for site-specific gene modification. CRISPR/Cas9 is currently considered the most popular tool in the genome-editing era. The system, which originated as a component of prokaryotic innate immunity system [55], recognizes target loci via sgRNA sequence: this 20-bp sgRNA requires an adjourning NGG sequence known as the protospacer adjacent motif (PAM) site for Cas9 recognition [56–58] (Figure 3C). In brief, the editing efficiency of Cas9 to a target gene is deeply related to the selection of sgRNA, including the distance between its cleavage site and the translation start site, the GC content, the strand selection and the last four nucleotides before the PAM site of the sgRNA. All of the factors listed here should be taken into consideration to enhance the performance of Cas9-mediated genome editing [59].
Cas9 has grown in popularity for Drosophila gene modification because of the following advantages over other methods. First, both ZFN and TALEN require the engineering of proteins for the specificity of gene targeting; instead, a change in 20-bp sgRNA is sufficient for Cas9 to distinguish genes and NGG sequences are abundant in the fly genome. Second, several methods have been tested to deliver the Cas9 system to fly embryos: (1) introducing of plasmid vectors or in vitro transcribed RNA encoding Cas9 and sgRNA [60]; (2) injecting sgRNA plasmid DNA into Cas9 transgenic flies, which significantly increases the editing flexibility and efficiency [17]; (3) establishing a transgenic model of Cas9-sgRNA complex, which shows higher efficiency and stability [61]. Third, similar to P[acman], the introduction of selection markers, such as coinjection of a donor vector carrying 3xP3-RFP (or 3xP3-DsRed), with Cas9 can speed up the screening process and Cas9 can create conditional knock-out lines when combined with the Cre-loxP system [60, 62]. These exogenous DNA (3xP3-RFP/3xP3-DsRed or loxP sequence, for examples) are flanked with homology arms about 1 kb long in both ends on the donor plasmid for efficient knock-in [60, 62], and in this way, Cas9-based knock-in has shown better successful rate compared with previous Ends-In techniques [42]. Furthermore, Cas9 can be used as an alternative tactic to create deletion mutations from the pre-existing P-element insertions [60]. With the efficiency up to 88% of injected embryos having mutations, Cas9 can generate mutations rapidly [16]. One thing worthy of attention is the off-target effects when using Cas9-based approaches. The target binding capacity of Cas9 remains the same with 1-3 bp mismatches in target sequence unless mutations are located in the PAM domain. This is significantly different from that of TALEN, which can only tolerate 1-2 bp mismatches [57]. Even though both TALEN and Cas9 can accommodate some mismatches in target sequence, it has been shown that Cas9 has more off-target problems in the mammalian genome [63, 64]. High concentration of sgRNA has been shown to impair Cas9 targeting [64]. It is possible that, in some cases, the off-target effects may outweigh the ease of construct design of the Cas9 system. Different gene disruption strategies should be carefully compared before each project based on the context of targets and the goal of the project.
Conclusions
Since Thomas Morgan and follow researchers have characterized and studied the heritable mutants of thousands of fruit flies, Drosophila has served as a key model system in a wide range of biological researches. The new resources and strategies for gene disruption will undoubtedly contribute to the understanding at the systematic complexity of the gene networks in this small yet sophisticated organism, and to the molecular mechanisms underlying these important biological processes. The pros and cons are discussed above. EMS mutagenesis provides an unbiased screen platform but is labor-intensive. P-elements are easy to trace and to manipulate, but their preference for insertion sites is far from being random. The method involving HR is site-specific but relatively less efficient; however, the combination of ZFN, TALEN or CRISPR/Cas9 with homologous recombination has made the editing efficiency more promising. While gene targeting is simplified by the use of CRISPR/Cas9, it is now feasible to establish a disrupting library that covers all annotated protein-coding genes in Drosophila melanogaster. With the goal of producing a whole-genome mutant collection, scientists have declared the capacity to generate deletion mutants in 50–100 genes per month with an average turnaround time of two months [65]. With the combined efforts of multiple laboratories, it would be possible to generate all of the mutants in a few years, and permit the functional dissection of individual genes in clean genetic knockout background.
The manipulation of exogenous DNA templates in the Cas9 cleavage site could be versatile (Figure 3D). This “knock-in” process allows the generation of a conditional knockout allele by introducing loxP sites, the analysis of the expression pattern by inserting a traceable marker, or the generation of point mutations in the endogenous locus for the structure-function analysis. Together, these genetic tools are valuable assets for researchers not only to uncover the physiological functions of Drosophila genes, but also to offer insights to decipher the complex mechanisms of human biology and diseases in the future.
Acknowledgements
We would like to thank Drs. Adam Haberman, Shu-Yi Huang, Tzu-Yang Lin, June-Tai Wu, and all members of the Chan lab for critical comments on this manuscript. This work was supported by grants from the National Science Council of Taiwan (NSC 101-2320-B-002-051-MY2), the Ministry of Science and Technology of Taiwan (MOST 103-2320-B-002-025), and National Taiwan University (NTUMC 102R39012 and 103R39012) to CCC.
Abbreviations
- EMS
Ethyl methanesulfonate
- ZFN
Zinc-finger nuclease
- TALEN
Transcription activator-like effector nuclease
- CRISPR
Clustered regularly interspaced short palindromic repeats
- DSBs
Double-strand breaks
- NHEJ
Non-homologous end-joining
- HR
Homologous recombination
- BDGP
Berkeley Drosophila Genome Project
- MiMIC
Minos-Mediated Integration Cassette
- FRTs
FLP Recombinase Target sites
- sgRNA
Small guide RNA
- PAM
Protospacer adjacent motif
- ZFP
Zinc-finger protein
- RVDs
Repeat variable di-residues.
Footnotes
Competing interests
The authors declared that they have no competing interests.
Authors’ contributions
SCL and CCC planned the manuscript outline. SCL wrote the draft and generated the figures, YYC and CCC revised and did proof reading. All authors read and approved the final manuscript.
Contributor Information
Shih-Ching Lin, Email: roy047lin@ntu.edu.tw.
Yu-Yun Chang, Email: chang220@umn.edu.
Chih-Chiang Chan, Email: chancc1@ntu.edu.tw.
References
- 1.Reiter LT, Potocki L, Chien S, Gribskov M, Bier E. A systematic analysis of human disease-associated gene sequences in Drosophila melanogaster. Genome Res. 2001;11:1114–1125. doi: 10.1101/gr.169101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandey UB, Nichols CD. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zou S, Meadows S, Sharp L, Jan LY, Jan YN. Genome-wide study of aging and oxidative stress response in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2000;97:13726–13731. doi: 10.1073/pnas.260496697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker KD, Thummel CS. Diabetic larvae and obese flies-emerging studies of metabolism in Drosophila. Cell Metab. 2007;6:257–266. doi: 10.1016/j.cmet.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu B, Vogel H. Drosophila models of neurodegenerative diseases. Annu Rev Pathol. 2009;4:315–342. doi: 10.1146/annurev.pathol.3.121806.151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polesello C, Roch F, Gobert V, Haenlin M, Waltzer L. Modeling cancers in drosophila. Prog Mol Biol Transl Sci. 2011;100:51–82. doi: 10.1016/B978-0-12-384878-9.00002-9. [DOI] [PubMed] [Google Scholar]
- 7.Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, George RA, Lewis SE, Richards S, Ashburner M, Henderson SN, Sutton GG, Wortman JR, Yandell MD, Zhang Q, Chen LX, Brandon RC, Rogers YH, Blazej RG, Champe M, Pfeiffer BD, Wan KH, Doyle C, Baxter EG, Helt G, Nelson CR, et al. The genome sequence of Drosophila melanogaster. Science (New York, NY) 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- 8.Weng MP, Liao BY. DroPhEA: drosophila phenotype enrichment analysis for insect functional genomics. Bioinformatics (Oxford, England) 2011;27:3218–3219. doi: 10.1093/bioinformatics/btr530. [DOI] [PubMed] [Google Scholar]
- 9.St Johnston D. The art and design of genetic screens: drosophila melanogaster. Nat Rev Genet. 2002;3:176–188. doi: 10.1038/nrg751. [DOI] [PubMed] [Google Scholar]
- 10.Bauer H, Demerec M, Kaufmann BP. X-Ray Induced Chromosomal Alterations in Drosophila Melanogaster. Genetics. 1938;23:610–630. doi: 10.1093/genetics/23.6.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie HB, Golic KG. Gene deletions by ends-in targeting in Drosophila melanogaster. Genetics. 2004;168:1477–1489. doi: 10.1534/genetics.104.030882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong WJ, Golic KG. Ends-out, or replacement, gene targeting in Drosophila. Proc Natl Acad Sci U S A. 2003;100:2556–2561. doi: 10.1073/pnas.0535280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bibikova M, Golic M, Golic KG, Carroll D. Targeted chromosomal cleavage and mutagenesis in Drosophila using zinc-finger nucleases. Genetics. 2002;161:1169–1175. doi: 10.1093/genetics/161.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson SP. Sensing and repairing DNA double-strand breaks. Carcinogenesis. 2002;23:687–696. doi: 10.1093/carcin/23.5.687. [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Li C, Yu Z, Huang P, Wu H, Wei C, Zhu N, Shen Y, Chen Y, Zhang B, Deng WM, Jiao R. Efficient and specific modifications of the Drosophila genome by means of an easy TALEN strategy. J Genet Genom = Yi chuan xue bao. 2012;39:209–215. doi: 10.1016/j.jgg.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Bassett AR, Tibbit C, Ponting CP, Liu JL. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 2013;4:220–228. doi: 10.1016/j.celrep.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gratz SJ, Cummings AM, Nguyen JN, Hamm DC, Donohue LK, Harrison MM, Wildonger J, O'Connor-Giles KM. Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease. Genetics. 2013;194:1029–1035. doi: 10.1534/genetics.113.152710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu Z, Ren M, Wang Z, Zhang B, Rong YS, Jiao R, Gao G. Highly efficient genome modifications mediated by CRISPR/Cas9 in Drosophila. Genetics. 2013;195:289–291. doi: 10.1534/genetics.113.153825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pastink A, Heemskerk E, Nivard MJ, van Vliet CJ, Vogel EW. Mutational specificity of ethyl methanesulfonate in excision-repair-proficient and -deficient strains of Drosophila melanogaster. Mol Gen Genet: MGG. 1991;229:213–218. doi: 10.1007/BF00272158. [DOI] [PubMed] [Google Scholar]
- 20.Blumenstiel JP, Noll AC, Griffiths JA, Perera AG, Walton KN, Gilliland WD, Hawley RS, Staehling-Hampton K. Identification of EMS-induced mutations in Drosophila melanogaster by whole-genome sequencing. Genetics. 2009;182:25–32. doi: 10.1534/genetics.109.101998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen D, Ahlford A, Schnorrer F, Kalchhauser I, Fellner M, Viragh E, Kiss I, Syvanen AC, Dickson BJ. High-resolution, high-throughput SNP mapping in Drosophila melanogaster. Nat Methods. 2008;5:323–329. doi: 10.1038/nmeth.1191. [DOI] [PubMed] [Google Scholar]
- 22.Liao GC, Rehm EJ, Rubin GM. Insertion site preferences of the P transposable element in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2000;97:3347–3351. doi: 10.1073/pnas.97.7.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis A, Brookfield JY. Movement of Drosophila melanogaster transposable elements other than P elements in a P-M hybrid dysgenic cross. Mol Gen Genet MGG. 1987;208:506–510. doi: 10.1007/BF00328147. [DOI] [Google Scholar]
- 24.Cooley L, Kelley R, Spradling A. Insertional mutagenesis of the Drosophila genome with single P elements. Science (New York, NY) 1988;239:1121–1128. doi: 10.1126/science.2830671. [DOI] [PubMed] [Google Scholar]
- 25.Zhai RG, Hiesinger PR, Koh TW, Verstreken P, Schulze KL, Cao Y, Jafar-Nejad H, Norga KK, Pan H, Bayat V, Greenbaum MP, Bellen HJ. Mapping Drosophila mutations with molecularly defined P element insertions. Proc Natl Acad Sci U S A. 2003;100:10860–10865. doi: 10.1073/pnas.1832753100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochman H, Gerber AS, Hartl DL. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potter CJ, Luo L. Splinkerette PCR for mapping transposable elements in Drosophila. PLoS One. 2010;5:e10168. doi: 10.1371/journal.pone.0010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tower J, Karpen GH, Craig N, Spradling AC. Preferential transposition of Drosophila P elements to nearby chromosomal sites. Genetics. 1993;133:347–359. doi: 10.1093/genetics/133.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spradling AC, Bellen HJ, Hoskins RA. Drosophila P elements preferentially transpose to replication origins. Proc Natl Acad Sci U S A. 2011;108:15948–15953. doi: 10.1073/pnas.1112960108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bentley A, MacLennan B, Calvo J, Dearolf CR. Targeted recovery of mutations in Drosophila. Genetics. 2000;156:1169–1173. doi: 10.1093/genetics/156.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winkler S, Schwabedissen A, Backasch D, Bokel C, Seidel C, Bonisch S, Furthauer M, Kuhrs A, Cobreros L, Brand M, Gonzalez-Gaitan M. Target-selected mutant screen by TILLING in Drosophila. Genome Res. 2005;15:718–723. doi: 10.1101/gr.3721805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellen HJ, Levis RW, He Y, Carlson JW, Evans-Holm M, Bae E, Kim J, Metaxakis A, Savakis C, Schulze KL, Hoskins RA, Spradling AC. The Drosophila gene disruption project: progress using transposons with distinctive site specificities. Genetics. 2011;188:731–743. doi: 10.1534/genetics.111.126995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venken KJ, Schulze KL, Haelterman NA, Pan H, He Y, Evans-Holm M, Carlson JW, Levis RW, Spradling AC, Hoskins RA, Bellen HJ. MiMIC: a highly versatile transposon insertion resource for engineering Drosophila melanogaster genes. Nat Methods. 2011;8:737–743. doi: 10.1038/nmeth.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Creighton HB, McClintock B. A correlation of cytological and genetical crossing-over in Zea Mays. Proc Natl Acad Sci U S A. 1931;17:492–497. doi: 10.1073/pnas.17.8.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lawrence JG, Retchless AC. The interplay of homologous recombination and horizontal gene transfer in bacterial speciation. Meth Mol Biol (Clifton, NJ) 2009;532:29–53. doi: 10.1007/978-1-60327-853-9_3. [DOI] [PubMed] [Google Scholar]
- 36.Dymecki SM. Flp recombinase promotes site-specific DNA recombination in embryonic stem cells and transgenic mice. Proc Natl Acad Sci U S A. 1996;93:6191–6196. doi: 10.1073/pnas.93.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellaiche Y, Mogila V, Perrimon N. I-SceI endonuclease, a new tool for studying DNA double-strand break repair mechanisms in Drosophila. Genetics. 1999;152:1037–1044. doi: 10.1093/genetics/152.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rong YS, Titen SW, Xie HB, Golic MM, Bastiani M, Bandyopadhyay P, Olivera BM, Brodsky M, Rubin GM, Golic KG. Targeted mutagenesis by homologous recombination in D. melanogaster. Genes Dev. 2002;16:1568–1581. doi: 10.1101/gad.986602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang J, Zhou W, Watson AM, Jan YN, Hong Y. Efficient ends-out gene targeting in Drosophila. Genetics. 2008;180:703–707. doi: 10.1534/genetics.108.090563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou W, Huang J, Watson AM, Hong Y. W::Neo: a novel dual-selection marker for high efficiency gene targeting in Drosophila. PLoS One. 2012;7:e31997. doi: 10.1371/journal.pone.0031997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan CC, Scoggin S, Hiesinger PR, Buszczak M. Combining recombineering and ends-out homologous recombination to systematically characterize Drosophila gene families: Rab GTPases as a case study. Communicative Integr Biol. 2012;5:179–183. doi: 10.4161/cib.18788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao G, McMahon C, Chen J, Rong YS. A powerful method combining homologous recombination and site-specific recombination for targeted mutagenesis in Drosophila. Proc Natl Acad Sci U S A. 2008;105:13999–14004. doi: 10.1073/pnas.0805843105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venken KJ, Carlson JW, Schulze KL, Pan H, He Y, Spokony R, Wan KH, Koriabine M, de Jong PJ, White KP, Bellen HJ, Hoskins RA. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nat Methods. 2009;6:431–434. doi: 10.1038/nmeth.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chan CC, Scoggin S, Wang D, Cherry S, Dembo T, Greenberg B, Jin EJ, Kuey C, Lopez A, Mehta SQ, Perkins TJ, Brankatschk M, Rothenfluh A, Buszczak M, Hiesinger PR. Systematic discovery of Rab GTPases with synaptic functions in Drosophila. Curr Biol. 2011;21:1704–1715. doi: 10.1016/j.cub.2011.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moore JK, Haber JE. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2164–2173. doi: 10.1128/mcb.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durai S, Mani M, Kandavelou K, Wu J, Porteus MH, Chandrasegaran S. Zinc finger nucleases: custom-designed molecular scissors for genome engineering of plant and mammalian cells. Nucleic Acids Res. 2005;33:5978–5990. doi: 10.1093/nar/gki912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beumer K, Bhattacharyya G, Bibikova M, Trautman JK, Carroll D. Efficient gene targeting in Drosophila with zinc-finger nucleases. Genetics. 2006;172:2391–2403. doi: 10.1534/genetics.105.052829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carroll D, Beumer KJ, Trautman JK. High-efficiency gene targeting in Drosophila with zinc finger nucleases. Meth Mol Biol (Clifton, NJ) 2010;649:271–280. doi: 10.1007/978-1-60761-753-2_17. [DOI] [PubMed] [Google Scholar]
- 49.Chen S, Oikonomou G, Chiu CN, Niles BJ, Liu J, Lee DA, Antoshechkin I, Prober DA. A large-scale in vivo analysis reveals that TALENs are significantly more mutagenic than ZFNs generated using context-dependent assembly. Nucleic Acids Res. 2013;41:2769–2778. doi: 10.1093/nar/gks1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wood AJ, Lo TW, Zeitler B, Pickle CS, Ralston EJ, Lee AH, Amora R, Miller JC, Leung E, Meng X, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Meyer BJ. Targeted genome editing across species using ZFNs and TALENs. Science (New York, NY) 2011;333:307. doi: 10.1126/science.1207773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pattanayak V, Ramirez CL, Joung JK, Liu DR. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nat Methods. 2011;8:765–770. doi: 10.1038/nmeth.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beumer KJ, Trautman JK, Christian M, Dahlem TJ, Lake CM, Hawley RS, Grunwald DJ, Voytas DF, Carroll D. Comparing zinc finger nucleases and transcription activator-like effector nucleases for gene targeting in Drosophila. G3 (Bethesda, Md) 2013;3:1717–1725. doi: 10.1534/g3.113.007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science (New York, NY) 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 54.Morbitzer R, Romer P, Boch J, Lahaye T. Regulation of selected genome loci using de novo-engineered transcription activator-like effector (TALE)-type transcription factors. Proc Natl Acad Sci U S A. 2010;107:21617–21622. doi: 10.1073/pnas.1013133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ishino Y, Shinagawa H, Makino K, Amemura M, Nakata A. Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product. J Bacteriol. 1987;169:5429–5433. doi: 10.1128/jb.169.12.5429-5433.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science (New York, NY) 2012;337:816–821. doi: 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mali P, Aach J, Stranges PB, Esvelt KM, Moosburner M, Kosuri S, Yang L, Church GM. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat Biotechnol. 2013;31:833–838. doi: 10.1038/nbt.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science (New York, NY) 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science (New York, NY) 2014;343:80–84. doi: 10.1126/science.1246981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gratz SJ, Ukken FP, Rubinstein CD, Thiede G, Donohue LK, Cummings AM, O'Connor-Giles KM. Highly Specific and Efficient CRISPR/Cas9-Catalyzed Homology-Directed Repair in Drosophila. Genetics. 2014;196:961–971. doi: 10.1534/genetics.113.160713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kondo S, Ueda R. Highly improved gene targeting by germline-specific Cas9 expression in Drosophila. Genetics. 2013;195:715–721. doi: 10.1534/genetics.113.156737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xue Z, Ren M, Wu M, Dai J, Rong YS, Gao G. Efficient gene knock-out and knock-in with transgenic Cas9 in Drosophila. G3 (Bethesda, Md) 2014;4:925–929. doi: 10.1534/g3.114.010496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cradick TJ, Fine EJ, Antico CJ, Bao G. CRISPR/Cas9 systems targeting beta-globin and CCR5 genes have substantial off-target activity. Nucleic Acids Res. 2013;41:9584–9592. doi: 10.1093/nar/gkt714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pattanayak V, Lin S, Guilinger JP, Ma E, Doudna JA, Liu DR. High-throughput profiling of off-target DNA cleavage reveals RNA-programmed Cas9 nuclease specificity. Nat Biotechnol. 2013;31:839–843. doi: 10.1038/nbt.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kondo S. New horizons in genome engineering of Drosophila melanogaster. Genes Genet Syst. 2014;89:3–8. doi: 10.1266/ggs.89.3. [DOI] [PubMed] [Google Scholar]


